Abstract
Objective:
To assess the technical and oncologic results of anatomic hepatic bi- and trisegmentectomies.
Summary Background Data:
Regardless of their size, some tumors require extensive hepatectomy only because they are located centrally or in the vicinity of major portal pedicles or hepatic veins. Anatomic bi- and trisegmentectomy might represent an alternative to extensive hepatectomies in such cases.
Methods:
Of 435 liver resections, 32 cases (7%) included 2 or 3 adjacent segments (left lateral sectionectomies, ie, bisegmentectomies 2–3, excluded). There were 16 central hepatectomies (segments 4, 5, and 8), 7 right posterior sectionectomies (segments 6 and 7) and 2 central anterior (segments 4b and 5), 1 central posterior (segments 4a and 8), 2 right superior (segments 7 and 8), 3 right inferior (segments 5 and 6), and 1 left anterior (segments 3 and 4b) bisegmentectomies. Indications were malignant disease in 29 patients, including 15 with cirrhosis and 2 with benign tumors. External landmarks, selective devascularization, and intraoperative ultrasound were used to achieve anatomic resection.
Results:
Mortality, transfusion, and morbidity rates were 0%, 26%, and 19%, respectively. Mean section margin was 9 mm (range, 1-40 mm). Isolated intrahepatic recurrence occurred in 7 patients (24%) and 3 (43%) underwent repeat hepatectomy.
Conclusion:
Anatomic bi- or trisegmentectomy is a safe alternative to extensive liver resection in selected patients, avoiding unnecessary sacrifice of functional parenchyma and enhancing the opportunity to perform repeat resections in cases of recurrence.
Surgical resection is the standard treatment for malignant liver tumors and selected benign lesions. Large tumors require extensive resection (ie, hemihepatectomy or trisectionectomys), whereas smaller lesions can be treated with more limited resections such as segmentectomies. Segmental resections have been developed thanks to a better knowledge of hepatic anatomy and the increased use of intraoperative ultrasound.1–3 Monosegmentectomies can be used for peripheral lesions, but some tumors might require extensive liver resection only because they are located centrally or in the vicinity of major portal pedicles or hepatic veins. Anatomic bi- and trisegmentectomy could represent an alternative to extensive hepatectomies in such cases. Advantages include prevention of postoperative liver failure, especially in patients with underlying liver disease, and the increased opportunity to perform repeat resections in cases of recurrent malignancy. However, the operative results and oncologic value of such resections have been little studied. We review our experience with these less usual anatomic liver resections.
PATIENTS AND METHODS
From 1991 to 2001, 435 patients underwent liver resection for malignant or benign liver disease. Of these, 31 (7%) had 32 resections, including 2 or 3 adjacent anatomic segments defined according to the IHPBA terminology derived from Couinaud’s classification4; these are the subject of this retrospective study. The commonly performed left lateral sectionectomy (bisegmentectomy 2–3) was excluded from this study.
Technique
General Principles
Anatomic bi- or trisegmentectomies were defined according to a classification summarized in Table 1. Our technical approach to liver resection has been described previously.5 In summary, a bilateral subcostal incision with upper midline extension was used in all cases. Intraoperative ultrasonography was always used to screen lesions missed on preoperative evaluation and to confirm the vascular anatomy of the planned type of resection. The liver was mobilized and hepatic veins controlled extrahepatically.5 Portal triad and hepatic veins were encircled by umbilical tapes, and clamping, when required, was applied by a tourniquet. When the underlying liver was normal and a clamping time of less than 30 minutes was anticipated, continuous vascular occlusion was applied. In all other cases, intermittent clamping (15-min clamping periods separated by 5-min intervals) was used. Transection of the liver parenchyma was undertaken using a crushing technique with a hemostat clamp. Vessels and bile ducts were secured using bipolar cautery, hemoclips, or polyglycolic ligatures according to their size.
TABLE 1. Classification of Types of Anatomic Hepatic Resections in 31 Patients who Underwent 32 Bi- or Trisegmentectomies for Hepatic Tumors
Specific Technical Features
Preoperative anatomic location of the lesions used ultrasonography, computerized tomography, and magnetic resonance examinations. Intraoperative anatomic definition of the limits of segments and sectors to be resected was obtained by a combination of 1) external anatomic landmarks including umbilical fissure (ie, round and falciform ligaments) and gallbladder fossa; 2) selective devascularization or clamping to create ischemic margins; and 3) intraoperative ultrasound used to identify the plane of the middle and right hepatic veins and the plane of portal bifurcation. Resections were performed anatomically.
