Abstract
Objective:
To compare the clinical presentation and results of treatment of postcholecystectomy bile duct injuries in patients with and without arterial injuries.
Summary Background Data:
Incidence and impact of arterial injuries in patients with a postcholecystectomy biliary injury are unknown, although they are claimed to increase the risk of septic complications, difficulty of biliary repair and risk of recurrent stricture.
Methods:
Fifty-five patients referred for postcholecystectomy biliary strictures and who underwent surgical repair were prospectively evaluated by celiac and superior mesenteric angiography. Circumstance and presenting symptoms of the biliary injury in patients with and without vascular injury as well as intra- and postoperative outcome in the 43 patients who underwent a Hepp–Couinaud biliary repair were compared.
Results:
Incidence of vascular injury was 47%, the most frequent of which was right-sided hepatic artery disruptions (36%). Indication of cholecystectomy (cholecystitis, 42 vs. 45%), technique of resection (laparoscopy, 80 vs. 79%) as well as delay of recognition and presenting symptom of the biliary injury were comparable in patients with and without vascular injury. Among patients undergoing a biliary repair, the level of the biliary injury (Bismuth’s type III or IV 63% vs. 54%), duration of surgery, and incidence of postoperative complications (21 vs. 21%) were also comparable in patients with and without arterial injury. One patient in each group experienced recurrent biliary stricture.
Conclusions:
The discovery of a disruption of the right branch of the hepatic artery should not affect management of the biliary stricture when if a Hepp–Couinaud repair is performed.
Common bile duct injury is a severe complication of cholecystectomies that results in biliary leak or stenosis. It occurs in 0.2–0.3% of patients undergoing an open procedure1,2 and in 0.5–0.8% of those undergoing a laparoscopy.2
Vascular injury is another surgical complication of cholecystectomies, the most frequent of which is the disruption of the right branch of the hepatic artery.3,4 Unlike biliary injuries, it does not usually lead to significant complications5,6 and therefore probably remains unnoticed in most patients. Its incidence after cholecystectomy has been estimated to be 7% in an autopsy series of cadavers who had undergone an open procedure.4 This incidence seems increased in patients with a bile duct injury, ranging between 12% and 39%.7–9 As angiographic studies are usually not routinely performed, the exact figure is however unknown.
Although the consequence of these arterial injuries in patients with bile duct injuries have not been clearly assessed, uncontrolled reports suggest that they may induce liver necrosis or abscess,10 increase the risk of bleeding at the time of the biliary repair,9 and favor recurrent stenosis.10,11
The aim of the present study was therefore to assess (1) the incidence of vascular injuries in patients with a bile duct injury who had undergone routine angiography in the era of laparoscopic cholecystectomies and (2) its impact on the clinical presentation, and results of surgical repair, of the biliary injury.
PATIENTS AND METHODS
Between 1990 and 1999 (so as to allow adequate follow-up), 60 patients underwent surgical repair at our institution of a major postcholecystectomy bile duct injury. Patients with biliary leakage from the cystic duct or from the gallbladder bed were considered to have a minor injury and were therefore not included. Fifty-five of these patients were prospectively evaluated by celiac and superior mesenteric angiography. The other 5 patients did not undergo this exploration because they had, or were at risk, of kidney failure or had a known history of severe allergy to contrast media. They were excluded from further analysis. These 55 patients comprised 19 men and 36 women with a mean age of 49 ± 16 years. The operative records of the cholecystectomy were obtained from the referring institution as well as all details of the clinical course and treatment procedures performed prior to referral. This allowed in particular stratification of the time of recognition of the injury into 3 groups: intraoperative, early (within 2 weeks of the cholecystectomy), and delayed thereafter.
The management of bile duct injuries was standardized during the study period. Bile collections were drained percutaneously and biliary leaks were allowed to heal before undertaking the biliary repair. Preoperative workup included abdominal ultrasound, computed tomography scan, as well as percutaneous cholangiography (performed the day before surgery), and/or more recently magnetic resonance imaging–cholangiography. The level of the injury was defined using the classification of Bismuth.12
Biliary repair was performed using the Hepp–Couinaud technique in 43 patients as previously described.13 The bile duct stenosis was resected at the time of surgery for pathologic examination. No attempt was made to repair the arterial injury. The 12 other patients underwent right hepatectomy because of right lobar atrophy and a Roux-en-Y anastomosis on the left bile duct. All patients were regularly followed after surgery at the out-patient clinic on an at least yearly basis with liver function tests and abdominal ultrasound.
