Abstract
Objective:
To assess long-term quality of life in a population-based sample of rectal cancer patients.
Summary Background Data:
Quality of life in rectal cancer patients who suffer reduced bowel and sexual function is very important. Few studies, however, have long term follow-up data or sufficient sample sizes for reliable comparisons between operation groups.
Patients and Methods:
A 4-year prospective study of rectal cancer patients’ quality of life was assessed by using the European Organization for Research and Treatment of Cancer QLQ-30 and CR38 questionnaires.
Results:
A total of 329 patients returned questionnaires. Overall, anterior resection patients had better quality of life scores than abdominoperineal extirpation patients. High-anterior resection patients had significantly better scores than both low-anterior resection and abdominoperineal extirpation patients. Low-anterior resection patients, however, overall had a better quality of life than abdominoperineal extirpation patients, especially after 4 years. Abdominoperineal extirpation patients’ quality of life scores did not improve over time. Stoma patients had significantly worse quality of life scores than nonstoma patients. Quality of life improved greatly for patients whose stoma was reversed.
Conclusions:
Anterior resection and nonstoma patients, despite suffering micturition and defecation problems, had better quality of life scores than abdominoperineal extirpation and stoma patients. Comparisons between abdominoperineal extirpation and anterior resection patients should consider the effect of temporary stomas. Improvements in quality of life scores over time may be explained by reversal of temporary stomas or physiologic adaptation.
Anterior resection and nonstoma patients, despite suffering micturation and defaecation problems, had better quality of life scores than APE and stoma patients. Quality of life also improved for those patients whose stoma was reversed.
Colorectal cancer is one of the most common cancers in Western industrialized countries.1,2 In the United States, 135,400 new cases have been estimated for 2001.3 Mortality rates for colon and rectal cancer, however, have declined in both the United States and Germany.4 Improvements in treatment and early detection indicate that more patients will live with the consequences of this disease.5,6 Rectal cancer surgery, however, is often complicated and can result in bowel and sexual function problems.7 Therefore, it is important to assess rectal cancer patients’ quality of life to determine whether such impairments disrupt everyday life. Changes in operation techniques must also be evaluated for their effect on quality of life.8 The main factors determining patient quality of life seem to be operation technique, abdominoperineal extirpation (APE) versus anterior resection (AR), tumor location, and the presence of a stoma or not. In general, stoma and APE patients experience poorer quality of life, although there are exceptions.2,9–12
Compared with breast cancer, for example, there are relatively few studies that have investigated quality of life in rectal cancer patients. Long-term prospective studies of rectal cancer survivors are sparse.1 A large proportion of papers report only specific functional status, such as sphincter function,5,13,14 not overall quality of life. A frequent limitation of rectal cancer studies is the small sample sizes. Few studies, therefore, have been able to compare effects such as age and gender.15,16 Further, results of comparisons between operation methods should be treated with caution because of low patient numbers.
This study aims to rectify some of the shortfalls of the previous literature by presenting results of a large, 4-year, prospective study of rectal cancer patients’ quality of life, as assessed by the European Organization for Research and Treatment of Cancer (EORTC) generic and rectal-specific cancer questionnaires. The issues of time, operation method, and stoma will be addressed.
METHODS
The Munich Cancer Registry records all cancer patients in Munich and the surrounding area, a population of around 2.3 million. In 1996, the government provided funding for a focused field study in rectal cancer patients with a 2-year recruitment period. The Munich Cancer Registry receives all original reports from all co-operating pathologists so that the number of rectal cancer patients in the region is known and complete. All essential clinical details, such as tumor classification, surgery, therapy, disease progression, and life status, were registered from standardized forms. Physicians also completed special questionnaires concerning diagnosis, primary therapy, follow-up, and palliative care. Dose and timing details of adjuvant therapy courses, however, are not routinely recorded. Additionally, original clinical reports were available for reliability checks. At the time of primary treatment during a 2-year recruitment period clinicians requested informed consent from patients to receive a quality of life survey. Questionnaires were then sent from the field study (independent of the clinician) to willing participants at regular intervals over 4 years.
The first part of the patient questionnaire established details of treatment, medicines being taken, and aftercare. These details were cross-referenced with clinical reports. The second part of the questionnaire was the EORTC QLQ-30, a recognized reliable and validated quality of life evaluation tool.17–19 Thirty questions combine to make 5 functional scales (physical, emotional, cognitive, social, and role functioning), a global quality of life measure, and symptom assessment, including pain, fatigue, diarrhea, and constipation. The third section of the survey was the EORTC CR38, also a reliable and validated measure specifically for rectal cancer patients.20,21 Thirty-eight questions combine to create 8 overall scales, including body image, sexual function and enjoyment, future perspective, and gastrointestinal and micturition problems. Men and women separately answer questions about sexual problems, and stoma patients respond to stoma-specific questions whereas nonstoma patients complete defecation-related questions.
The 68 EORTC questions are designed to be aggregated to create 27 variables for analysis. For ease of interpretation, we grouped them into 4 sections: functioning scores, symptom scores, sexual problems, and other. Patient responses were combined and converted to a 0-100 scale according to guidelines provided by the EORTC.22 From these guidelines, high functional scores (0-100) represent good function and high symptom (100-0) scores signify more problems. In our study, however, symptom scores have been reversed, as is recommended elsewhere,9 so that function and symptom scores can be represented and compared together. Thus, high scores in our report represent positive outcomes for all variables. Demographic details were also obtained, including marital status, education, and employment.
The data were considered in 2 ways: cross-sectionally with 4 cohorts at the 4 different time points and longitudinally with those patients who completed questionnaires repeatedly over the 4 time periods. Data were analyzed using SPSS for Windows (Version 10.0, Chicago, IL). Because a number of variables at various time points were skewed, a common phenomenon in quality of life data,23 nonparametric tests were used throughout, as recommended.24 Our own analysis and previous studies have shown that patients with disease progression have worse quality of life scores.25 Patients quality of life scores were only considered, therefore, before disease progression was recorded. Thus, those with metastases at diagnosis were not included.
First, χ2 tests were conducted to indicate the clinical and demographic differences between our 3 operation groups: APE, low AR (LAR), and high AR (HAR). LAR was defined as a tumor location below 8 cm and HAR at 8 cm or above. Friedmann tests were used to compare repeated measures over time and Mann–Whitney U or Kruskal–Wallis tests were conducted on the cross-sectional data. Overall quality of life scores were compared over time to establish the representativeness of the cross-sectional sample and to show changes over time. Overall APE, LAR, and HAR scores were first contrasted at each yearly time period. Then, the repeated measures analysis between years 1 and 2 was stratified by the 3 groups (APE, LAR, and HAR). Differences in quality of life between those with and without a stoma were then also examined. Each of the 4-year time points was considered separately so that stoma status, as reported by the patient at that time point, could be considered. Changes in scores for LAR and HAR patients with a reversed stoma, LAR and HAR patients with no stoma, and permanent stoma APE patients were also considered, although not statistically compared because of unequal and small patient numbers. Finally, the effect of radiation therapy and age on quality of life was investigated.
It should be noted that, with so many variables, multiple analyses were conducted, increasing the risk of type I statistical errors. All significance levels have been shown to enable both highly significant (P < 0.01) and less significant (P < 0.05) results to be considered. At this stage, insufficient research has been conducted to clearly indicate the most important quality of life variables in rectal cancer, and thus to reduce the number of analyses. This research remains exploratory and in this case adjustments are not recommended.26
RESULTS
Of the 1038 patients recorded with rectal cancer between 1996 and 1998, clinicians from 22 clinics received signed informed consent from 443. Participating patients were more likely to be from clinics treating over 25 patients per year (P < 0.001), less than 70 years of age (P < 0.001), and men (P < 0.01). Questionnaires were sent to the 443 patients and 74.3% (n = 329) returned at least 1 questionnaire. Those who did not respond were more likely to be more than 70 years of age (P < 0.001) and have disease progression (P < 0.01). Of these patients, 299 were operated by anterior resection or extirpation. The remaining 30 patients received only palliative care or were locally resected. In the first year, 217 patients completed eligible questionnaires and 53 patients were excluded because of disease progression. In year two, 169 patients completed their second questionnaire, 38 their first, and 76 patients were excluded through disease progression. In year three, 103 patients completed their third questionnaire, 20 their second and 10 their first questionnaire, 88 were excluded through disease progression. Finally in year four, 34 patients completed their first questionnaire, 3 their second, 15 their third and 48 patients who completed all 4 questionnaires, 99 were excluded through disease progression.
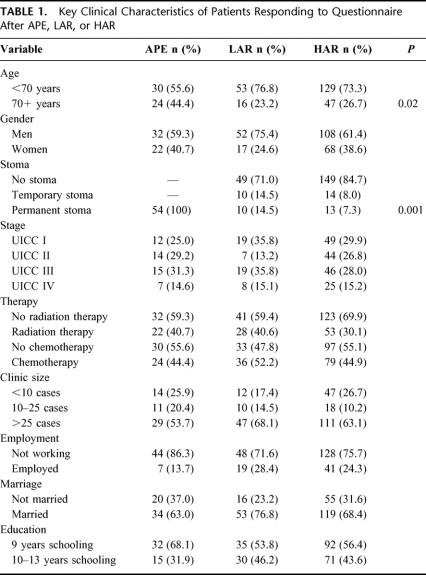
Table 1 presents the key clinical and demographic characteristics of the 3 operation groups APE, LAR, and HAR. Thirty-four patients were not given a UICC classification because they received neoadjuvant therapy to reduce the tumor size before surgery. Some patients were also reluctant to provide demographic details. There was a significant age difference among the groups, with APE patients more likely to be more than 70 years of age. Naturally, all APE patients had a permanent stoma, whereas a higher percentage of LAR patients had a stoma than HAR patients.
TABLE 1. Key Clinical Characteristics of Patients Responding to Questionnaire After APE, LAR, or HAR
Table 2 shows the mean scores for all 27 EORTC variables across the 4 years of the study. In the CR38 rectal-specific variables, sexual functioning and enjoyment, future perspective, and weight loss scores were particularly low. Although statistical comparisons can not be made between the defecation and stoma scores (because patients can not complete both these questions at the same time), stoma problems appear to be worse. Likewise, no statistical comparisons can be made between male and female sexual problems. Women, however, seem to report fewer female sexual problems, although notably few women responded to these questions.
TABLE 2. Mean Scores for all EORTC (QLQ30 & CR38) Questions Across the 4 Time Points (Cross Sectional) for Patients Without Disease Progression
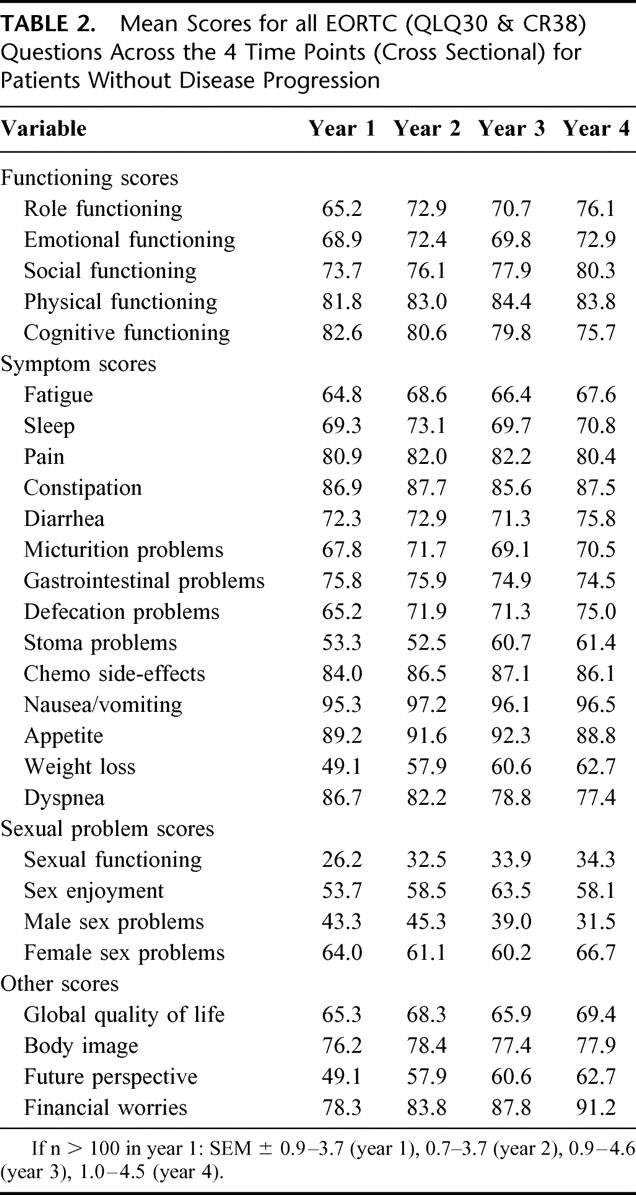
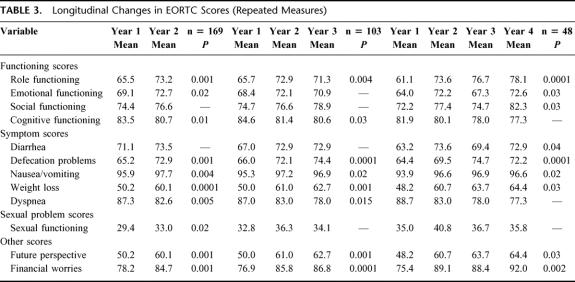
Table 3 presents the significant results of the analyses of scores over time for those patients who repeatedly completed questionnaires. Generally all scores improved over time, only cognitive function and dyspnea decreased significantly. Three of the 5 functioning scores showed significant improvements over time, 5 symptom scores improved, sexual functioning ameliorated, and both financial worries and future perspective got better. Between those who completed either two, 3 or 4 questionnaires, scores were very similar. The scores also compare closely with the cross sectional scores at each time point, indicating the representativeness of the sample.
TABLE 3. Longitudinal Changes in EORTC Scores (Repeated Measures)
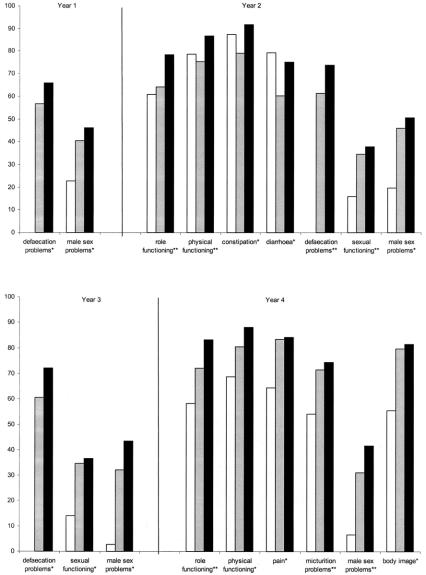
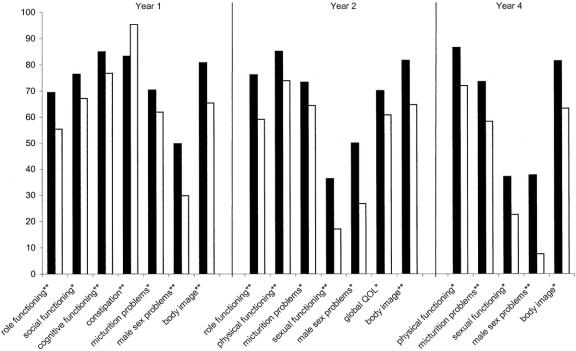
Figure 1 illustrates the significant differences in scores for patients operated by APE, LAR, or HAR at the 4 yearly time points. Over the 4-year time points, 10 variables were significantly worse for APE patients than AR patients and 3 were significantly worse for LAR patients. At year 2, HAR patients had better physical and role functioning scores than both LAR and APE patients. By year four, HAR patients had higher role functioning scores than LAR patients, likewise, LAR patients had better scores than APE patients. Considering symptom scores, in the first 3 years, LAR patients had significantly worse defecation scores than HAR patients. In year four, APE patients suffered more micturition problems than both LAR and HAR patients. Under sexual problem scores, across all 4 time points, male APE patients reported more male sexual problems than HAR or LAR patients. Likewise, LAR and HAR patients had better sexual functioning scores at years 2 and three. Figure 2 further illustrates the trend for LAR and HAR patients to generally experience better quality of life than APE patients.
FIGURE 1. Significant differences between APE, LAR and HAR patients’ EORTC scores (4 years cross-sectional). Solid box, HAR (≥8 cm) patients; shaded box, LAR (<8 cm) patients; open box, APE patients. *P < 0.05, **P < 0.01.
FIGURE 2. Principal EORTC scores for APE, LAR, and HAR patients (4 years cross-sectional). Solid box, HAR (≥8 cm) patients; shaded box, LAR (<8 cm) patients; open box, APE patients.
Ten of the EORTC scores that had shown improvements in the earlier repeated measures analysis were then compared repeatedly over the first 2 years for each group, APE and LAR and HAR. None of the variables improved significantly for APE patients. Emotional functioning (P < 0.02) and future perspective (P < 0.03) improved significantly for LAR patients alone. Body image (P < 0.02), nausea and vomiting (P < 0.02), and sexual functioning (P < 0.02) improved significantly for HAR patients alone. Role functioning (P < 0.05 and 0.001), defecation problems (P < 0.007 and 0.001), and weight loss (P < 0.02 and 0.03) significantly improved for both LAR and HAR patients, respectively.
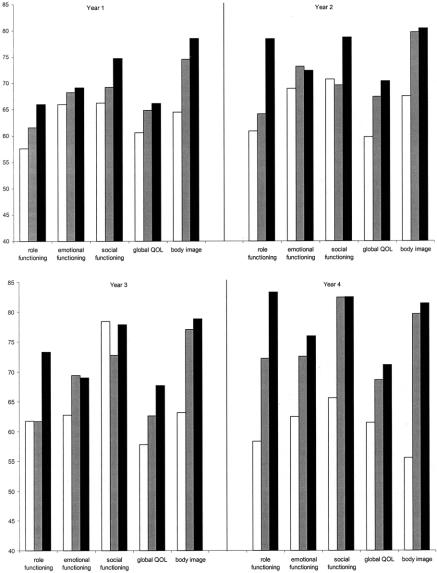
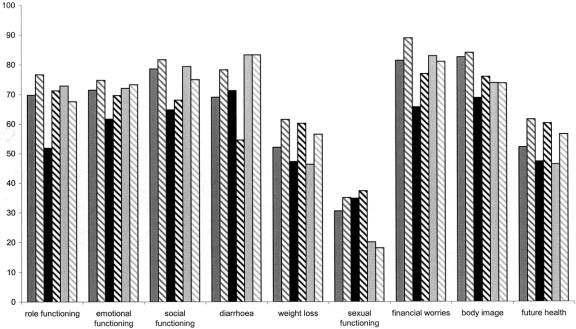
Figure 3 presents the significant differences in quality of life scores for patients with and without a stoma. Over half the stoma patients were APE patients (53.5%), 26.7% were HAR patients and 19.8% LAR patients. In total, over the 4 years, 9 variables were significantly worse for stoma patients. Four of the 5 functioning scores were significantly worse for stoma patients; role, social, physical, and cognitive functioning. Worse symptom scores were reported for micturition problems by stoma patients across 3 time points. Constipation, however, was experienced more by nonstoma patients at year 1 only. Among sexual problems, sexual functioning and male sex problems were consistently worse for stoma patients. Stoma patients also had significantly lower body image scores at 3 time points.
FIGURE 3. Significant differences between stoma and nonstoma patients’ EORTC scores (4 years cross sectional). Solid box, nonstoma patients; open box, stoma patients. *P < 0.05, **P < 0.01.
Figure 4 demonstrates the improvement in quality of life for those patients who had a stoma at the time of their first year questionnaire which was then reversed by the time of their second year questionnaire. It can be clearly seen that the improvement in role functioning and emotional functioning was much greater for reversed stoma patients than no stoma and permanent stoma groups. At the same time diarrhea problems increased for reversed stoma patients alone.
FIGURE 4. Differences in EORTC scores over time (repeated measures) by stoma type. Medium shaded box, no stoma year 1; medium hatched box, no stoma year 2; solid box, temporary stoma year 1; dark hatched box, reversed stoma year 2; lightly shaded box, permanent stoma year 1; lightly hatched box, permanent stoma year 2.
Finally, sexual functioning was consistently significantly worse for patients more than 70 years of age. In contrast, they had significantly higher emotional functioning in year 2. Radiation therapy patients also had significantly worse quality of life across 16 variables in year one. These differences persisted only for defecation problems.
DISCUSSION
Although the population-based sample of rectal cancer patients in the Munich region was complete, not all patients were approached by clinicians for quality of life data. In terms of operation strategy, the quality of life sample was representative. If it was biased in any way, it was toward younger patients but this would have had little effect on overall quality of life. If progression patients had been included, however, there would have been more patients with poorer quality of life and these patients would also have been more likely to have had APE. Thus, if anything, our report underestimates the differences in quality of life between APE and AR patients. Within the survey, questions relating to sexual problems were sometimes avoided. Low response rates to sexual questions, however, have been observed elsewhere.9,20 Although not all patients completed questionnaires at each time point, comparisons between those who completed multiple questionnaires showed similar scores, indicating that our cross sectional comparisons were also representative. Essentially, however, our study is descriptive and cannot establish causality. Further, without multivariate parametric statistics, only possible in nonskewed data, we cannot fully control for confounding variables. Nonetheless, with such large patient numbers stratification has been possible and possible confounders will be discussed.
Compared with responses to the EORTC QLQ-30 from a general German population sample,27 rectal cancer patients in our study, even after 4 years, had poorer functioning and more problems. The largest differences were in role and social functioning, fatigue, sleep, and diarrhea. Despite having survived the cancer, patients are scarred by their experience. A person never really gets over cancer because the threat of recurrence hangs over the individual and their family for the rest of the person’s life.28 On a more positive note, however, doctors can inform patients that many aspects of quality of life do improve over time, as shown by our repeated measures analyses, especially because there is some evidence that a positive approach from the doctor can reduce symptom burden.29
In our study, anterior resection patients and those without a stoma, despite suffering diarrhea, constipation, micturition, and defecation problems had better quality of life than APE or stoma patients without anorectal function. A previous study also found no relationship between bowel function and quality of life scores.30 A study that only measured functioning would, therefore, wrongly conclude that AR and non stoma patients were worse off. This is clearly not the case for overall quality of life, which should also be measured when comparing rectal cancer treatments. Further, differences in diarrhea, constipation and micturition problems in our study were not consistently statistically significant, indicating that they played a minor role.
For both AR and nonstoma patients, across most variables, there was a consistent trend toward better quality of life. In particular, there were significant differences in role, social and physical functioning, and better body image in patients without a stoma. In other words, stoma patients’ everyday work and hobby activities were limited (role functioning) and their social and family life disrupted (social functioning). They were less able to get about and look after themselves (physical functioning), and they felt less attractive (body image). In addition, scores for stoma problems were worse than those for diarrhea, constipation, micturition, defecation, and gastrointestinal problems. The importance of stoma on quality of life can further be demonstrated by the improvement in scores when a stoma was reversed. Despite an almost 20-point increase in diarrhea, for example, role functioning improved by 20 points. Essentially, quality of life improved to a greater extent for those HAR and LAR patients with a reversed stoma than for those with no stoma or APE patients with a permanent stoma.
In general, our findings for stoma patients reflect previous studies. An earlier large study concluded that stoma patients had worse quality of life.31 In Camilleri–Brennan and Steele’s review, stoma patients had reduced well-being and body image, whereas Sprangers et al, in their review, reported more urinary and sexual problems, greater distress and lower social functioning among stoma patients.2,10 Nonstoma patients, however, seemed to report more constipation.2
In our study, those with a high AR had less frequent or painful bowel movements than those with low AR where sphincter function may be impaired to a greater extent.7 LAR patients alone reported significantly more diarrhea and constipation than APE patients. This, however, did not lead to LAR patients, overall, having a worse quality of life than APE patients. Moreover, our work indicates that in the long term, APE patients had a worse quality of life. After 4 years, there were many significant differences between APE and LAR patients. Additionally, LAR patients improved their quality of life scores over time where APE patients, unfortunately, did not.
These results are in contrast to a recent study, also using the EORTC QLQ-30 and CR38, which concluded that “patients undergoing low AR have a lower QoL than those undergoing APE.”12 No statistical analysis was reported, however, to this effect; only LAR and HAR were statistically compared in their paper. Some of the differences found may be the result of the smaller patient sample and “precisely defined patient group” reported by Grumann et al. None of the AR patients in their study appeared to have stomas; they had no stoma problem scores, for example.12 This is in contrast to our sample where 30% of LAR and 15% HAR patients had a stoma postoperation. Other sample differences were also evident. The average age for APE patients was 61.4 and 62.2 years for AR patients.12 APE and AR patients in our quality of life sample were older (66.4 and 64.0 years, respectively). Other studies, reviewed by Camilleri-Brennan and Steele, reported that APE patients experienced more sexual and urinary problems, whereas LAR patients endured more bowel problems than standard AR patients.10 Some studies also reported that APE patients experienced poorer quality of life than AR patients but that LAR patients suffered reduced bowel function more than HAR patients.1,15,16 In a review of European studies, however, some papers reported no significant differences between APE and AR.9
Throughout our study, sexual functioning scores were particularly low. Without healthy population scores for sexual functioning, it is difficult to estimate the magnitude of this finding. Importantly, the survey asked to what extent the patient was interested in sex and sexually active, not what effect did their illness have on their sexual functioning. There were, however, consistently significant differences between APE patients and AR patients. LAR and HAR patients had similar scores. If nerve damage were the principal reason for lower sexual functioning one would expect APE and LAR scores to be worse than HAR scores. This was not the case in our study, suggesting that age was a stronger influence. More APE patients were older and the only consistent significant difference in quality of life between patients more or less than 70 years of age was in their sexual functioning scores, with older patients reporting worse sexual functioning. In our study stomas did not appear to contribute to sexual functioning scores as those with a temporary stoma had better sexual functioning than those with a permanent stoma and their functioning did not increase following stoma reversal. In particular, sexual functioning scores did not improve over time when other important scores, such as role functioning, greatly improved. This suggests that sexual functioning may not affect overall quality of life in rectal cancer patients.
As mentioned above, the only consistently significant age differences in quality of life were sexual functioning scores. There was also a trend, found in other research,32,33 that older patients had better psychologic functioning than younger patients. It has been suggested that cancer may have a greater impact on active and other wise healthy younger adults.34,35 With this in mind, one might expect the significantly older APE patients to have better quality of life. This was not reflected in our results where APE patients had consistently lower quality of life scores. This indicates that presence of a stoma, not age, may be the influential factor.
Finally, those who received radiation therapy had significantly worse quality of life in the first year. Cessation of radiation therapy may, therefore, have contributed to improvements in quality of life over time and differences in defecation between LAR and HAR patients. Similar percentages of LAR and APE patients received radiation therapy, yet there were significant differences in quality of life between these groups. Further those with a temporary or permanent stoma had similar levels of radiation therapy, yet when stomas were reversed a large improvement in quality of life occurred where stoma patients’ quality of life remained stable or deteriorated despite cessation of radiation therapy. Moreover, the effects of radiation therapy were confined to the first year where operation and stoma differences remained into the fourth year. Radiation therapy is delivered according to disease stage and this may have been reflected in the early quality of life scores and did not persist because when disease progression was recorded, quality of life scores were excluded from our analysis.
In conclusion, APE and stoma patients seemed to have a consistently lower quality of life. Nonstoma patients and HAR patients (most likely without a stoma) experienced better functioning and fewer symptoms. LAR patients, however, had higher quality of life scores than APE patients, even after 4 years. Improvements in scores over time were only evident in the LAR and HAR groups; reversal of temporary stomas may have contributed to this effect. The use of temporary stomas should, therefore, be carefully considered; the experience of having a stoma, even if temporary, seems to affect quality of life. The issue of temporary stomas is important and has not been adequately addressed in previous research. Studies comparing APE and AR, where possible, should include a stoma comparison. Those patients who are inconvenienced by a stoma, however, appear to have a better quality of life with regular irrigation.31 Many patients (60%) in our sample were poorly informed about stoma irrigation techniques. Indeed, patients report fewer symptoms and less anxiety when they feel better informed.29,36 Doctors should spend as much time as possible helping patients understand the disease, the prognosis and the implications of different operation methods. Interventions are also required to address poor self esteem, particularly in stoma patients. Thus, quality of life data should be used to improve treatment and care, to assist intervention planning, and to help patients anticipate side-effects.11
ACKNOWLEDGMENTS
We thank especially all patients who completed questionnaires and showed us how important it was to them to support our study. We also thank Fr. E. Liebetruth, Fr. A. Hucke, and Fr. B. Stegmann in particular for processing the data and all colleagues of the MCR for the co-operation and the reliable infrastructure. Such a work-intensive observational study is impossible without dedicated staff. Additionally, we thank all the following hospitals and departments that participated in the documentation of the medical data: Klinikum rechts der Isar der TU, Chirurgische Klinik und Poliklinik: Prof. Siewert, PD Dr. Nekarda, Dr. Vogelsang, Dr. Snopkowski; Klinikum der Ludwig Maximilians Universität—Groβhadern, Chirurgische Klinik und Poliklinik: Prof Jauch, Prof. Schildberg (em.), PD Dr. Heiss, Dr. Lau-Werner, PD Dr. Müller, Dr. Hornung; Klinikum der Ludwig Maximilians Universität—Innenstadt, Chirurgische Klinik und Poliklinik: Prof. Mutschler, Prof. Siebeck, Dr. Schorr; Städt. Krankenhaus München-Neuperlach, 1. Chirurgische Abteilung: Prof. Günther, Dr. Staimmer, Dr. Bergmann, Dr. Holzfurtner, Dr. Langer; Städt. Krankenhaus München-Schwabing, Visz. Chirurgische Abteilung: Prof. Waldner, Dr. Göring; Städt. Krankenhaus München-Harlaching, Chirurgische Abteilung: Prof. Horn, Dr. Kluge; Städt. Krankenhaus München-Bogenhausen, Abteilung für Allgemein- und Unfallchirurgie: Prof. Heitland, Dr. Wilhelm; Kreisklinik Fürstenfeldbruck, Chirurgische Abteilung: Dr. Knapp, Dr. Kauffmann, Dr. Gyβling; Rotkreuzkrankenhaus, Chirurgische Abteilung: Prof. Schoenberg, Dr. Paskuda, Dr. Fuchs; Krankenhaus des Dritten Ordens, Abteilung Allgemein- und Gefäβchirurgie, Schilddrüsenchirurgie: Dr. Pütterich, Dr. Löppert; Krankenhaus der Barmherzigen Brüder, Chirurgische Abteilung: Prof. Reuter, Dr. Papadakis; Maria-Theresia-Klinik, Chirurgische Abteilung: Dr. Hoffmann, Dr. Zimmermann, Dr. Grunow, Dr. Konietzny; Privatklinik Dr. Rinecker, Chirurgie: Dr. Rinecker, Dr. Göring; Kreiskrankenhaus München-Pasing, Kreiskrankenhaus München-Pasing: Dr. Laqua, Dr. Kieβling; Kreiskrankenhaus Landshut-Achdorf, Chirurgische Abteilung: Prof. Raab; Klinikum Landshut, Chirurgische Abteilung: Dr. Filler; Privatklinik Bogenhausen, Chirurgie: Dr. Huber, Dr. Osterholzer, Dr. Schmick; Kreiskrankenhaus Starnberg, Chirurgische Abteilung: Prof. Stahlknecht, Dr. Schmitz; Chirurgische Klinik Seefeld: Dr. Hermes, Dr. Hofinger; Kreisklinik Dachau, Abteilung für Allgemeinchirurgie: Dr. Birkhofer, Dr. Hildebrand; Kreiskrankenhaus Ebersberg, Chirurgische Abteilung: Prof. Dostal, Dr. Molitor, Dr. Sobez; Krankenhaus der Missionsbenediktinerinnen Tutzing, Chirurgische Abteilung: Dr. Wiesmeier, Dr. Dietl; Kreiskrankenhaus Erding, Abteilung für Viszeral- und Thoraxchirurgie: Dr. Boedecker, Dr. Nagel, Dr. Maier; Privatklinik Josephinum, Chirurgische Abteilung: Dr. Holzmann, Dr. Grube, Dr. Sassen; Kreiskrankenhaus Freising, Chirurgische Abteilung: Dr. Zeller, Dr. Hirster; Klinik Dr. Wolfart, Chirurgische Abteilung: Dr. Hungbauer, Dr. Czerny; Klinik Dr. Schreiber, Chirurgische Abteilung: Dr. Schreiber; Krankenhaus Martha Maria, Chirurgie: Dr. Fürst, Prof. Spelsberg; Kreiskrankenhaus München-Perlach, Chirurgische Abteilung, Dr. Burghart, Dr. Scharff; Klinik Olympiapark, Chirurgie: Dr. Buess.
Footnotes
Reprints: Jutta Engel, MD, Tumorregister München, Institut für Med. Informationsverarbeitung, Biometrie und Epidemiologie, Ludwig Maximilians Universität München, Machioninistrasse 15, D-81377 München, Germany. E-mail: engel@ibe.med.uni-muenchen.de.
Supported by the Federal Ministry of Health. The Munich Field Study is integrated in the Munich Cancer Registry (MCR), which is part of the Munich Comprehensive Cancer Center (MCCC), an institution of the Ludwig Maximilians Universität. Additional funding was given by the Deutsche Krebshilfe, the Bavarian Ministry of Health, and the Wilhelm Sander Stiftung.
REFERENCES
- 1.Renner K, Rosen HR, Novi G, et al. Quality of life after surgery for rectal cancer: do we still need a permanent colostomy? Dis Colon Rectum. 1999;42:1160–1167. [DOI] [PubMed] [Google Scholar]
- 2.Sprangers MA, Taal BG, Aaronson NK, et al. Quality of life in colorectal cancer. Stoma vs. nonstoma patients. Dis Colon Rectum. 1995;38:361–369. [DOI] [PubMed] [Google Scholar]
- 3.Greenlee RT, Hill-Harmon MB, Murray T, et al. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. [DOI] [PubMed] [Google Scholar]
- 4.Becker N, Muscat JE, Wynder EL. Cancer mortality in the United States and Germany. J Cancer Res Clin Oncol. 2001;127:293–300. [DOI] [PubMed] [Google Scholar]
- 5.Jehle EC, Haehnel T, Starlinger MJ, et al. Level of the anastomosis does not influence functional outcome after anterior rectal resection for rectal cancer. Am J Surg. 1995;169:147–152; discussion 152–153. [DOI] [PubMed] [Google Scholar]
- 6.Pahlman L. The rectal cancer debate. Eur J Surg Oncol. 2001;27:439. [DOI] [PubMed] [Google Scholar]
- 7.Guillem JG, Cohen AM. Current issues in colorectal cancer surgery. Semin Oncol. 1999;26:505–513. [PubMed] [Google Scholar]
- 8.McLeod RS. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. 2001;233:157–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koller M, Lorenz W. Quality of life research in patients with rectal cancer: traditional approaches versus a problem-solving oriented perspective. Langenbecks Arch Surg. 1998;383:427–436. [DOI] [PubMed] [Google Scholar]
- 10.Camilleri-Brennan J, Steele RJ. Quality of life after treatment for rectal cancer. Br J Surg. 1998;85:1036–1043. [DOI] [PubMed] [Google Scholar]
- 11.Sprangers MAG. Quality-of-life assessment in colorectal cancer patients: evaluation of cancer therapies. Semin Oncol. 1999;26:691–696. [PubMed] [Google Scholar]
- 12.Grumann MM, Noack EM, Hoffmann IA, Schlag PM. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. 2001;233:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuoka N, Moriya Y, Akasu T, Fujita S. Long-term outcome of urinary function after extended lymphadenectomy in patients with distal rectal cancer. Eur J Surg Oncol. 2001;27:165–169. [DOI] [PubMed] [Google Scholar]
- 14.McDonald PJ, Heald RJ. A survey of postoperative function after rectal anastomosis with circular stapling devices. Br J Surg. 1983;70:727–729. [DOI] [PubMed] [Google Scholar]
- 15.Marquis R, Lasry JC, Heppell J, et al. [Quality of life of patients after restorative surgery for cancer of the rectum]. Ann Chir. 1992;46:830–838. [PubMed] [Google Scholar]
- 16.Lewis WG, Martin IG, Williamson ME, et al. Why do some patients experience poor functional results after anterior resection of the rectum for carcinoma? Dis Colon Rectum. 1995;38:259–263. [DOI] [PubMed] [Google Scholar]
- 17.Osoba D, Aaronson N, Zee B, et al. Modification of the EORTC QLQ-C30 (version 2.0) based on content validity and reliability testing in large samples of patients with cancer. The Study Group on Quality of Life of the EORTC and the Symptom Control and Quality of Life Committees of the NCI of Canada Clinical Trials Group. Qual Life Res. 1997;6:103–108. [DOI] [PubMed] [Google Scholar]
- 18.McLachlan SA, Devins GM, Goodwin PJ. Validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ-C30) as a measure of psychosocial function in breast cancer patients. Eur J Cancer. 1998;34:510–517. [DOI] [PubMed] [Google Scholar]
- 19.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. [DOI] [PubMed] [Google Scholar]
- 20.Sprangers MAG, Te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). Eur J Cancer. 1999;35:238–247. [DOI] [PubMed] [Google Scholar]
- 21.Cull AM. Cancer-specific quality of life questionnaires: the state of the art in Europe. Eur J Cancer. 1997;33(suppl 6):S3–S7. [DOI] [PubMed] [Google Scholar]
- 22.Fayers P, Aaronson N, Bjordal K, et al. On behalf of the EORTC Quality of Life Study Group. QLQ-C30 Scoring Manual: 1st Edition, Brussels, 1995.
- 23.King MT. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res. 1996;5:555–567. [DOI] [PubMed] [Google Scholar]
- 24.Curran D, Aaronson N, Standaert B, et al. Summary measures and statistics in the analysis of quality of life data: an example from an EORTC-NCIC-SAKK locally advanced breast cancer study. Eur J Cancer. 2000;36:834–844. [DOI] [PubMed] [Google Scholar]
- 25.Camilleri-Brennan J, Steele RJ. The impact of recurrent rectal cancer on quality of life. Eur J Surg Oncol. 2001;27:349–353. [DOI] [PubMed] [Google Scholar]
- 26.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 27.Schwarz R, Hinz A. Reference data for the quality of life questionnaire EORTC QLQ-C30 in the general German population. Eur J Cancer. 2001;37:1345–1351. [DOI] [PubMed] [Google Scholar]
- 28.Mossman J, Boudioni M, Slevin ML. Cancer information: a cost-effective intervention. Eur J Cancer. 1999;35:1587–1591. [DOI] [PubMed] [Google Scholar]
- 29.Little P, Everitt H, Williamson I, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ. 2001;323:908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallbook O, Hass U, Wanstrom A, et al. Quality of life measurement after rectal excision for cancer. Comparison between straight and colonic J-pouch anastomosis. Scand J Gastroenterol. 1997;32:490–493. [DOI] [PubMed] [Google Scholar]
- 31.Schaube J, Scharf P, Herz R. [The quality of life after extirpation of the rectum for carcinoma]. Dtsch Med Wochenschr. 1996;121:153–157;discussion 158. [DOI] [PubMed]
- 32.Kenny P, King LM, Shiell A, et al. Early stage breast cancer: costs and quality of life one year after treatment by mastectomy or conservative surgery and radiation therapy. Breast. 2000;9:37–44. [DOI] [PubMed] [Google Scholar]
- 33.King MT, Kenny P, Shiell A, et al. Quality of life three months and one year after first treatment for early stage breast cancer: influence of treatment and patient characteristics. Qual Life Res. 2000;9:789–800. [DOI] [PubMed] [Google Scholar]
- 34.Warmuth MA, Bowen G, Prosnitz LR, et al. Complications of axillary lymph node dissection for carcinoma of the breast: a report based on a patient survey. Cancer. 1998;83:1362–1368. [DOI] [PubMed] [Google Scholar]
- 35.Bentzen SM, Dische S. Morbidity related to axillary irradiation in the treatment of breast cancer. Acta Oncol. 2000;39:337–347. [DOI] [PubMed] [Google Scholar]
- 36.Mills ME, Sullivan K. The importance of information giving for patients newly diagnosed with cancer: a review of the literature. J Clin Nurs. 1999;8:631–642. [DOI] [PubMed] [Google Scholar]