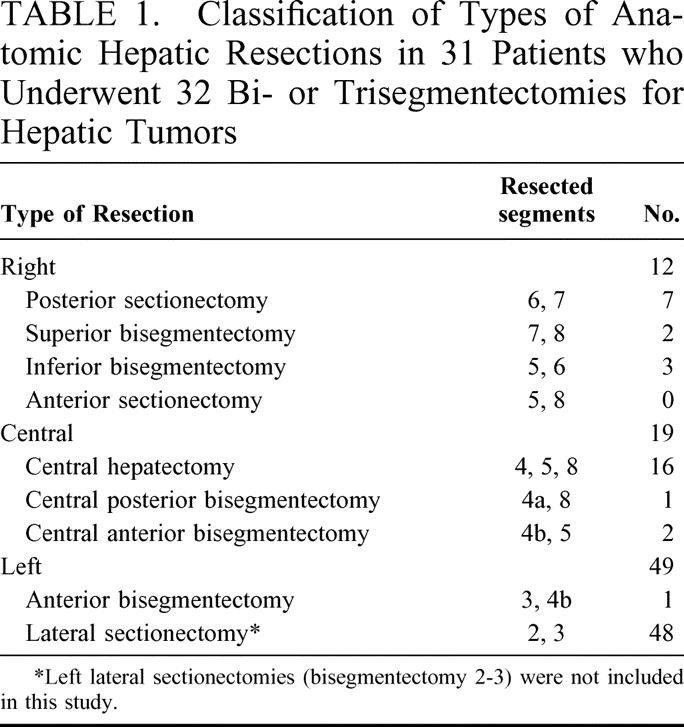
The 2 most commonly performed operations were central hepatectomy (resection of segments 4, 5, and 8; 16 patients) and right posterior sectionectomy (bisegmentectomy 6–7; 7 patients), which accounted together for 72% of all resections (Fig. 1).
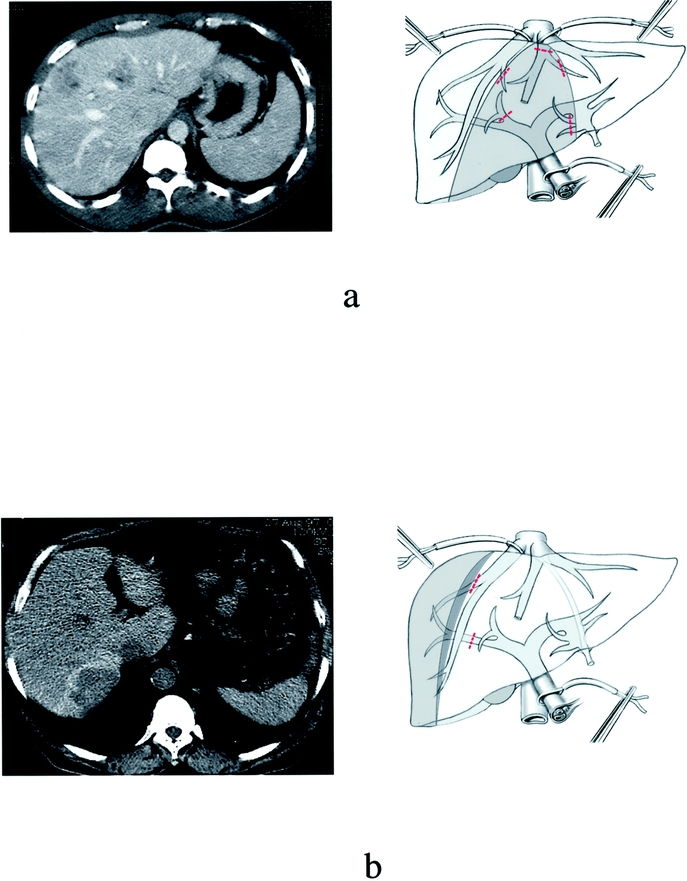
FIGURE 1. Examples of central hepatectomy and right posterior sectionectomy for liver tumors performed in 16 patients and 7 patients, respectively; preoperative imaging (left side column) and corresponding diagrams (on the right side column). (a) Planned central hepatectomy (segments 4, 5, and 8) for bifocal hepatocellular carcinoma (HCC) on chronic hepatitis B. (b) Planned right posterior sectionectomy (segments 6 and 7) for cholangiocellular carcinoma.
Central Hepatectomy
This procedure involved the resection of liver territory drained by the middle hepatic vein. In the sagittal plane, suprahilar clamping of the right anterior sectional portal pedicle and division of the pedicles of segment 4 in the umbilical fissura plane provided inflow control. This resulted in 2 lines of demarcation along the right intersectional plane and along the falciform ligament (left intersectional plane). The liver parenchyma was transected along both ischemic lines resulting in 2 cut surfaces. The middle hepatic vein was divided at the end of the transection. Because the double transection line could take more time than a single one, alternate clamping was used in most cases, including the left portal pedicle for the left transection between segments 4 and 2–3, and right vascular exclusion for the right transection (ie, clamping of right portal pedicle and right hepatic vein)5 (Fig. 2a). This permitted reduced clamping time for each remaining hemiliver. Central resection could be less extensive, either including segments 4b and 5 (anterior central) or segments 4a and 8 (posterior central). In either case, intraoperative ultrasound and external anatomic landmarks provided delimitation of the resection margins.
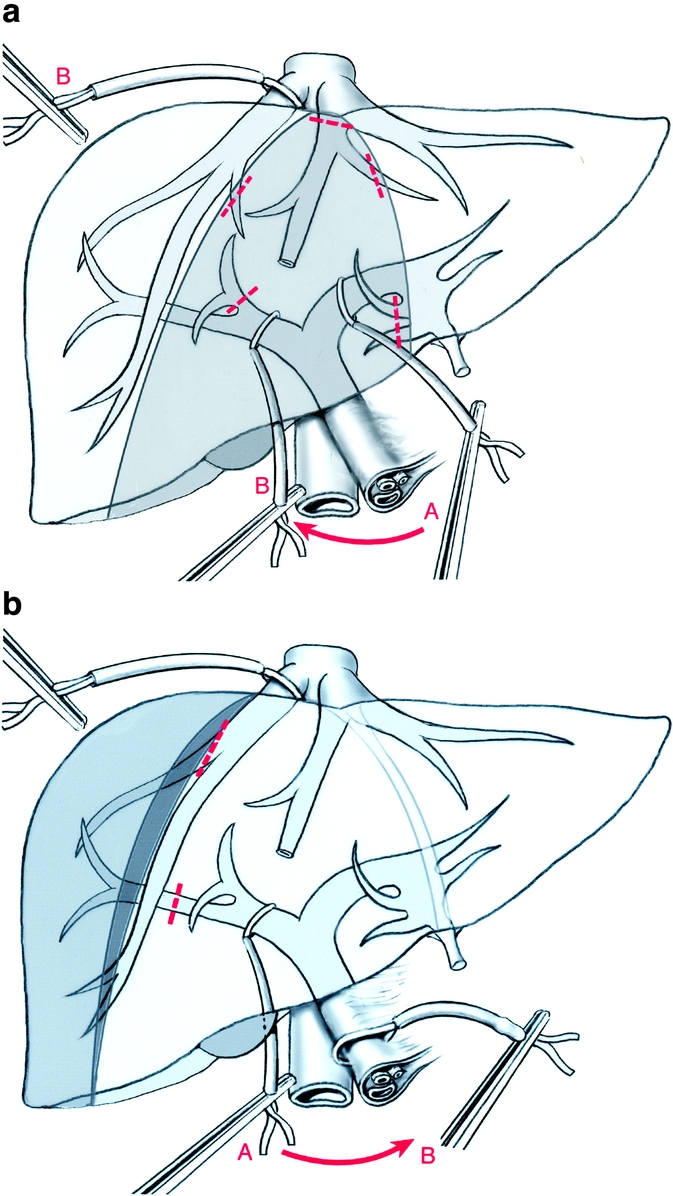
FIGURE 2. Diagrams of vascular clamping in 16 patients who had central hepatectomy and 7 patients who had right posterior resection, respectively. (a) Alternate clamping for central hepatectomy: the left side transection is first performed under selective left portal pedicle clamping (A) followed by the right-side transection performed under right posterior vascular exclusion (ie, clamping of the right portal pedicle and right hepatic vein) (B). (b) Right posterior vascular exclusion for right posterior sectionectomy: the resection is performed under clamping of the right portal pedicle (A) and right hepatic vein (posterior right vascular exclusion). In case of bleeding, a Pringle maneuver can be easily applied (B).
Right Posterior Sectionectomy
Resection of the right posterior section (segments 6 and 7) required complete mobilization of the right liver and division of the retrohepatic veins. Delimitation of resection was obtained by clamping the right posterior sectional pedicle. Resection was performed using right portal pedicle clamping or portal triad clamping (Fig. 2b). The right hepatic vein had to be preserved but could be clamped in combination with inflow clamping, resulting in right liver vascular exclusion.5
Other Resections
On the right side, other resections included right inferior bisegmentectomy (segments 5 and 6) and right superior bisegmentectomy (segments 7 and 8; Fig. 3). The latter required the presence of an accessory right inferior hepatic vein to drain remaining segment 6.3 On the left side, left anterior resection included segments 3 and 4b.
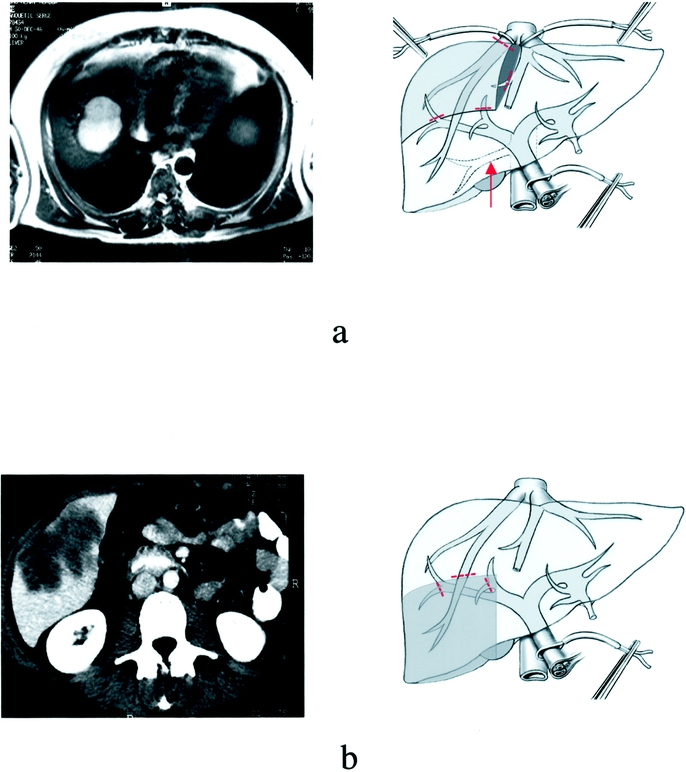
FIGURE 3. Examples of right superior and right inferior bisegmentectomy for liver tumors: preoperative imaging (left column) and corresponding diagrams (on the right column). (a) Planned right superior bisegmentectomy (segments 7 and 8) for liver dome HCC (the presence of a right inferior hepatic vein [arrow] is required for this resection). (b) Planned right inferior bisegmentectomy (segments 5 and 6) for colorectal liver metastasis
RESULTS
Thirty-one patients had 32 resections, as summarized in Table 1. One patient synchronously underwent 2 resections, including right posterior sectionectomy and left anterior resection as defined previously (Fig. 4). There were 23 males (74%) and 8 females (26%). The mean age was 56 years (range, 25-69 y). The indications for liver resection are summarized in Table 2. Eleven patients (35%) had cirrhosis and 4 patients (13%) had marked fibrosis of various origins, including viral (n = 6), alcoholic (n = 5), or hemochromatosis (n = 4).
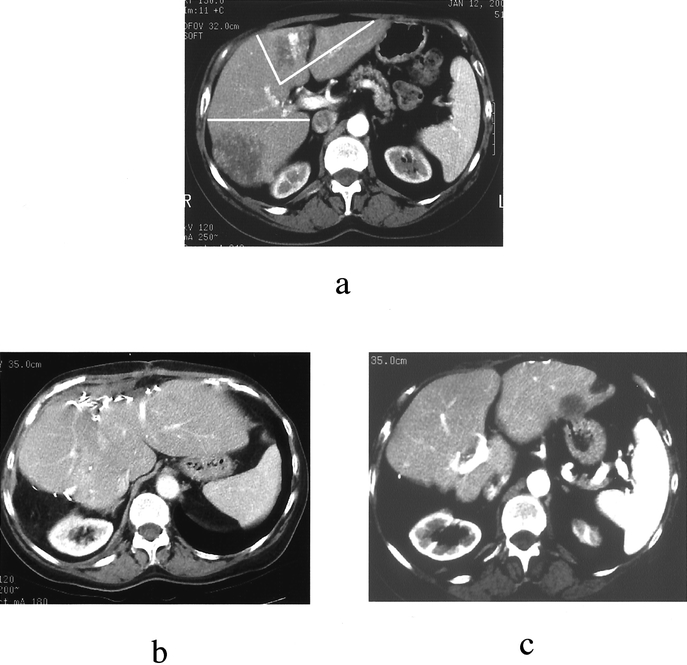
FIGURE 4. A 65-year-old woman with liver metastases of colorectal origin. (a) Preoperative liver computerized tomography (CT) showing bilobar disease; clear lines delimitate the planned resections, ie, right posterior sectionectomy (segments 6 and 7) and left anterior bisegmentectomy (segments 4b and 3). (b) Twelve-month follow-up CT showing liver hypertrophy without recurrence. (c) Eighteen-month follow-up CT showing isolated segment 2 recurrence. Repeat hepatectomy (completion of left lateral sectionectomy, ie, segmentectomy 2) was performed
TABLE 2. Indications for 32 Anatomic Hepatic bi or trisegmentectomies in 31 patients with hepatic tumors
Mortality was nil. Median transfusions were 1 unit (range, 0–9 U), and 23 patients (74%) did not require any transfusion. Mean operative time was 250 minutes (range, 180–420 min). Mean operative time was 280 minutes (range, 180–420 min) for central hepatectomy and 265 minutes (range, 210–360 min) for right posterior resection. Postoperative course was uneventful in 25 patients (81%). Seven complications occurred in 6 patients (19%), including 2 subphrenic collections treated percutaneously, 2 ascites, 1 transient liver failure, and 2 pneumonias.
In 29 patients operated on for malignant disease, all resections were macroscopically complete (Fig. 5). Macroscopic exposure of the tumor during transection did not occur. Mean margin was 9 mm (range, 1–40 mm). On histology, margins were 10 mm or more in 16 resections (55%), 3–9 mm in 8 (28%), and 2 mm or less in 5 (17%).
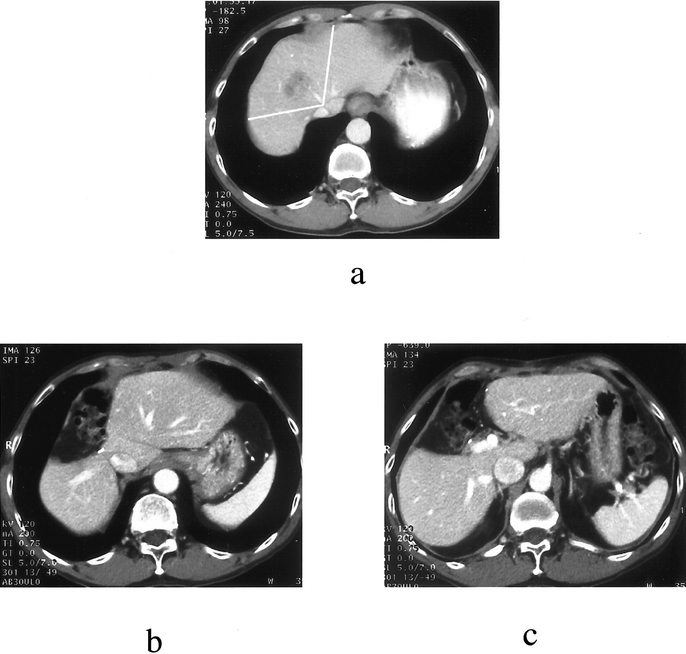
FIGURE 5. A 45-year-old man with hepatocellular carcinoma and hepatitis B-related cirrhosis. (a) Preoperative CT scan showing a tumor in segment 8 encroaching segment 4 by crossing the middle hepatic vein. Clear lines delimitate the planned resection, ie, central hepatectomy (segments 4, 5, and 8). Right posterior margin was 5 mm. (b) Postoperative CT scan (12 months): liver dome view. (c) Postoperative CT scan (12 mo): liver hilum view
Mean follow up was 18 months (range, 6–73 mo). Eleven patients had recurrence (38%). Isolated intrahepatic recurrence occurred in 7 patients (24% of all patients and 64% of recurrences) of which 6 were localized in remote areas and 1 at the site of primary resection. This patient had an initial section margin of 4 mm and also had multifocal hepatic recurrence. Three of the patients with isolated hepatic relapse (43%) underwent reresection and 1 underwent orthotopic liver transplantation.
DISCUSSION
Liver resection is the only treatment that offers the prospect of long-term survival and potential cure to patients with liver cancer. The treatment of small peripheral lesions by limited resections and of large tumors by extensive hepatectomies is the standard. However, some lesions located in the depth of the right lobe or in central segments might require an extensive resection only because of their location and vascular connections. In those cases, anatomic bi- and trisegmentectomies can be considered, provided curative resection can be achieved. This study confirms that, in selected cases, these procedures are valuable alternatives to extensive liver resection by preserving liver function and enhancing the possibility of repeat hepatectomy.6,7
Such operations are based on an accurate analytic knowledge of the anatomy of liver segments, including their inflow as well as their outflow supply. This is well exemplified by 3 of the operations described in this report. Central hepatectomy requires access to the right anterior sectional portal pedicle and resects the territory drained by the middle hepatic vein; right posterior sectionectomy requires access to the right posterior sectional portal pedicle and preservation of the right hepatic vein; right superior bisegmentectomy removes the main right hepatic vein and therefore requires the presence of a right inferior hepatic vein to drain segment 6.3 Therefore, these operations are technically more demanding. They require more extensive dissection of vascular pedicles, expertise in intraoperative ultrasound, and larger transection surfaces. They represented only 7% of our 10-year series of liver resections, but this rate has increased gradually to reach 20% of the resections in the last 2 years. We now consider this approach systematically for posterior or central lesions. Posterior tumors, which otherwise would require right hepatectomy, and central tumors, which otherwise would require extended right or left hepatectomy (trisectionectomy), can be treated by right posterior sectionectomy and central hepatectomy, respectively, thus sparing 2 liver segments.
The procedures as used in the present study proved to be safe. There were no deaths and morbidity rate (19%) as well as transfusion requirements (median 1 unit and 74% of patients not transfused) compare favorably with those of the literature for more conventional resections. Interestingly, only 1 patient had transient postoperative liver failure, although 48% of the patients had either cirrhosis or marked fibrosis.
Tumor clearance was macroscopically satisfactory and no intraoperative exposure of tumors within the transection line occurred. However, resection margins were less than 10 mm in 45% of the cases and 2 mm or less in 17%. Because of their design, these operations are more likely to be associated with smaller margins, but the value of the resection margin as a prognostic factor has probably been overestimated.8–10 Indeed, several studies, including large numbers of patients with hepatocellular carcinoma,9,11,12 cholangiocellular carcinoma,13 or colorectal metastases,8,10,14 showed no negative effect on survival of a less than 10-mm margin. In a recent report,10 a highly sensitive method of detection of micrometastases by genetic alteration study was used in the vicinity of resected colorectal liver metastases. It showed that surgical margins of less than 2 mm were associated with only a 6% risk of confirmed margin related-recurrence. Moreover, this study10 showed that surgical margins did not affect either the overall survival rate or the overall recurrence rate. However, no margin-related recurrence was observed when section margins were wider than 10 mm. Reviews of published series show that surgical margins of 10 mm or more were achieved in approximately one third of the patients with more conventional surgical procedures.10 In our series, only 1 patient developed recurrence at the site of resection (in association with multifocal recurrence), whereas all other intrahepatic recurrences occurred at a remote site. In addition, it should be emphasized that in several of our patients, either because of underlying liver disease anticipating insufficient remnant liver or bilobar tumor location, we had to choose between resection with a less than 10-mm margin or no resection at all.
In fact, most recurrences occurred in remote areas of the liver, of which 43% underwent repeat resection, had a higher figure than those usually reported in the literature.15–18 This is a valuable advantage because repeat resection clearly increases survival figures in colorectal liver metastases19–21 and, to a lesser extent, in cases of HCC.15
We conclude that anatomic bi- or trisegmentectomies are safe alternatives to extensive liver resections in selected patients. They avoid unnecessary sacrifice of functional parenchyma, which prevents postoperative liver failure in patients with underlying liver disease and enhances the possibility of repeat liver resection.
Footnotes
Reprints: Daniel Cherqui, MD, Service de Chirurgie Digestive, Hôpital Henri Mondor, 51, Avenue du Maréchal de Lattre de Tassigny, 94010 Créteil Cedex, France. E-mail: daniel.cherqui@hmn.ap-hop-paris.fr.
REFERENCES
- 1.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. [DOI] [PubMed] [Google Scholar]
- 2.Billingsley KG, Jarnagin WR, Fong Y, et al. Segment-oriented hepatic resection in the management of malignant neoplasms of the liver. J Am Coll Surg. 1998;187:471–481. [DOI] [PubMed] [Google Scholar]
- 3.Makuuchi M, Hasegawa H, Yamazaki S, et al. Four new hepatectomy procedures for resection of the right hepatic vein and preservation of the inferior right hepatic vein. Surg Gynecol Obstet. 1987;164:69–72. [PubMed] [Google Scholar]
- 4.The terminology committee of the IHPBA. The Brisbane 2000 terminology of hepatic anatomy and resections. HPB. 2000;333–339. [Google Scholar]
- 5.Cherqui D, Malassagne B, Colau PI, et al. Hepatic vascular exclusion with preservation of the caval flow for liver resections. Ann Surg. 1999;230:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scudamore CH, Buczkowski AK, Shayan H, et al. Mesohepatectomy. Am J Surg. 2000;179:356–360. [DOI] [PubMed] [Google Scholar]
- 7.Wu CC, Ho WL, Chen JT, et al. Mesohepatectomy for centrally located hepatocellular carcinoma: an appraisal of a rare procedure. J Am Coll Surg. 1999;188:508–515. [DOI] [PubMed] [Google Scholar]
- 8.Elias D, Cavalcanti A, Sabourin JC, et al. Results of 136 curative hepatectomies with a safety margin of less than 10 mm for colorectal metastases. J Surg Oncol. 1998;69:88–93. [DOI] [PubMed] [Google Scholar]
- 9.Poon RT, Fan ST, Ng IO, et al. Significance of resection margin in hepatectomy for hepatocellular carcinoma: a critical reappraisal. Ann Surg. 2000;231:544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokudo N, Miki Y, Yanagisawa A, et al. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma. Arch Surg. 2002;137:833–840. [DOI] [PubMed] [Google Scholar]
- 11.Ochiai T, Takayama T, Inoue K, et al. Hepatic resection with and without surgical margins for hepatocellular carcinoma in patients with impaired liver function. Hepatogastroenterology. 1999;46:1885–1889. [PubMed] [Google Scholar]
- 12.Bilimoria MM, Lauwers GY, Doherty DA, et al. Underlying liver disease, not tumor factors, predicts long-term survival after resection of hepatocellular carcinoma. Arch Surg. 2001;136:528–535. [DOI] [PubMed] [Google Scholar]
- 13.Weimann A, Varnholt H, Schlitt HJ, et al. Retrospective analysis of prognostic factors after liver resection and transplantation for cholangiocellular carcinoma. Br J Surg. 2000;87:1182–1187. [DOI] [PubMed] [Google Scholar]
- 14.Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakajima Y, Ko S, Kanamura T, et al. Repeat liver resection for hepatocellular carcinoma. J Am Coll Surg. 2001;192:339–344. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki S, Sakaguchi T, Yokoi Y, et al. Impact of repeat hepatectomy on recurrent colorectal liver metastases. Surgery. 2001;129:421–428. [DOI] [PubMed] [Google Scholar]
- 17.Muratore A, Polastri R, Bouzari H, et al. Repeat hepatectomy for colorectal liver metastases: a worthwhile operation? J Surg Oncol. 2001;76:127–132. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto J, Kosuge T, Shimada K, et al. Repeat liver resection for recurrent colorectal liver metastases. Am J Surg. 1999;178:275–281. [DOI] [PubMed] [Google Scholar]
- 19.Scheele J, Stangl R, Schmidt K, et al. Recurrent tumor after R0 resection of colorectal liver metastases. Incidence, resectability and prognosis. Chirurgie. 1995;66:965–973. [PubMed] [Google Scholar]
- 20.DeMatteo RP, Palese C, Jarnagin WR, et al. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. 2000;4:178–184. [DOI] [PubMed] [Google Scholar]
- 21.Yamada H, Katoh H, Kondo S, et al. Repeat hepatectomy for recurrent hepatic metastases from colorectal cancer. Hepatogastroenterology. 2001;48:828–830. [PubMed] [Google Scholar]