We first investigated in the entire group of 55 patients the incidence of vascular injury and its impact on the clinical presentation of the biliary injury. We next investigated in the 43 patients who had undergone a Hepp–Couinaud biliary repair whether the presence of an arterial injury had an impact on the intra- and postoperative course.
RESULTS
Vascular injuries were present in 26 of the 55 patients (47%). These included disruption of the right branch of the hepatic artery or of a replaced right hepatic artery in 20 patients (36%), pseudoaneurysm of the right branch of the hepatic artery in 2 patients (4%), and portal vein injury with (n = 3) or without (n = 1) hepatic artery injury in 4 patients (7%). Disruption of the arterial supply to the right liver right branch of the hepatic artery was associated in all patients with an omega-shaped collateral circulation originating from the left branch of the hepatic artery and revascularizing the distal stump of the right branch of the hepatic artery (Fig. 1).
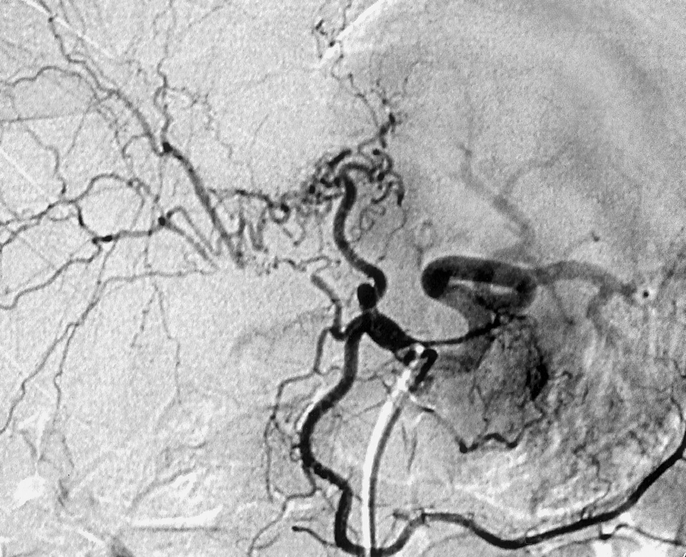
FIGURE 1. Celiac angiography showing interruption of the right branch of the hepatic artery (plain arrow) and revascularization of its distal stump by a collateral circulation originating from the left branch of the hepatic artery (dotted arrow).
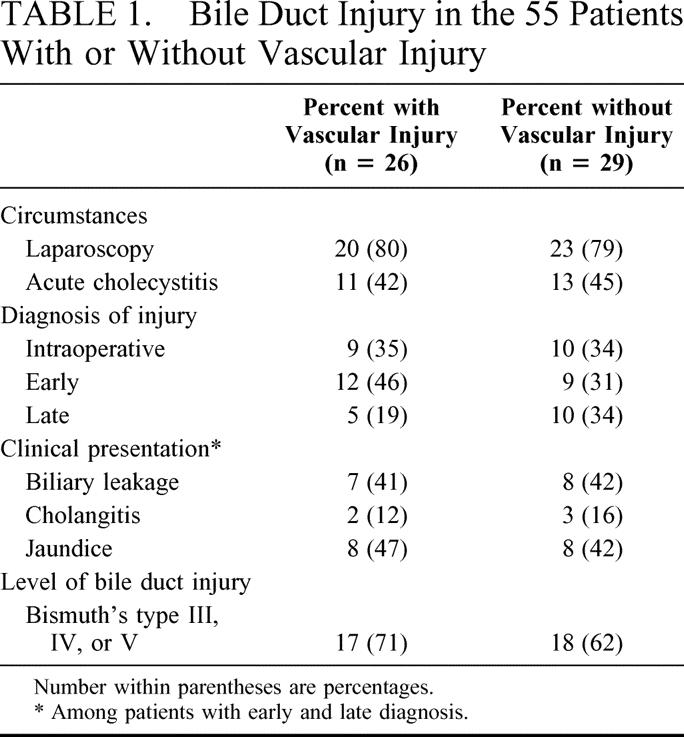
The circumstance of the bile duct injury was comparable in patients with and without vascular injury. Acute inflammation of the gallbladder was recorded at the time of cholecystectomy in 42% and 45% of the patients, respectively, and most patients in both groups had undergone a laparoscopic surgery (80 vs. 79%). The clinical presentation of the biliary injury in each group is summarized in Table 1. The bile duct injury had been recognized during the course of cholecystectomy in 19 patients and managed at that time by either an end-to-end ductal anastomosis over a T-tube (n = 13) or a biliary-enteric anastomosis (n = 6). In the remaining 36 patients, diagnosis of the bile duct injury was delayed and the revealing symptom was biliary leakage, jaundice, or cholangitis. Most of these patients (75%) had undergone an invasive procedure before referral, including end-to-end primary repair over a T-tube (n = 6), biliary-enteric anastomosis (n = 11), surgical external drainage without attempt at reconstruction (n = 5), or endoscopic stenting or dilatation (n = 5). Revealing symptoms, timing, and nature of these procedures, as well as mean time interval between cholecystectomy and referral (overall = 16 ± 22 months; range = 1-103 months), were comparable in patients with and without vascular injury.
