Abstract
Objective:
To determine whether the number of hepatocytes containing AFP mRNA shed into the bloodstream during transarterial chemoembolization (TAE) affects the incidence and pattern of recurrence of hepatocellular carcinoma (HCC).
Patients and Methods:
We developed a Taqman procedure to quantify AFP mRNA prospectively in 52 consecutive patients before and after TAE. Results are expressed in hepatocytes /mL.
Results:
Thirteen of the patients (24.5%) were positive for AFP mRNA (42 ± 19 hepatocytes/mL) before TAE and 13 (24.5%) (80 ± 32 hepatocytes/mL) after TAE; the difference was not significant. The presence of AFP mRNA in the bloodstream before TAE was associated with larger nodules (85.2 ± 73.8 mm versus 34.8 ± 26.1 mm; P = 0.006). Six of the patients were excluded from the analysis because they underwent curative surgery or were lost to follow-up. The circulating levels of AFP mRNA released in the 46 remaining patients after TAE did not affect metastasis-free survival. A significant number of extrahepatic metastases were found in patients exhibiting at least 1 AFP mRNA-positive blood sample either before or after TAE. However, the TAE procedure did not increase the risk of extrahepatic recurrences.
Conclusion:
Cells containing AFP mRNA are inconsistently released into the circulation during TAE. The amount of these cells released does not affect the recurrence of HCC.
We use a Taqman procedure to prospectively quantify circulating AFP mRNA in 52 consecutive patients who underwent transarterial chemoembolisation (TAE).
The only hope for the long-term survival of patients with hepatocellular carcinoma (HCC) is surgical resection or transplantation. However, only a small proportion of the patients diagnosed with HCC actually benefit from curative surgery. Most patients with HCC undergo palliative approaches. The most frequently used palliative approach is transarterial embolization (TAE), which is either used alone or in combination with chemotherapy (the so-called chemoembolization). Some studies have shown that this approach can lead to a beneficial downstaging or total necrosis of the tumor, improving the metastasis-free survival of patients who subsequently underwent curative surgery.1-12 However, TAE remains a controversial approach for the treatment of patients with HCC. Indeed, some controlled trials have failed to show a significant benefit in long-term survival in treated patients compared with untreated patients.13-19 The main problems with TAE are the mortality rate because of liver injury and the increased occurrence of hepatic or extrahepatic recurrences.20-25 The most common extrahepatic metastasis of HCC is pulmonary metastasis.17 Some studies have reported that hematogenous dissemination might occur from the primary tumor if TAE results in tumor necrosis, which facilitates the release of cancer cells from the primary tumor.26-29
We previously described the amplification of AFP mRNA by means of reverse transcription (RT) and a nested polymerase chain reaction (PCR). This method is highly sensitive for the detection of residual HCC cells in peripheral blood.30 However, the qualitative (positive versus negative) detection of HCC cells in samples from individual patients is of limited value in predicting the risk of disease progression.30,31 Because the level of AFP mRNA is increased in HCC tissue compared with in normal hepatocytes,32 the quantification of AFP transcripts seems to be a more reliable indicator of disease progression. We developed a highly sensitive assay based on TaqMan technology to quantify AFP mRNA in “real time”. We used this technique to determine whether circulating AFP mRNA can be used as a marker of the delivery of tumor cells into the bloodstream during TAE and whether this is responsible for the formation of extrahepatic metastases. We correlated the presence of circulating AFP mRNA with the recurrence and metastasis-free survival of the patients.
PATIENTS AND METHODS
Patients and Samples
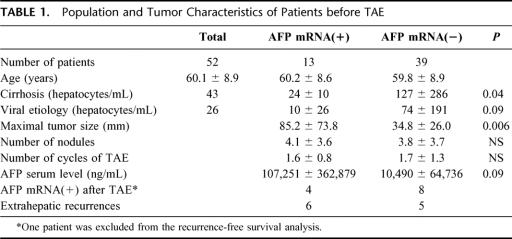
Peripheral blood samples were obtained prospectively from 52 patients with HCC who underwent TAE. Patients with indications of increased risk (poor liver function, serum bilirubin level > 50 μmol/L, serum creatinin level > 150 μmol/L, tumor penetration of liver capsule and occluded portal vein) were excluded from the study. The characteristics of the patients are summarized in Table 1. None of the patients had known extrahepatic metastasis, as assessed by systematic abdominal ultrasound, thoracoabdominal computed tomography (CT) scan, and bone scintigraphy. Peripheral vein puncture was performed just before and after the first cycle of TAE (at 24 hours and then 1 to 3 times before day 5 when the patients gave a blood sample that was used to assess liver function). The patients underwent systematic abdominal ultrasound, thoracoabdominal CT scan, and bone scintigraphy every 3 or 6 months until 27 months following TAE. The maximal diameter of each hepatic nodule was recorded on CT scans 6 weeks following the TAE procedure. Clinical events including surgery, recurrence, or death were recorded. Most of the patients were male (n = 43) and the mean age was 60.1 ± 8.9 years (range, 41-77 years).
TABLE 1. Population and Tumor Characteristics of Patients before TAE
A group of 28 control blood samples from healthy subjects was assessed in parallel.
Transarterial Embolization
Hepatocellular carcinomas were treated with hepatic arterial chemotherapy under fluoroscopic control via a catheter in a femoral artery. A conventional celiomesenteric arteriography was used to check arterial anatomy and portal venous flow. Papaverine (40 mg) (Renaudin, France) was injected into the mesenteric artery to optimize portal vein flow. Coaxial microcatheters were used in cases of substetonic or tortuous artery. Alternatively, small arteries were injected to decrease the risk of reflux into other arteries. In the case of diffuse HCC or multifocal HCC (right and left side of the liver), chemoembolization material was injected into the hepatic artery between the gastroduodenal and the cystic arteries.
Intraarterial chemotherapy was given by injecting 10-12 mL of iodized oil (lipiodol Ultra Fluide; Laboratoire Guerbet, Aulnay-sous-Bois, France) mixed with an emulsion of 20 to 70 mg of cisplatin (Cysplatyl; Lilly, St. Cloud, France).
Embolization was performed by applying a mixture of contrast agent and 4-mm-diameter resorbable gelatin sponge Curaspon® (Curaspon Healthcare, Zwanenburg, The Netherlands) until stasis was achieved in the tumor vessels. Embolization was not carried out for patients with occluded main portal veins, hepatofugal portal flow or inadequate residual liver function. During chemoembolization, conscious sedation was induced with 1 - 2 mg Hypnovel (Midazolam, Laboratories Roche, Neuilly/Seine, France) and 50 - 100 mg Fentanyl (Laboratories Dakota Pharm, Sanofi-Synthelabo, Le Plessis-Robinson, France). Sedated patients were continuously monitored. After the intraarterial procedure, the patients were allowed to recover with 24 hours bed rest. During the first 6 hours after intraarterial embolization, patients underwent hourly clinical evaluation. Liver function tests were carried out every 2 days until the patients were discharged.
Cell Material and RNA Isolation
Nucleated cells were isolated from 10 mL of peripheral blood (precisely quantified) collected in tubes that had been treated with EDTA using tetradecyltrimethylammonium bromide, as previously described.30 Total RNA was extracted from the pellet, and the RNA was dissolved in RNaase-free water (40 μL). The efficiency of RNA extraction was assessed by amplifying the 18s rRNA by use of a commercially available assay (Applied Biosystems, Foster City, CA).
Human hepatocytes were isolated from 3 cadaveric organ donors using collagenase as described by Pichard et al33 Total RNA was extracted from 107 hepatocytes and used to produce a standard curve. The quantity of AFP mRNA was then assessed in each hepatocyte preparation, yielding reproductible data
Real-Time Quantification of AFP mRNA
cDNA was synthesized in a volume of 10 μL using TaqMan reverse transcription reagents (Applied Biosystems). RT buffer 10X (1 μL), 5.5 mM MgCl2, 500 μM each dNTP, 200 nM AFP RT-primer (5′TCTGGATTTCAGTAAAATTAACTTTGGTAA3′), 8 U RNase inhibitor, and 25 U Multiscribe reverse transcription were mixed with 3 μL RNA extracted from peripheral blood samples. The cycling conditions were 10 minutes at 25°C, 20 minutes at 42°C, and 5 minutes at 95°C. The reverse transcription product (2.5 μL) was used for each subsequent round of PCR. A standard curve was constructed with pure and diluted cDNA synthesized with RNA from 40,000 hepatocyte equivalents. A new standard curve was prepared for each series of samples. The PCRs, TaqMan analysis, and subsequent calculations were performed using the ABI Prism 7700 Sequence Detection System (Applied Biosystems), which detects the signal from the fluorogenic probes during PCR.
AFP cDNAs were amplified using gene-specific primers that anneal to different exons to give PCR products of 111 bp. Primers and probes were chosen with the assistance of the Primer Express computer program (Applied Biosystems). The sense and antisense primers were 5′AGCAGCTTGTTAAATCAACATGCA3′ and 5′AAAATTAACTTTGGTAAACTTCTGACTCAGT3′. The probe was 5′6-FAM-TGGGACCCGAACTTTCCAAGCCATC-TAMRA 3′.
The PCR mixture contained 400 nM of each AFP primer, 200 nM probe, 5 mM MgCl2, 200 μM dATP, dCTP and dGTP, 400 μM dUTP, 0.625 U of AmpliTaq Gold, 0.25 μM Amperase UNG (uracyl N-glycosylase), and 1× TaqMan buffer in a total volume of 25 μL. All of the PCR reagents were purchased for TaqMan PCR core reagents (Applied Biosystems) The mixture was incubated for 2 minutes at 50°C to permit UNG cleavage. AmpliTaq Gold was then activated by incubation for 10 minutes at 95°C. Each of the 50 PCR cycles consisted of 15s denaturation at 95°C and hybridization and DNA synthesis for 1 minute at 60°C. Experiments were performed in triplicate for each standard and sample data point.
The threshold cycle (Ct), which is proportional to the initial number of target copies in each sample, was defined as the fractional cycle number at which the fluorescence generated by the cleavage of the probe passed a fixed threshold. The ΔRn corresponded to the change in the intensity of the fluorescent reporter, before and after amplification normalized to the fluorescence of an internal positive reference. The amount of AFP cDNA in each sample was determined from the standard curve. The results are expressed as normal hepatocyte equivalents per mL of whole blood and simplified as “hepatocytes per mL”.
Statistical Analysis
Statistical analysis was performed using the Statview software system (Statview F-4.5) and the Student t test. The χ2 test and Fisher exact test were used to determine whether there were any statistical differences in clinical data in patients whose blood contained circulating AFP mRNA and in those whose blood did not. A correlation test was used to assess the relationship between the quantity of circulating AFP mRNA and AFP serum levels or the delay of recurrence. The probability of survival without recurrent disease in the 2 groups was determined by use of the Kaplan-Meier technique and the significance of the difference was analyzed by the log-rank test. A P value of less than 0.05 was considered to have statistical significance.
RESULTS
Performance of Real-Time Quantitative RT-PCR
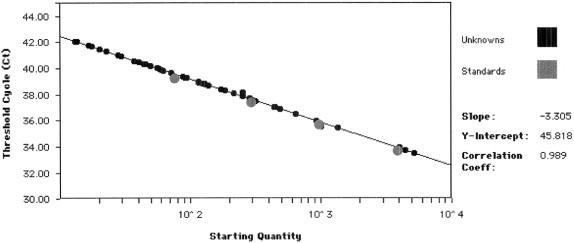
RT-PCR was optimized by assessing the effects of changes in several parameters including the concentrations of MgCl2, TaqMan buffer, primers, and probe. The optimum concentration for each component was that giving the best compromise between a high ΔRn and a low Ct value. A standard curve was constructed with total RNA extracted from human 107 hepatocytes and serially diluted 1:10 in total human leukocyte RNA extracted from normal subjects (Fig. 1). A strong linear relationship was found between Ct and the log number of stained copies in each case (r2 = 0.999). The efficiency of the reaction was always over 90%, as assessed by the slope of the standard curve as recommended by the manufacturer.
FIGURE 1. AFP mRNA standard curve obtained by real-time RT-PCR. Standard curve plotting log starting copy number versus Ct. Gray dots, data for standard curve point samples using fresh isolated human hepatocytes; small black dots, data for samples from unknown patients.
Sensitivity
We tested the sensitivity of the PCR by testing serial dilutions of human hepatocyte RNA. The last dilution that gave linear results was 10−4, corresponding to 10.9 pg of AFP mRNA (0.66 hepatocytes/mL). Thus, this method can detect at least 1 hepatocyte/106 cells.
Specificity
We quantified AFP mRNA in the peripheral blood of 28 healthy subjects. The results were all negative (ie, below 0.66 hepatocytes /mL).
Use of the Quantitative PCR Assay Before TAE in Patients with HCC
The 52 patients with HCC were tested before the TAE procedure. Thirteen samples (24.5%) were found to be positive, with 42 ± 19 hepatocyte /mL (range, 2-173). The total blood AFP mRNA concentrations were compared with the morphologic characteristics of the tumor as assessed by CT scan and ultrasound (number and diameter of the largest nodule) of the corresponding HCC tumors (Table 1). A positive correlation was found between concentrations of circulating AFP mRNA and the size of the nodules. The mean diameter of the largest nodule was 85.2 ± 73.8 mm in patients positive for circulating AFP mRNA versus 34.8 ± 26.1 mm in negative patients (P = 0.006). AFP mRNA-positive patients tended to have higher concentrations of serum AFP protein than did negative patients (107,251 ± 362,259 versus 10,490 ± 69,736 ng/mL; P = 0.09). The amount of circulating AFP mRNA was significantly lower in patients with underlying cirrhosis than in patients with HCC but no cirrhosis (P = 0.04). When cirrhosis was caused by a viral infection (HCV and/or HBV), the levels of circulating AFP mRNA tended to be significantly lower (P = 0.09). Of the thirteen patients who were positive for AFP mRNA before TAE, 12 were analyzed for recurrence and 6 presented an extrahepatic metastasis during follow-up.
Use of the Quantitative PCR Assay After TAE in Patients with HCC
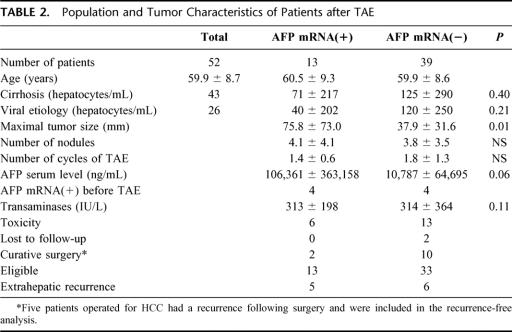
Thirteen patients (24.5%) were positive for circulating AFP mRNA at least once after TAE. The mean value was 80 ± 32 hepatocytes/mL (range, 1-877; Table 2). A significant correlation was obtained between the release of AFP mRNA cells into the bloodstream and the maximal size of the tumor (75.8 ± 73.0 versus 38.0 ± 32.6 mm, P = 0.012). The number of AFP mRNA-positive cells released into the bloodstream following TAE was not significantly higher than that released before TAE (P = 0.63, paired t test).
TABLE 2. Population and Tumor Characteristics of Patients after TAE
Ten patients were positive 24 hours after TAE and 2 became positive later. Eight patients remained positive for at least 2 consecutive blood samples and only 1 patient gave 3 different positive samples.
TAE Complications
Post-TAE syndrome (abdominal pain, fever > 38°C, nausea) was observed in 19 patients (36.5%, 6 of the 13 AFP mRNA-positive patients and 13 of the 39 AFP mRNA-negative patients). Two patients suffered from liver failure with an increase of ascites and serum bilirubin concentration, but without encephalopathy. Five patients presented transient thoracic pain. One patient had severe hypoxia. No treatment-related deaths occurred at the time or during the 4 weeks following TAE.
There was no significant difference in the mean peak value of transaminases during the first 3 days post-TAE between patients with circulating AFP mRNA (352 ± 203 IU/L) and those without (302 ± 367 IU /L). The peak value of transaminases is considered to be a biologic marker for TAE-induced cytolysis.
Effect of TAE on Recurrence of HCC
Two patients who were negative for circulating AFP mRNA were lost within the first month of follow-up and were excluded from the analysis. Nine patients underwent curative surgery, including 6 patients who underwent orthotopic liver transplantation; the size of their tumor had been reduced sufficiently to allow surgery by the TAE cycles. These patients could not be compared with patients who did not undergo surgery. However, we only excluded patients who did undergo surgery when no recurrence was observed following surgery: 4 patients were operated within 2 months following the end of TAE and no recurrence was observed within the 12 to 27 months of post surgery follow-up. However, we included the 5 remaining patients in the recurrence-free survival analysis, including 2 patients who had undergone an orthotopic liver transplantation. These patients were included because they presented a recurrence after curative surgery, suggesting the presence of circulating micrometastases. Thus, a total of 46 patients were analyzed.
The occurrence of metastases, as assessed by CT scan, was considered for the analysis to determine the metastasis-free survival in the 46 remaining patients. Figure 2 shows the Kaplan-Meier curves of metastasis-free survival. Thirteen patients were positive for circulating AFP mRNA after TAE and 33 patients were negative. At 12 months, 73% of AFP mRNA-positive patients had developed a metastasis, compared with 39% of the negative patients. The median delay of metastasis-free survival was 11 months in the positive patients and 20.5 months in the negative group (P = 0.79, Logrank analysis).
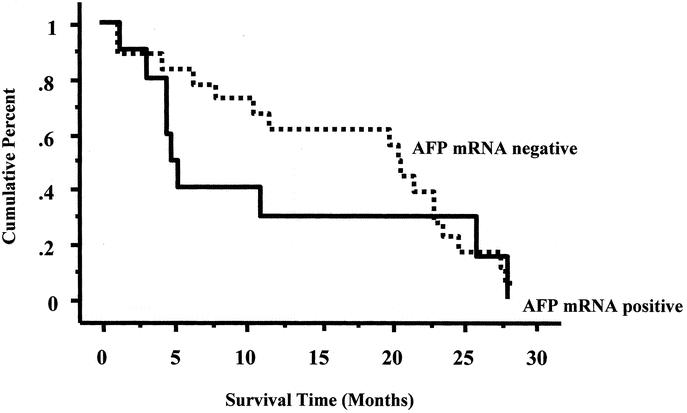
FIGURE 2. Kaplan-Meier curves for metastasis-free survival for the 2 groups of patients are shown: 1) patients without AFP mRNA and 2) patients positive for AFP mRNA after TAE.
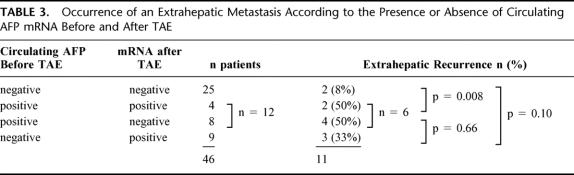
Eleven patients (24%) suffered extrahepatic metastases (Table 3). These patients could be divided into 3 groups. The first group included patients who never had circulating AFP mRNA either before or after TAE, 8% of these patients exhibited an extrahepatic metastasis. The second group included patients who were positive before TAE and were either positive or negative after TAE. An extrahepatic recurrence was observed in 6 out of the 12 patients in this group (50%); the percentage of recurrence was significantly different from the first group (P = 0.008). The third group included patients who were negative before TAE and then became positive after TAE, one third (33%) of these patients had an extrahepatic metastasis; the difference was not significant compared with the first or the second groups.
TABLE 3. Occurrence of an Extrahepatic Metastasis According to the Presence or Absence of Circulating AFP mRNA Before and After TAE
We also analyzed the occurrence of extrahepatic metastases in the 21 patients who were positive for AFP mRNA either before of after TAE (Table 3); 9 of these patients had extrahepatic metastases, compared with 2 of the patients who never had a positive sample (P = 0.06).
Effect of the Amount of Circulating AFP mRNA on Recurrence
The amount of circulating AFP mRNA varied from 1 to 877 hepatocytes/mL. We found no relationship between the amount of circulating AFP mRNA and the delay before recurrence for each patient (P = 0.65).
DISCUSSION
We developed a sensitive technique and used it to analyze the amount and the timing of the shedding of cells containing AFP mRNA into the bloodstream in patients with HCC following transarterial chemoembolization. Our data show that approximately 80 hepatocytes per mL containing AFP mRNA are released inconsistently into the circulation during TAE. The number of hepatocytes containing AFP mRNA released into the bloodstream after TAE did not affect the probability of recurrence.
HCC mainly recurs as a result of hematogenous dissemination from tumor cells. Screening for the disseminated or even circulating cells may provide important preoperative clinical information. There is evidence that different therapeutic management strategies, eg, surgery or needle liver biopsy, in HCC patients and in patients with other liver diseases result in higher circulating concentrations of AFP mRNA, but this does not necessarily indicate the presence of malignant cells.30,31,34-36 Indeed, AFP mRNA has been found in normal hepatocytes.30-32 Moreover, a minimum number of circulating cancer cells are needed for the development of distant metastases in animal models.37,38 The quantification of circulating HCC cells might help us to gain more insight into the dynamics of the micrometastatic process and to overcome specificity problems. The sensitivity of our quantitative assay is similar to that of conventional nested RT-PCR using reverse transcription and Taq polymerase (data not shown). The advantage of this quantitative assay compared with other quantitative methods39-42 is that the standard curve is constructed using freshly isolated human hepatocytes instead of cell lines. Indeed, isolated human hepatocytes provide a closer view of the physiological situation in terms of analytical processes (RNases, necrosis, etc.). Furthermore, the results can be expressed more easily as the number of released “normal” hepatocytes per mL of whole blood and not compared with the level of expression of a reference gene that may be subject to variations due to the cancer.43 Interestingly, we did not find circulating AFP mRNA in normal control subjects.
Our quantitative assay showed that an average of 80 hepatocytes are released per mL. It is difficult to compare this number of cells to other results as they are not generally expressed in the same units.39-42 The high interpatient variations may explain why we, and others,28,31,34-36,39,40,42,44 did not observe circulating hepatocytes in all HCC patients. One possible reason for the sporadic detection of AFP mRNA is that tumor cell dissemination is a highly dynamic process. AFP mRNA may only be present in the blood for short periods of time as the shed cells seem to be cleared by capillary systems, especially in the bone marrow. Rapid necrosis of liberated cells may be a major reason for the variable and low numbers of tumor cells found in the bloodstream. This is particularly true in the case of TAE, which induces massive hepatocyte necrosis. The amount of circulating cells may be near to the sensitivity threshold and PCR may fail to detect cells even after slight changes in cell numbers. However, 80 hepatocytes/mL probably represents a large number of circulating hepatocytes because this number relates to daily 10 mL samples. If the liver blood flow rate (2 mL/min) is considered, the number of circulating cells after TAE could be very high number.
Before TAE, 24.5% of HCC patients had circulating hepatocytes. This percentage is similar to that reported by us30 and others36,42 and lower than that reported by Louha et al,31 Matsumara et al,42 and Okuda et al35 The release of AFP mRNA was correlated with a greater size of the largest nodule, as previously reported.30,32,41,42 After TAE, 24.5% of patients had circulating hepatocytes. This percentage corresponded to that previously described by Wong et al41 Only a few of the patients exhibited circulating AFP mRNA 3 days post TAE, showing that cells are rapidly cleared up. These results are consistent with the release of tumorous hepatocytes and/or mRNA from necrotic hepatocytes. However, the release of AFP mRNA did not correspond to the degree of hepatocyte necrosis as assessed by the peak of serum transaminases. This is not surprising because necrosis is generally associated with the release of broken cells, the RNA of which is then degraded by RNases. This could explain why we found more circulating AFP mRNA in patients who had developed HCC on noncirrhotic livers with nonviral etiologies.
The use of TAE in our series of 52 HCC patients resulted in side effects in 19 patients but no direct mortality and allowed curative surgery in 9 patients (17%). These data are consistent with previous results from our group9,10 and others.20,24,25 However, the release of AFP mRNA did not lead to a greater incidence of side effects or death.
The levels of circulating AFP mRNA after TAE did not affect metastasis-free survival. The analysis of the occurrence of extrahepatic metastasis post TAE showed that when patients were negative both before and after TAE, only 8% of them exhibited an extrahepatic recurrence. However, when patients were positive before TAE, the risk of extrahepatic recurrence increased to 50%. Nine out of 46 patients (20%) presenting circulating AFP mRNA cells after TAE were negative before TAE; only 33% of them had an extrahepatic recurrence. Therefore, the release of AFP mRNA-positive cells into the bloodstream may be associated with an increased risk of extrahepatic recurrence, independent of TAE. Similarly, Matsumara et al found AFP mRNA in the blood of 6 out of 6 patients with metastases of extrahepatic organs.44 Because AFP mRNA has been found in normal hepatocytes, we cannot discriminate between the release of normal cells and that of tumor cells. No genes have yet been shown to be specifically and systematically altered in HCC with no illegitimate transcription within leukocytes that can be used to study HCC dissemination. The quantification of the transcripts of a gene more specifically implicated in the metastatic process, particularly in the homing of metastases or the loss of cell adhesion,45-47 but not transcribed at a low level within leukocytes, could be a better method for assessing the metastatic potential of tumors.
In conclusion, a significant correlation was obtained between circulating AFP mRNA and tumor size before TAE, consistent with a release of hepatocytes. The presence of AFP mRNA-positive cells in the bloodstream either before or after TAE procedure did affect the prognosis. However, TAE itself did not affect the number of AFP mRNA-positive cells shed into the bloodstream or the pattern of recurrence of HCC.
ACKNOWLEDGMENTS
The authors acknowledge the valuable contributions of members of the Department of Anesthesiology at Paul Brousse Hospital.
Footnotes
Reprints: Antoinette Lemoine, MD, Service de Biochimie et Biologie Moléculaire, Hôpital universitaire Paul Brousse, 14 avenue Paul Vaillant Couturier, 94804 Villejuif Cedex, E-mail: France.antoinette.lemoine@pbr.ap-hop-paris.fr.
Grants: This work was supported by Faculté de Médecine Paris Sud (UPRES-EA 1596), Fondation pour l’Avenir and Fondation Adrienne et Pierre Sommer.
REFERENCES
- 1.Okamura J, Horikawa S, Fujiyama T, et al. An appraisal of transcatheter arterial embolization combined with transcatheter arterial infusion of chemotherapeutic agent for hepatic malignancies. World J Surg. 1982;6:352–357. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura H, Tanaka T, Hori S, et al. Transcatheter embolization of hepatocellular carcinoma: assessment of efficacy in cases of resection following embolization. Radiology. 1983;147:401–405. [DOI] [PubMed] [Google Scholar]
- 3.Yamada R, Sato L, Kawabata L, et al. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397–401. [DOI] [PubMed] [Google Scholar]
- 4.Kasugai H, Kojima J, Tatsuta M, et al. Treatment of hepatocellular carcinoma by transcatheter arterial embolization combined with intraarterial infusion of a mixture of cisplatin and ethiodized oil. Gastroenterology. 1989;97:965–971. [DOI] [PubMed] [Google Scholar]
- 5.Monden M, Okamura J, Sakon M, et al. Significance of transcatheter chemoembolization combined with surgical resection for hepatocellular carcinomas. Cancer Chemother Pharmacol. 1989;23:S90–5. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda K, Kumada H, Saitoshi S, et al. Effect of repeated transcatheter arterial embolization on survival time in patients with hepatocellular carcinoma: an analysis by the Cox proportional hazard model. Cancer. 1991;15:2150–2154. [DOI] [PubMed] [Google Scholar]
- 7.Vetter D, Wenger JJ, Bergier JM, et al. Transcatheter oily chemoembolization in the management of advanced hepatocellular carcinoma in cirrhosis: results of a western comparative study in 60 patients. Hepatology. 1991;13:427–433. [PubMed] [Google Scholar]
- 8.Yamashita Y, Takahashi M, Koga Y, et al. Prognostic factors in the treatment of hepatocellular carcinoma with transcatheter arterial embolization and arterial infusion. Cancer. 1991;67:385–391. [DOI] [PubMed] [Google Scholar]
- 9.Bismuth H, Morino M, Sherlock D, et al. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg. 1992;163:387–394. [DOI] [PubMed] [Google Scholar]
- 10.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578–1583. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. [DOI] [PubMed] [Google Scholar]
- 13.Hwang TL, Chen MF, Lee TY, et al. Resection of hepatocellular carcinoma after transarterial embolization. Reevaluation of the advantages and disadvantages of preoperative embolization. Arch Surg. 1987;122:756–759. [DOI] [PubMed] [Google Scholar]
- 14.Pelletier G, Roche A, Ink O, et al. A randomized trial in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990;11:181–184. [DOI] [PubMed] [Google Scholar]
- 15.Madden MV, Krige JEJ, Bailey S, et al. Randomized trial of targeted chemotherapy with lipiodol and 5-epidoxorubicin compared with symptomatic treatment for hepatoma. Gut. 1993;34:1598–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groupe d’étude et de traitement du carcinome hepatocellulaire. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med. 1995;332:1256–1261. [DOI] [PubMed] [Google Scholar]
- 17.Wu CC, Ho YZ, Ho WL, et al. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122–126. [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki S, Hasegawa H, Kinoshita H, et al. A prospective randomised trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. Jpn J Cancer Res. 1996;87:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelletier G, Ducreux M, Gay F, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129–134. [DOI] [PubMed] [Google Scholar]
- 20.Nagasue N, Galizia G, Kohno H, et al. Adverse effects of preoperative hepatic artery chemoembolization for resectable hepatocellular carcinoma: a retrospective comparison of 138 liver resections. Surgery. 1989;106:81–86. [PubMed] [Google Scholar]
- 21.Adachi E, Matsumata T, Nishizaki T, et al. Effects of preoperative transcatheter hepatic arterial chemoembolization for hepatocellular carcinoma. The relationship between postoperative course and tumor necrosis. Cancer. 1993;72:3593–3598. [DOI] [PubMed] [Google Scholar]
- 22.Yu YQ, Xu DB, Zhou XD, Lu JZ, Tang ZY, Mack P. Experience with liver resection after hepatic arterial chemoembolization for hepatocellular carcinoma. Cancer. 1993;71:62–65. [DOI] [PubMed] [Google Scholar]
- 23.Harada T, Matsuo K, Inoue T, et al. Is preoperative hepatic arterial chemoembolization safe and effective for hepatocellular carcinoma? Ann Surg. 1996;224:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paye F, Jagot P, Vilgrain V, et al. Preoperative chemoembolization of hepatocellular carcinoma: a comparative study. Arch Surg. 1998;133:767–772. [DOI] [PubMed] [Google Scholar]
- 25.Paye F, Farges O, Dahmane M, et al. Cytolysis following chemoembolization for hepatocellular carcinoma. Br J Surg. 1999;86:176–180. [DOI] [PubMed] [Google Scholar]
- 26.Bonfil RD, Bustuoabad OD, Ruggiero RA, et al. Tumor necrosis can facilitate the appearance of metastases. Clin Exp Metastasis. 1988;6:121–129. [DOI] [PubMed] [Google Scholar]
- 27.Nishizaki T, Matsumata T, Kanematsu T, et al. Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J Surg Res. 1990;49:92–97. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura M, Shiratori Y, Niwa Y, et al. Presence of alpha-fetoprotein mRNA in blood correlates with outcome in patients with hepatocellular carcinoma. J Hepatol. 1999;31:332–339. [DOI] [PubMed] [Google Scholar]
- 29.Liou TC, Shih SC, Kao CR, et al. Pulmonary metastasis of hepatocellular carcinoma associated with transarterial chemoembolization. J Hepatol. 1995;23:563–568. [DOI] [PubMed] [Google Scholar]
- 30.Lemoine A, Le Bricon T, Salvucci M, et al. Prospective evaluation of circulating hepatocytes by alpha-fetoprotein mRNA in humans during liver surgery. Ann Surg. 1997;226:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louha M, Nicolet J, Zylberberg H, et al. Liver resection and needle liver biopsy cause hematogenous dissemination of liver cells. Hepatology. 1999;29:879–882. [DOI] [PubMed] [Google Scholar]
- 32.Niwa Y, Matsumura M, Shiratori Y, et al. Quantitation of alpha-fetoprotein and albumin messenger RNA in human hepatocellular carcinoma. Hepatology. 1996;23:1384–1392. [DOI] [PubMed] [Google Scholar]
- 33.Pichard L, Fabre I, Fabre G, et al. Cyclosporin A drug interactions. Screening for inducers and inhibitors of cytochrome P-450 (cyclosporin A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab Dispos. 1990;18:595–606. [PubMed] [Google Scholar]
- 34.Louha M, Poussin K, Ganne N, et al. Spontaneous and iatrogenic spreading of liver-derived cells into peripheral blood of patients with primary liver cancer. Hepatology. 1997;26:998–1005. [DOI] [PubMed] [Google Scholar]
- 35.Okuda N, Nakao A, Takeda S, et al. Clinical significance of alpha-fetoprotein mRNA during perioperative period in HCC. Hepatogastroenterology. 1999;46:381–386. [PubMed] [Google Scholar]
- 36.Kienle P, Weitz J, Klacs R, et al. Detection of isolated disseminated tumor cells in bone marrow and blood samples of patients with hepatocellular carcinoma. Arch Surg. 2000;135:213–218. [DOI] [PubMed] [Google Scholar]
- 37.Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973;9:223–227. [DOI] [PubMed] [Google Scholar]
- 38.Mayhew E, Glaves D. Quantitation of tumorigenic disseminating and arrested cancer cells. Br J Cancer. 1984;50:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumura M, Ijichi M, Shiratori Y, et al. Simple quantitative assay of alpha-fetoprotein mRNA in liver tissue using the real-time detection polymerase chain reaction assay - its application for clinical use. Hepatol Res. 2001;20:84–96. [DOI] [PubMed] [Google Scholar]
- 40.Funaki NO, Tanaka J, Imamura M. Quantitative analysis of alpha-fetoprotein mRNA in circulating peripheral blood of patients with hepatocellular and alpha-fetoprotein-producing gastric carcinomas. Life Sci. 1998;62:1973–1984. [DOI] [PubMed] [Google Scholar]
- 41.Wong IH, Lau WY, Leung T, et al. Hematogenous dissemination of hepatocytes and tumor cells after surgical resection of hepatocellular carcinoma: a quantitative analysis. Clin Cancer Res. 1999;5:4021–4027. [PubMed] [Google Scholar]
- 42.Aselmann H, Wolfes H, Rohde F, et al. Quantification of a1-fetoprotein mRNA in peripheral blood and bone marrow: a tool for perioperative evaluation of patients with hepatocellular carcinoma. Langenbeck’s Arch Surg. 2001;386:118–123. [DOI] [PubMed] [Google Scholar]
- 43.Finnegan MC, Goepel JR, Hancock BW, et al. Investigation of the expression of housekeeping genes in non-Hodgkin’s lymphoma. Leuk Lymphoma. 1993;10:387–393. [DOI] [PubMed] [Google Scholar]
- 44.Matsumura M, Niwa Y, Kato N, et al. Detection of alpha-fetoprotein mRNA, an indicator of haematogenous spreading hepatocellular carcinoma, in the circulation: a possible predictor of metastatic hepatocellular carcinoma. