Abstract
Objective:
To evaluate the feasibility of an aggressive surgical approach incorporating major hepatic resection after biliary drainage and preoperative portal vein embolization for patients with hilar bile duct cancer.
Summary Background Data:
Although many surgeons have emphasized the importance of major hepatectomy in terms of curative resection for patients with hilar bile duct cancer, this procedure results in a high incidence of postoperative morbidity and mortality in patients with cholestasis-induced impaired liver function.
Methods:
A retrospective cohort study was conducted in 140 patients with hilar bile duct cancer treated from 1990 through 2001. Resectional surgery was performed in 79 patients, 69 of whom underwent major hepatic resection. Thirteen patients underwent concomitant pancreaticoduodenectomy. Preoperative biliary drainage was carried out in all 65 patients who had obstructive jaundice. Portal vein embolization was conducted in 41 of 51 patients undergoing extended right hepatectomy. Short- and long-term outcomes were evaluated.
Results:
No patient experienced postoperative liver failure (maximum total bilirubin level, 5.4 mg/dL). The in-hospital mortality rate was 1.3% (1 in 79, resulting from cerebral infarction). A histologically negative resection margin was obtained more frequently when the scheduled extended hepatic resection was conducted (75% vs 44%, P = 0.0178). The estimated 5-year survival rate was 40% when histologically negative resection margins were obtained, but only 6% if the margins were positive. Multivariate analysis identified the resection margin and nodal status as independent factors predictive of survival.
Conclusions:
Extensive resection, mainly extended right hemihepatectomy, after biliary drainage and preoperative portal vein embolization, when necessary, for patients with hilar bile duct cancer canbe performed safely and is more likely to result in histologically negative margins than other resection methods.
Hilar bile duct cancer remains one of the most difficult management problems in terms of staging and radical treatment. It has long been recognized that surgical resection with complete removal of all cancer tissues offers patients the only chance for cure and long-term survival.1-3 In theory, radical excisional therapy for patients with hilar bile duct cancer often necessitates extensive hepatic resection.4,5
Indeed, local or hilar resections, including the extrahepatic suprapancreatic biliary tract, have been reported to result in a high percentage (76%) of localized regional recurrence even after formal curative resections.6 Although many surgeons have emphasized the importance of hepatic resections,7-19 the percentage of patients undergoing major hepatic resection is still limited, presumably because of the fear of a high incidence of liver failure associated with extensive hepatic resection.
This concern is heightened because the majority of patients with hilar bile duct cancer experience cholestasis-induced impairment of liver function.4,7,16 In addition, controversy appears to exist regarding the selection of patients for whom extensive hepatic resection is indicated, especially those with Bismuth-Corlette (B-C) type I or II tumors,17,18 the type of hepatectomy indicated, ie, right- or left- sided hepatectomy, and whether routine caudate lobe resection is necessary.4,5,9,19 This lack of consensus arises largely from the difficulty in precisely diagnosing the proximal tumor extension before resectional surgery or even during laparotomy.
Over the last 10 years, we have routinely adopted, whenever possible, an aggressive resectional approach comprising extended liver, mainly right hemiliver, resection together with caudate lobe resection as the treatment of choice for potential cure of patients with hilar bile duct cancer. We have applied portal vein embolization (PVE)24,25 and biliary decompression26 as preoperative adjuncts when indicated. In this study, we reviewed our 11-year experience with hilar bile duct cancer in terms of the resectability rate, surgical morbidity and mortality, and long-term outcome.
PATIENTS AND METHODS
From January 1990 to December 2001, 140 patients with hilar bile duct cancer were admitted to the First Department of Surgery, Shinshu University Hospital. There were 100 men and 40 women and their mean age was 68.4 ± 0.7 years (median, 70 y; range, 39-87 y). Hilar bile duct cancer was defined as a tumor in the upper common, right, or left hepatic duct. Papillary cholangiocarcinoma was considered hilar if the base of the tumor originated in the bile duct areas defined previously. Patients with diffuse bile duct involvement were included if the confluence was involved, whereas those with tumors involving the hepatic hilar region but predominantly located in the hepatic parenchyma or gallbladder were excluded.
The principle of our treatment strategy is as follows. The standard curative operation consists of extended right or left hepatectomy, resection of the entire caudate lobe and extrahepatic bile duct, and dissection of the lymph nodes and connective tissues in the hepatoduodenal ligament, posterior to the upper portion of the pancreatic heads, and around the common hepatic artery in an en bloc fashion. The decision of whether right- or left-sided hepatectomy is indicated is made according to the predominant site of the lesion, which is located approximately by ultrasonography (US) and CT scanning. Extended right hemihepatectomy is indicated when the predominant site of involvement is the right hepatic duct or when both hepatic ducts are invaded equally, namely, B-C types I, II, IIIa, and IV, whereas extended left hemihepatectomy is indicated for patients in whom the left hepatic duct is predominantly involved, ie, B-C type IIIb. Extended right hemihepatectomy signifies resection of the right hemiliver, entire caudate lobe, and inferior part of segment IV, whereas extended left hemihepatectomy indicates resection of the left hemiliver, including the spigelian lobe and right hilar region. Gross extrahepatoduodenal ligament lymph node, hepatic, or distant metastases are also evaluated by US and/or CT and their presence is considered evidence of unresectability.
The majority of the patients in our series had been compromised with obstructive jaundice, and biliary decompression was carried out using either percutaneous transhepatic biliary drainage (PTBD)22 or endoscopic retrograde biliary drainage (ERBD) methods. Our policy is to perform unilateral biliary decompression of the lobe that is to remain after resection, even when the communication between the right and left bile duct is interrupted by tumor extension. Longitudinal tumor extension was assessed by direct cholangiography. In patients with B-C type IV cancers, an extended right hemihepatectomy is indicated if the tumor extension is confined to the second segmental ramification of the left bile duct. Conversely, if the tumor was definitely thought to extend peripherally to the second segmental ramification of the left bile duct, the patient was considered to be inoperable. A concomitant pancreaticoduodenectomy was indicated if the tumor’s distal border was considered to be in the lower bile duct and/or massive peripancreatic head lymph node metastases was suspected.
The volume of the entire liver and lobe to be resected were calculated from serial transverse CT scan images. If the scheduled hepatectomy consisted of the removal of more than 60% of the total hepatic parenchyma, preoperative portal vein embolization (PVE) was indicated to decrease the risk of postoperative hepatic failure as described earlier.20,21 PVE using an ileocolic approach was routinely performed through a small incision in the right lower abdomen. At the time of the PVE, the patient was examined for the presence of peritoneal dissemination or lymph node metastases around the end of the ileum; however, only a limited abdominal survey can be performed through the small incision. If the survey’s results were positive, the patient’s condition was considered to be inoperable and the PVE was aborted. Hepatic angiography was carried out in all patients who were possible candidates for radical surgery to evaluate the radial spread of the tumor. The resectional surgery was scheduled 2 to 3 weeks after the PVE procedure once liver hypertrophy had been confirmed by successive CT scans and the serum total bilirubin level was less than 2 mg/dL. Frozen-section histologic assessment of the resection margins was performed during surgery. Additional tissues were resected, if possible, when residual microscopic carcinoma was suspected. If the frozen-section histology revealed that, despite the preoperative imaging studies, the carcinoma had invaded the intrapancreatic bile duct, for which further bile duct resection was impossible, a pancreaticoduodenectomy was indicated at that time.
In principle, patients who underwent resectional surgery did not receive any adjuvant chemotherapy or radiotherapy.
Patient survival was calculated using the Kaplan-Meier method. For the survival results reported here, all deaths related or unrelated to tumor recurrence or secondary to postsurgical complications were regarded as the end points. Differences between the survival rates of groups were assessed by the log-rank test and those at P <0.05 were accepted as statistically significant. Multivariate regression analysis was performed using the Cox proportional hazards model and variables associated with P <0.15 were entered into the final model adopted.23 Data were analyzed using StatView 5.0J software (SAS Institute Inc., Cary, NC).
RESULTS
Results of Preoperative Evaluations and Surgical Procedures
Biliary drainage was performed in 125 of 140 patients. Twenty-four patients were considered to have unresectable disease after the examinations and reviewing the various preoperative studies (Fig. 1). Eleven of these 24 had locally advanced lesions with extensive biliary involvement (n = 9) or portal and/or arterial invasion (n = 2), 8 had distant metastases (liver, lung, and distant lymph node metastases in 4, 1, and 3, respectively), and 5 were considered unfit for the planned major operation as a result of their comorbid conditions. Ten patients were judged to be unresectable as a result of the findings at the time of laparotomy for PVE by the ileocolic approach or during the period between PVE and resectional surgery. Six of these patients were inoperable as a result of peritoneal dissemination, 3 as a result of rapid tumor growth after PVE, and 1 because liver metastases developed after PVE. Thirty-one of these 34 patients considered inoperable before laparotomy for resectional surgery underwent biliary drainage as a palliative procedure.
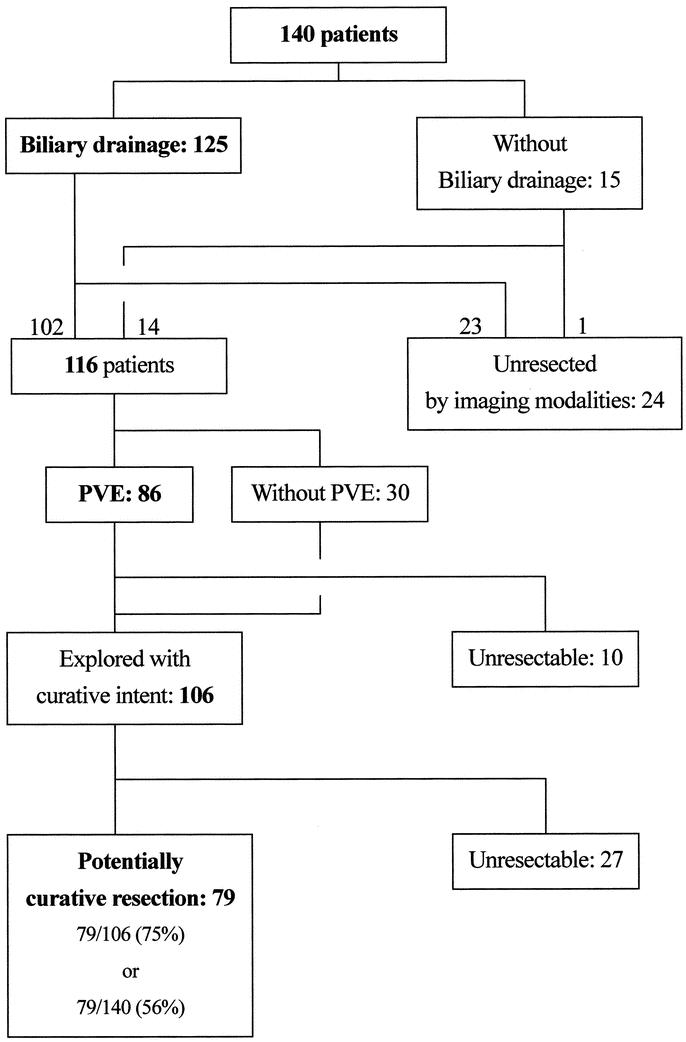
FIGURE 1. Flow diagram of 140 patients admitted to our institute. The group of patients who underwent potentially curative operations includes those in whom the resection margin was revealed to be histologically positive (R1 resection). PVE, preoperative portal vein embolization.
Consequently, 106 patients were considered to have potentially resectable disease and surgical intervention was attempted (Fig. 1). During exploration, 27 patients had findings that precluded curative resection: 11 had distant metastases (5, 4, and 2 to the liver, peritoneum, and paraaortic lymph nodes, respectively) and 16 had locally advanced tumors with extensive biliary involvement.
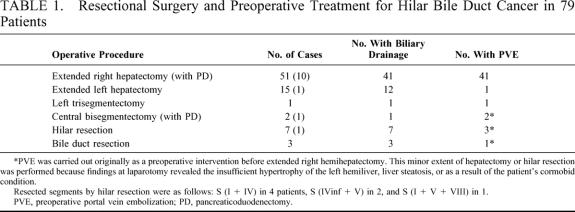
Finally, 79 patients underwent potentially curative resectional surgery (Table 1). Three patients underwent extrahepatic bile duct resection without hepatic resection, whereas 76 (96% of those undergoing resection) underwent hepatectomy in addition to resection of the extrahepatic biliary apparatus. Seven of these patients underwent hilar resection, ie, resection of segments IV and I. These local or hilar resections were adopted as a result of deterioration in the patients’ general conditions, including prolonged cholestasis. Extended hepatic resections were carried out in 69 patients (87% of all those undergoing resectional surgery and 91% of those undergoing hepatic resection) (Table 1). En bloc caudate lobe resection was conducted in all these 69 patients. PVE was not necessary in 10 patients undergoing extended right hepatectomy owing to atrophy of the corresponding lobe secondary to obstruction of the lobar portal or biliary branch. Three extended left hepatectomies were performed, although the predominant tumor was not located at the left bile duct, because the left lobar volumes were less than 25% of the entire liver volume and these lobes were not expected to enlarge sufficiently, even though PVE had been performed. Similarly, in 1 each of the patients undergoing extended left hemihepatectomy, central bisegmentectomy, or hilar resection, the scheduled extended right hemihepatectomy was abandoned because the findings during laparotomy revealed that only segment IV, segments IV, V, and VIII, or segments V and VIII had atrophied, presumably as a result of technical error during the PVE procedure. Five and 2 patients underwent portal vein or hepatic artery resection and reconstruction, respectively. Thirteen patients underwent concomitant pancreaticoduodenectomy in addition to hepatectomy (Table 1) to obtain tumor-free resection margins. Regarding the 51 patients who underwent an extended right hepatectomy, 39 patients had previously undergone biliary drainage, whereas 41 patients had previously undergone PVE. Thirty-three patients had previously undergone both biliary drainage and PVE (Table 1).
TABLE 1. Resectional Surgery and Preoperative Treatment for Hilar Bile Duct Cancer in 79 Patients
Perioperative Results, Morbidity, and Mortality
Sixty-five of the 79 patients who underwent a potentially curative resection had previously undergone biliary drainage. Three complications were observed in 3 patients: peritonitis from the dislodgment of PTBD catheter was observed in 2 patients and pancreatitis associated with an ERBD procedure was observed in 1 patient. In the 2 cases of PTBD catheter dislodgment, emergency laparotomies were performed for peritoneal lavage and the reinsertion of the catheter. The pancreatitis in the remaining patient was treated in a conservative manner.
The respective mean and median blood loss volumes of all the patients who underwent resectional surgery were 1212 ± 70 mL and 1060 mL (range, 300-4040 mL). Eighteen patients (23%) required packed red blood cell transfusion perioperatively (within 1 day of surgery) and the mean and median volumes transfused were 162 ± 38 mL and 0 mL (range, 0-1600 mL).
The following surgery-related complications occurred in 11 patients (14%) who underwent resectional surgery: intraabdominal abscess (2); leakage from the bilioenteric anastomosis (1); leakage from the pancreaticogastric anastomosis (2); jejunal perforation (1); hepatic artery rupture (2); biloma (2); and bleeding from the cut surface of the liver (1). Six of these patients (7.6% of all those who underwent resectional surgery) required reoperation for these complications. The respective mean and median preoperative serum total bilirubin levels, including those of 17 patients who did not experience obstructive jaundice, were 1.2 ± 0.1 mg/dL and 1.0 mg/dL (range, 0.3-4.3 mg/dL), whereas the corresponding postoperative maximum levels were 2.4 ± 0.2 mg/dL and 2.2 mg/dL (range, 0.6-5.4 mg/dL). The maximum level was usually reached 1 to 2 days postoperatively and no patient showed prolonged hyperbilirubinemia (>10 mg/dL after 5 postoperative days24), an indicator of hepatic failure. One 71-year-old male patient developed a cerebral infarction 21 days after extended left hepatectomy and died on postoperative day 31.
Histopathology
Fifty-four of the 79 patients who underwent resectional surgery had histologically negative resection margins (R0 resection), whereas 25 patients had margins with tumor involvement (R1 resection). When the patients were classified according to whether the scheduled extended hepatectomy was performed, ie, extended right hepatectomy for B-C types I, II, IIIa, and IV and extended left hepatectomy for B-C type IIIb, versus all other types of resection, a negative margin was more likely to be achieved in the former group (75% [47 of 63] vs 44% [7 of 16], P = 0.0178). However, the tumor invasion depth and the degree of tumor differentiation were not related to the status of the resection margin.
Survival
At the time of analysis, 32 of the 79 patients who underwent resection were alive at a median follow-up time of 56 months; 29 of these 32 were free of disease, including R1 resection patients, and 3 had disease recurrence. Forty-five patients had died as a result of disease recurrence at a median of 24 months and 2 had died of other causes, 1 of a cerebral infarction (described above) and 1 probably of pneumonia. Fifteen of the 49 patients who underwent surgery 5 or more years ago actually survived for 5 years postoperatively and 14 of them (93%) had negative resection margins. In the meantime, 21 of 22 patients who only underwent exploratory laparotomy died a median of 6.2 months (range, 1.7-32.2 mo) postoperatively.
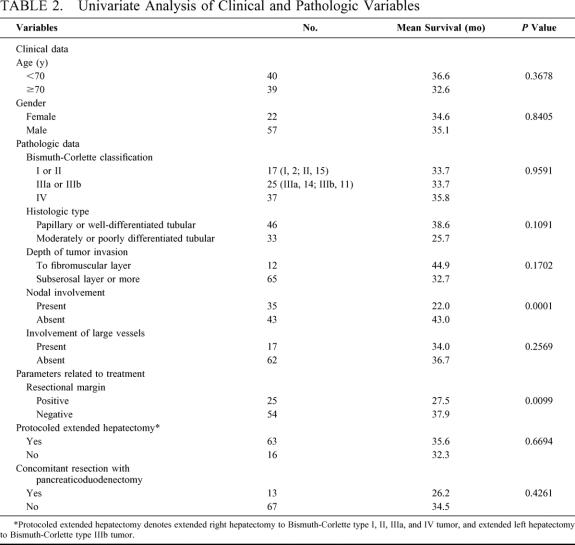
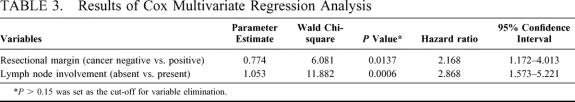
Various clinicopathologic factors potentially associated with patient survival are shown in detail in Table 2 and Figures 2 and 3. The survival curves, stratified according to the B-C tumor classification, did not differ significantly among the groups. Resection with a histologically negative margin (R0 resection) resulted in higher patient survival than that with a positive margin (R1 resection; Fig. 2; P = 0.0099). Comparison of the patients with positive resection margins with those who underwent exploration but were deemed unresectable because of locally advanced tumors showed the former survived longer than the latter (28 vs 10 mo; P <0.0001). Patients whose resected lymph nodes showed no cancer involvement survived longer than those with lymph node metastases (Fig. 3; P= 0.0001). Univariate analysis showed that other variables, such as cancer involvement of the portal vein and/or hepatic artery, tumor invasion depth, diffuse bile duct involvement requiring pancreaticoduodenectomy, and whether patients underwent the scheduled extended hepatectomy, were not significantly associated with patient survival (Table 2). Multivariate analysis using the Cox proportional hazards model identified a negative resection margin and absence of lymph node involvement by the tumor as factors that independently contributed to prolonged patient survival (Table 3).
TABLE 2. Univariate Analysis of Clinical and Pathologic Variables
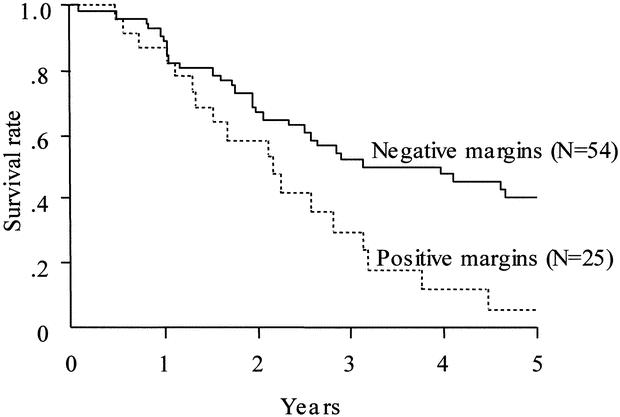
FIGURE 2. Patient survival stratified according to the resection margin status. The solid line indicates survival of patients with histologically negative margins (n = 54; median survival, 37.4 mo; 1-, 3-, and 5-y survival rates = 90.4%, 52.0%, and 39.9%, respectively). The dotted line indicates survival of patients with histologically positive margins (n = 25; median survival, 26.3 mo; 1-, 3-, and 5-y survival rates = 87.1%, 24.2%, and 6.0%, respectively). Survival was significantly longer in patients with negative than positive margins (P = 0.0099).
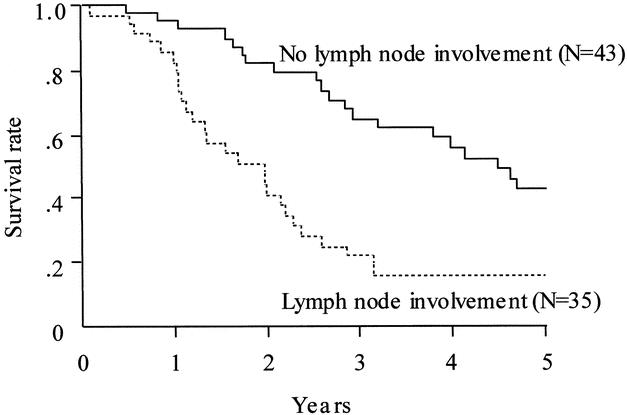
FIGURE 3. Patient survival stratified according to the lymph node involvement status. The solid line indicates survival of patients without lymph node involvement (n = 43; median survival, 53.7 mo; 1-, 3-, and 5-y survival rates = 95.1%, 64.7%, and 42.4%, respectively). The dotted line indicates survival of patients with lymph node involvement (n = 35; median survival, 23.5 mo; 1-, 3-, and 5-y survival rates = 82.4%, 22.2%, and 15.9%, respectively). Survival was significantly longer in patients without than with lymph node involvement (P = 0.0001).
TABLE 3. Results of Cox Multivariate Regression Analysis
DISCUSSION
The most striking characteristics of the present series of patients with hilar bile duct cancer is the low in-hospital death rate (1 of 79) even though extended, mainly right, hepatectomy was carried out routinely in a high proportion of patients (69 of 79). The proportions of all the patients who underwent resectional surgery and who also underwent hepatic resection, major hepatic resection, and extended right hepatectomy were 96%, 87%, and 65%, respectively. The low mortality rate can be further emphasized in view of the fact that the present series included 13 patients who underwent additional pancreaticoduodenectomy. To date, several centers have reported results of surgery for patients with hilar bile duct cancer, including considerable numbers who underwent major hepatectomy. The in-hospital mortality rates in these series ranged from 2.3% to 18%.2,8,10-15,19,24 Therefore, discussion has mainly focused on the rationale for application of extended hepatectomy and the safety of this procedure.
Many surgeons appear to agree with the importance of concomitant hepatectomy when performing resectional surgery for hilar bile duct cancer in terms of both obtaining a negative resection margin and increasing the number of resectable patients.4,7,11,13,19 Nevertheless, consensus does not appear to have been reached regarding the kind of hepatectomy that should be performed (right or left hepatectomy, or hilar resection) and the types of patients for whom hepatectomy is indicated.
In the present series, we performed extended right hemihepatectomy routinely for all patients with B-C types I, II, IIIa and IV tumors and left hemihepatectomy for those with B-C type IIIb. Caudate lobectomy was added systematically for all patients who underwent extended hepatic resections. In most cases of hilar bile duct cancer in which the right and left hepatic ducts are involved to a similar extent (B-C types I, II, and IV), right hepatectomy is, in theory, more likely to be associated with a negative resection margin than left hepatectomy based on the following anatomic considerations.
First, the extrahepatic part of the left hepatic duct is longer with a more distant segmental ramification than that of the right hepatic duct.25 Second, the common bile duct is on the right side of the hepatoduodenal ligament with the right hepatic artery passing behind its proximal portion. Therefore, the right hepatic artery is frequently invaded by cancer at this site, whereas the left (and middle) hepatic artery travels along the left side of the hepatoduodenal ligament and is not associated with the bile duct until the end of the transverse portion. Third, systematic caudate lobectomy, which is usually necessary for curative resection for hilar bile duct cancer,4,12,13 can be carried out more easily in patients undergoing right-sided than left-sided hepatectomy. Finally, in patients for whom portal venous resection at the hepatic hilum is necessary as a result of tumor invasion, it is easier to perform venous reconstruction with the left than the right portal vein, because of the long extrahepatic portion of the transverse portion of the former.27 However, we performed left hepatectomy for patients in whom the left hepatic duct was dominantly invaded, ie, B-C type IIIb. Accordingly, 11 of 69 patients underwent extended left hepatectomy. Furthermore, we decided to carry out left-sided hepatectomy for 3 other patients, even though their cancers were located at the confluence, because CT-based volumetry revealed that the proportion of the left lobar volume to the total liver volume was unacceptably small (<25%) and it was thought that, despite PVE, it would not exceed 40%. In addition, we had to perform 1 extended left hepatectomy and 1 central bisegmentectomy in patients scheduled for extended right hepatectomy as a result of technical errors with PVE.
Other local or hilar resections were performed because we decided that the patients’ impaired general conditions did not allow major hepatectomy. As expected, the likelihood of obtaining a histologically negative margin increased when the patients underwent their scheduled extensive hepatic resections according to the protocol (75% vs 44%).
With respect to the surgical procedure, some surgeons have insisted that local excision can be performed for patients with B-C type I cancers; or the smallest necessary hepatic segmentectomy, including hilar resection, should be carried out after precise evaluation of the extent of cancer invasion in each segmental bile duct.4 We argue against these proposals and claim that major hepatectomy should be performed for all patients with B-C type I to IV tumors, if their condition permits, based on the following considerations.
Although direct cholangiography is thought to play a major role in preoperative imaging diagnosis,28-30 it is not accurate enough to provide precise information about longitudinal cancer extension. This anxiety is compounded by the fact that hilar bile duct cancers often show submucosal tumor extension at their proximal margins, which, in theory, might not be visualized by cholangiography.12,31 Preoperative histologic evaluations such as biopsy or brush cytology are not accurate enough either, as a result of their limited sensitivities.32-34
Moreover, skip-type lesions, which are often encountered in patients with hilar bile duct cancer, render assessment by imaging and biopsy modalities difficult. This aggressive approach can be justified in view of the fact that resectional therapy, even with a histologically positive margin, still offers a significant benefit over palliative treatment in terms of both survival and quality of life.7,16
In the present series, 5 patients in whom resection resulted in a positive margin (R1 resection) survived for over 3 years and, at the time of this writing, 1 of them was alive with disease recurrence 6 years 2 months after surgery. The estimated survival rates after R1 resection (24% and 6% at 3 and 5 y, respectively) were significantly higher than those of 16 patients who only underwent exploratory laparotomy as a result of local tumor advancement (0% and 0%, respectively, P <0.0001).
The apparently high frequency of a positive resection margin, 25% even in patients undergoing scheduled extensive hepatectomy, despite the routine performance of major hepatic resection can be explained by the aggressive resectional approach to patients with locally advanced tumors (B-C type IV). The results of our multivariate analysis, which identified a negative histologic margin and negative lymph node involvement as independent factors contributing to a beneficial outcome, appears to agree quite well those reported previously, whereas whether the scheduled extended hepatectomy was carried out was not an independent prognostic factor. This result can be explained by the consideration that to perform the extended right or left hepatectomy according to the dominant side of tumor location and to secure the negative histologic margin is both clinically and statistically equivalent.
In summary, the limitations of the currently available preoperative diagnostic modalities only allow us to evaluate tumor extension approximately, ie, whether it is right-side dominant, left-side dominant, or both sides are invaded equally. Therefore, we insist that the surgical strategy for hilar bile duct cancer should simply comprise right or left hepatectomy according to the predominant side of carcinoma invasion. Caudate lobectomy is also an essential component of radical surgery, because the incidence of cancer involvement of the caudate branch is high and it is difficult to predict this involvement before the resected specimen is assessed histologically.
The current aggressive attitude toward extensive hepatectomy can be justified only on the basis of low surgical morbidity and mortality. We think the low morbidity and mortality rates experienced in this study are related to the routine application of preoperative biliary drainage and PVE after a precise evaluation of the hepatic lobar volume.
The role of preoperative biliary drainage has been a matter of debate.35-41 Several studies, including randomized, controlled ones, showed that preoperative drainage offered no advantage.38-41 However, these studies were designed to evaluate the role of preoperative biliary drainage mainly in patients who were scheduled to undergo pancreatoduodenectomy,35-40 and they only included a small proportion of patients undergoing hepatectomies who were considered most likely to benefit from preoperative drainage by preventing cholestasis-induced impairment of liver function.35-41 Indeed, Blumgart et al., who have opposed the need for preoperative drainage, claimed that even the resection of a caudate lobe is not a minor undertaking in a jaundiced patient.16 Furthermore, a major disadvantage of preoperative drainage reported in previous studies was development of catheter-related infections, the majority of which, we think, can be ascribed to technical inexperience. To avoid catheter-related infectious complications, we have made it a principle to drain only the hemihepatic lobe destined to remain after hepatectomy, because the rate of infection increases with the number of stents.4 We also recommend a unilateral approach in view of the enhanced hypertrophic process in the future remnant lobe.42 Similarly, we do not always carry out meticulous evaluation of the intrahepatic biliary tree by direct cholangiography43 in view of the limitations of this procedure and because of the fear that the risk of infection increases when contrast medium is forcefully injected into the biliary system.
Although PVE has been widely accepted as a preoperative adjunct to induce compensatory hypertrophy in the future remnant liver, strict indication criteria have not yet been established in randomized, controlled studies. The incidence of liver failure after hepatectomy for hilar bile duct cancer has been reported to range from 2.5% to 29% (median 11% in the cited references),2,4,7,8,11-15,19,24 and the overall surgical mortality cited in these reports varied from 2.3% to 18% (median, 9.2%). The variation in these values can be explained by the different criteria used to define liver failure and the varying degrees of extensive surgical aggressiveness. In view of the fact that 69 (91%) of the 76 hepatectomies in the present series were major hepatic resections, the lack of liver failure and 1.3% (1 in 79) incidence of in-hospital mortality can be considered a remarkably low figure. Jamagin et al. reported that infectious complications comprised the majority of postoperative morbidity and insisted that liver failure, which by itself was a rare complication, was the underlying cause of very few deaths.
However, because the liver constitutes a major part of the reticuloendothelial system and plays a central role in metabolism, the demerit of impaired postoperative liver function can never be underestimated. Patients with borderline liver function are more likely to develop infectious complications than those with normal liver function, and anastomotic leakage is often difficult to stop in such patients.
Despite the high proportion of major hepatectomies, the resection rate and overall 5-year survival rate were within the ranges previously reported (37-80% and 10-33%, respectively).2,4,7,8,11-15,19,24 However, these figures cannot simply be compared, because the institutions concerned had different referral systems and the patient populations and disease stages differed. Obviously, a better staging system that can be used by all institutions that will enable direct comparisons to be made is necessary.
In conclusion, the results of the present series of patients have provided a rationale for the routine application of extensive hepatectomy after biliary drainage and preoperative portal vein embolization for patients with hilar bile duct cancer. This procedure can be performed safely and is more likely than other resection techniques to be associated with a histologically negative resection margin.
Footnotes
Reprints: Seiji Kawasaki, MD, Professor & Surgeon-in-Chief, Second Department of Surgery, Juntendo University, Faculty of Medicine, 2-1-1, Hongo, Bunkyo-ku, Tokyo 113-8421,Japan. E-mail: kawasaki@med.juntendo.ac.jp.
REFERENCES
- 1.Hadjis NS, Blenkharn JI, Alexander N, et al. Outcome of radical surgery in hilar cholangiocarcinoma. Surgery. 1990;107:597–604. [PubMed] [Google Scholar]
- 2.Pichlmayr R, Weinmann A, Klempnauer J, et al. Management of proximal cholangiocarcinoma by surgical resection and radiotherapy. A single-center experience. Ann Surg. 1996;224:628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baer HU, Stain SC, Dennison AR, et al. Improvement in survival by aggressive resections of hilar cholangiocarcinoma. Ann Surg. 1993;217:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyagawa S, Makuuchi M, Kawasaki S. Outcome of extended right hepatectomy after biliary drainage in hilar bile duct cancer. Arch Surg. 1995;130:759–763. [DOI] [PubMed] [Google Scholar]
- 6.Mittal B, Deutsh M, Iwatsuki S. Primary cancers of the extrahepatic biliary passages. Int J Radiat Oncol Biol Phys. 1985;11:849–855. [DOI] [PubMed] [Google Scholar]
- 7.Launois B, Terblanche J, Lakehal M, et al. Proximal bile duct cancer:high resectability rate and 5-year survival. Ann Surg. 1999;230:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figueras J, Llado L, Vallas C, et al. Changing strategies in diagnosis and management of hilar cholangiocarcinoma. Liver Transpl. 2000;6:786–794. [DOI] [PubMed] [Google Scholar]
- 9.Nimura Y, Hayakawa N, Kamiya J, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;12:535–543. [DOI] [PubMed] [Google Scholar]
- 10.Tashiro S, Tsuji T, Kanematsu K, et al. Prolongation of survival for carcinoma at the hepatic duct confluence. Surgery. 1993;113:270–278. [PubMed] [Google Scholar]
- 11.Miyazaki M, Ito H, Nakagawa K, et al. Aggressive surgical approaches to hilar cholangiocarcinoma:hepatic or local resection? Surgery. 1998;123:131–136. [PubMed] [Google Scholar]
- 12.Ogura Y, Kawarada Y. Surgical strategies for carcinoma of the hepatic bile duct confluence. Br J Surg. 1998;85:20–24. [DOI] [PubMed] [Google Scholar]
- 13.Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223:384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madariaga JR, Iwatsuki S, Todo S, et al. Liver resection for hilar and peripheral cholangiocarcinomas:a study of 62 cases. Ann Surg. 1998;227:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke EC, Jarnagin WR, Hochwald SN, et al. Hilar cholangiocarcinoma. Pattern of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;28:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140:170–176. [PubMed] [Google Scholar]
- 19.Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imamura H, Shimada R, Kubota M, et al. Preoperative portal vein embolization:an audit of 84 patients. Hepatology. 1999;29:1099–1105. [DOI] [PubMed] [Google Scholar]
- 21.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma:a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 22.Makuuchi M, Bandai Y, Ito T, et al. Ultrasonically guided percutaneous transhepatic bile drainage:a single-step procedure without cholangiography. Radiology. 1980;136:65–169. [DOI] [PubMed] [Google Scholar]
- 23.Cox D, Oakes D. Analysis of Survival Data. London: Chapman and Hall; 1983. [Google Scholar]
- 24.Nagino M, Kamiya J, Uesaka K, et al. Complications of hepatectomy for hilar cholangiocarcinoma. World J Surg. 2001;25:1277–1283. [DOI] [PubMed] [Google Scholar]
- 25.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. [DOI] [PubMed] [Google Scholar]
- 26.Imamura H, Makuuchi M, Sakamoto Y, et al. Anatomical keys and pitfalls in living donor liver transplantation. J Hepatobiliary Pancreat Surg. 2000;7:380–394. [DOI] [PubMed] [Google Scholar]
- 27.Weter LA, Ring EJ, Pellegrini CA, et al. Differential diagnosis of sclerosing cholangiocarcinomas of the common hepatic duct (Klatskin tumors). Am J Surg. 1991;161:57–62. [DOI] [PubMed] [Google Scholar]
- 28.Adam A, Benjamin IS. Assessment of diagnostic techniques for biliary obstruction and liver masses. In: Blumgart LH, ed. Surgery of the Liver and Biliary Tract, 2nd ed, vol 1. New York: Churchill Livingstone; 1994:401–413. [Google Scholar]
- 29.Cameron JL, Pitt HA, Zinner MJ, et al. Management of proximal cholangiocarcinomas by surgical resection and radiotherapy. Am J Surg. 1990;159:91–98. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto E, Nimura Y, Hayakawa N, et al. The pattern of infiltration at the proximal border of hilar bile duct carcinoma. A histological analysis of 62 resected cases. Ann Surg. 1998;227:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis:results of a prospective study. Gastrointest Endosc. 1995;42:565–572. [DOI] [PubMed] [Google Scholar]
- 32.Scoefl R, Haefner M, Wrba F, et al. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand J Gastroenterol. 1997;32:363–368. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama A, Imamura H, Shimada R, et al. Proximal bile duct stricture disguised as malignant neoplasm. Surgery. 1999;125:514–521. [PubMed] [Google Scholar]
- 34.Nakayama T, Ikeda A, Okuda K. Effect of percutaneous transhepatic drainage on liver function and postoperative mortality. Surg Gynecol Obstet. 1982;155:161–166. [PubMed] [Google Scholar]
- 35.Lygidakis NJ, van der Heyde MN, Lubbers MJ. Evaluation of preoperative biliary drainage in the surgical management of pancreatic head carcinoma. Acta Chir Scand. 1987;153:665–668. [PubMed] [Google Scholar]
- 36.Smith RC, Pooley M, Gorge CRP, et al. Preoperative percutaneous transhepatic internal drainage in obstructive jaundice:a randomized controlled trial examining renal function. Surgery. 1985;97:641–648. [PubMed] [Google Scholar]
- 37.McPherson GAD, Benjamin IS, Hodgson HJF, et al. Pre-operative percutaneous transhepatic biliary drainage:the results of a controlled trial. Br J Surg. 1984;71:371–375. [DOI] [PubMed] [Google Scholar]
- 38.Pitt HA, Gomes AS, Lois JF, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisters PWT, Hudec WA, Hess KR, et al. Effect of preoperative biliary decompression on pancreaticoduodenectomy-associated morbidity in 300 consecutive patients. Ann Surg. 2001;234:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hochwald SN, Burke EC, Jarnagen WR, et al. Association of preoperative biliary stenting with increased postoperative infectious complications in proximal cholangiocarcinoma. Arch Surg. 1999;134:261–266. [DOI] [PubMed] [Google Scholar]
- 41.Noie T, Sugawara Y, Imamura H, et al. Selective versus total drainage for biliary obstruction in the hepatic hilus:an experimental study. Surgery. 2001;130:74–81. [DOI] [PubMed] [Google Scholar]
- 42.Nimura Y. Staging of biliary carcinoma:cholangiography and cholangioscopy. Endoscopy. 1993;25:76–80. [DOI] [PubMed] [Google Scholar]
- 43.Kamiya J, Nimura Y, Hayakawa N, et al. Preoperative cholangiography of the caudate lobe:surgical anatomy and staging for biliary carcinoma. J Hepatobiliary Pancreat Surg. 1994;4:385–389. [Google Scholar]