TABLE 1. Bile Duct Injury in the 55 Patients With or Without Vascular Injury
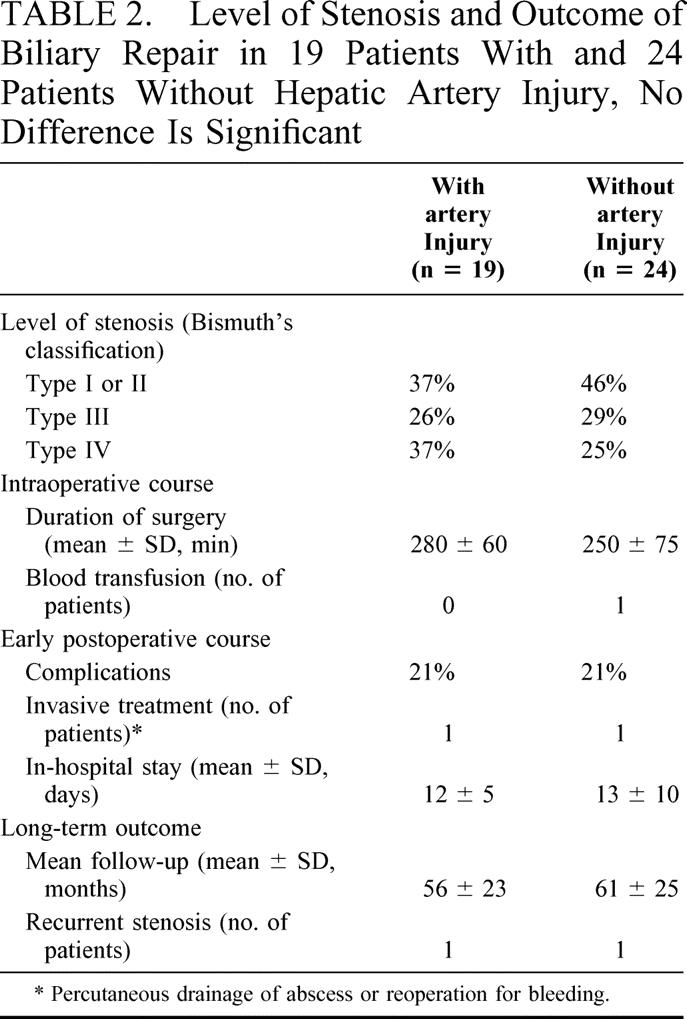
Among the 43 patients undergoing a Hepp–Couinaud biliary repair, 19 patients (44%) had an hepatic artery injury (none had a portal vein injury). Time interval between cholecystectomy and referral at our institution was 361 days in patients with and 377 days in patients without an arterial injury (not significant). Most patients (58%) had Bismuth’s type III or IV lesions, and the level of the biliary stenosis was not significantly different in the 2 groups (Table 2). An invasive procedure was performed after referral and before biliary repair in 15 of these patients (37% and 33% of those with and without an arterial injury, not significant). These included external drainage of the biliary tree or of a biloma (n = 13) or attempt at endoscopic treatment (n = 2). At surgery, no significant arterial collateral circulation was found in the portal pedicle in patients with hepatic artery injury except for an occasionally larger branch to segment 4 and their mean duration of surgery was comparable to that in patients without an injury (Table 2). A single patient, without vascular injury, required blood transfusion. The most frequent postoperative complication was fever that occurred with a similar incidence in the 2 groups. Four patients (2 in each group) experienced significant complications. These included 1 pulmonary infection and 1 intra-abdominal abscess that was successfully treated by percutaneous drainage in patients with an arterial injury. Among patients without arterial injury, 1 developed a biliary leak through the abdominal drainage that healed spontaneously and another an hemorrhage from a preoperative biliary drainage-induced subcapsular hematoma that required reoperation on postoperative day 4. All patients were discharged in good clinical condition and the duration of in-hospital stay was comparable in the 2 groups (Table 2). Examination of the resected stenosis found a neuroma in 56% and 62% of the patients respectively.
TABLE 2. Level of Stenosis and Outcome of Biliary Repair in 19 Patients With and 24 Patients Without Hepatic Artery Injury, No Difference Is Significant
Four patients (2 in each group) were lost after 1 year of follow-up with no biliary symptoms and normal liver function tests. Among the remaining patients, 2 (one in each group) experienced a recurrent stricture. The first patient with an hepatic artery injury was successfully treated by 1 session of endoscopic dilatation 5 years after the biliary repair. The second patient with no hepatic artery injury had progressive destructive cholangitis at and above the biliary anastomosis.
DISCUSSION
We have found that the incidence of vascular injuries in patients with a postcholecystectomy biliary injury was 47%. The most frequent of these was an interruption of the right branch of the proper hepatic artery or of a replaced right hepatic artery that was present in 36% of the patients. These arterial injuries had, however, no impact on the clinical presentation of the bile duct injury or on the difficulty or risk of failure of the biliary repair.
This incidence of vascular injuries in our patients is greater than the 12-16% incidence reported by others.7,14 This difference is most likely due to the fact that angiography was performed routinely in our patients and only selectively in previous studies. It is in contrast comparable to the 39% incidence reported 20 years ago in a series of patients with bile duct injuries after open cholecystectomies who had also undergone routine angiography.9 Because more than 80% of our patients had undergone a laparoscopic cholecystectomy, these results suggest that the laparoscopic approach is not associated with an additional risk of arterial injury. The presence, rather that the circumstance of a biliary injury is the predominant risk factor for the vascular injury.
The most frequent vascular injury was a right-sided hepatic artery disruption, as in previous studies,11 that most probably occurred at the time of cholecystectomy. These injuries have been alleged to increase the difficulty of biliary repair, the risk of intraoperative bleeding9 or postoperative septic parenchymal complications10 and to favor the development of recurrent strictures.3,10,11 As a matter of fact, bile ducts have an exclusive arterial supply15 that, if interrupted, may result in ischemic complications as best evidenced in liver transplant patients experiencing hepatic artery thrombosis.16
In contrast, the clinical presentation of the biliary injury and the postoperative course of biliary repair were comparable in our patients with and without arterial injuries. Approximately one third had their injury identified at the time of cholecystectomy, usually after conversion. This figure, which is comparable to that reported by others,14,17 was the same whether an arterial injury was present or not. The remaining patients had their injury revealed by a biliary leak in the early postoperative period or, subsequently, a jaundice. Revealing symptoms, and timing of diagnosis and previous attempts at treatment were again similar in patients with and without an arterial injury. Duration of surgery and of in-hospital stay as well as incidence of postoperative complications were neither influenced by this arterial injury. Finally, a comparable proportion of patients had either a neuroma or a disappearance of the bile duct wall at the level of the biliary stricture.
This lack of influence of the arterial injury in our patients is due to the fact that disruption was unilateral, in contrast to what happens after arterial thrombosis of liver graft. This allowed free communication between the left and right hepatic arteries via the hilar plate arterial plexus.18 This communication that develops very rapidly5 is very effective as all our patients had an adequate distal arterial supply to the right liver on angiographic studies. It also accounts for the previously reported lack of accuracy of doppler ultrasound in detecting interruption of the right arterial branch.11 Furthermore, this collateral circulation develops very rapidly.5
This collateral circulation within the hilar plate also provides adequate arterial blood supply to the biliary confluence and the extra hepatic portion of the left bile duct, where the anastomosis was performed using the Hepp–Couinaud technique. This may explain why the 95% success rate of the biliary repair was comparable in patients with and without hepatic artery injury.
It is therefore not surprising that the long term outcome of our patients with and without hepatic artery injury was comparable. This 95% success rate however requires the use of the Hepp–Couinaud technique so as to perform the biliary repair at, or above the hilar level. The previously reported association between failure of biliary repair and arterial injuries11 may simply reflect the relatively higher risk of arterial injuries in patients with high bile duct strictures which are the most difficult to treat. An alternative hypothesis is that patients with an arterial injury are more likely to have an ischemic mucosa at the level of the common bile duct and hence are at higher risk of recurrent stricture if the anastomosis is performed below the biliary confluence.
In conclusion, this prospective study reports a high incidence of interruption of the right hepatic artery in patients with postcholecystectomy bile duct injuries some of whom had previous attempts at biliary repair but fails to demonstrate its influence on the presentation or outcome of the biliary injury. Hence, angiographic studies, in particular by CT scan vascular reconstruction, may still be useful to rule out an exceptional arterial aneurysm, to identify an associated arterial and portal interruption in patients with parenchymal necrosis, or as a preoperative exploration if an hepatectomy is contemplated. However the discovery of a simple disruption of the right branch of the hepatic artery should not affect management if a Hepp-Couinaud biliary repair is performed.
Footnotes
Reprints: Jacques Belghiti, MD, Department of Surgery, Hospital Beaujon, 100 Boulevard du Général Leclerc, 92118 Clichy Cedex, France. E-mail: j.bel@bjn.ap-hop-paris.fr.
REFERENCES
- 1.Roslyn JJ, Binns GS, Hughes EF, et al. Open cholecystectomy. A contemporary analysis of 42,474 patients. Ann Surg. 1993;218:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101–125. [PubMed] [Google Scholar]
- 3.Madariaga JR, Dodson SF, Selby R, et al. Corrective treatment and anatomic considerations for laparoscopic cholecystectomy injuries. J Am Coll Surg. 1994;179:321–325. [PMC free article] [PubMed] [Google Scholar]
- 4.Halasz NA. Cholecystectomy and hepatic artery injury. Arch Surg. 1991;126:137–138. [DOI] [PubMed] [Google Scholar]
- 5.Mays ET, Wheeler CS. Demonstration of collateral arterial flow after interruption of hepatic arteries in man. N Engl J Med. 1974;290:993–996. [DOI] [PubMed] [Google Scholar]
- 6.Bengmark S, Rosengren K. Angiographic study of the collateral circulation to the liver after ligation of the hepatic artery in man. Am J Surg. 1970;119:620–624. [DOI] [PubMed] [Google Scholar]
- 7.Wudel LJ, Wright JK, Pinson CW, et al. Bile duct injury following laparoscopic cholecystectomy: a cause for continued concern. Am Surg. 2001;67:557–563. [PubMed] [Google Scholar]
- 8.Davidoff AM, Pappas TN, Murray EA, et al. Mechanisms of major biliary injury during laparoscopic cholecystectomy. Ann Surg. 1992;215:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bismuth H. How to treat a postoperative stenosis? In: Bismuth H, Lazorthes F, eds. Operative Injury of the Common Bile Duct. Paris: Masson; 1981:47–107. [Google Scholar]
- 10.Gupta N, Soloman H, Fairchild R, Kaminski DL. Management and outcome of patients with combined bile duct and hepatic artery injuries. Arch Surg. 1998;133:176–181. [DOI] [PubMed] [Google Scholar]
- 11.Koffron A, Ferrario M, Parsons W, et al. Failed primary management of iatrogenic biliary injury: incidence and significance of concomitant hepatic arterial disruption. Surgery. 2001;130:722–8. [DOI] [PubMed] [Google Scholar]
- 12.Bismuth H, Franco D, Corlette MB, et al. Long-term results of Roux-en-Y hepaticojejunostomy. Surg Gynecol Obstet. 1978;146:161–167. [PubMed] [Google Scholar]
- 13.Hepp J, Couinaud C. L’abord et l’utilisation du canal hépatique gauche dans les réparations de la voie biliaire principale. Presse Med. 1956;64:947–948. [PubMed] [Google Scholar]
- 14.Chapman WC, Halevy A, Blumgart LH, et al. Postcholecystectomy bile duct strictures. Management and outcome in 130 patients. Arch Surg. 1995;130:597–604. [DOI] [PubMed] [Google Scholar]
- 15.Northover JMA, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979;66:379–384. [DOI] [PubMed] [Google Scholar]
- 16.Hesselink EJ, Klompkmaker IJ, Pruim J, et al. Hepatic artery thrombosis after orthotopic liver transplantation: a fatal complication or an asymptomatic event. Transplant Proc. 1989;21:2462. [PubMed] [Google Scholar]
- 17.Lillemoe KD, Martin SA, Cameron JL, et al. Major bile duct injuries during laparoscopic cholecystectomy. Follow-up after combined surgical and radiologic management. Ann Surg. 1997;225:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vellar ID. The blood supply of the biliary ductal system and its relevance to vasculobiliary injuries following cholecystectomy. Aust N Z J Surg. 1999;69:816–820. [DOI] [PubMed] [Google Scholar]


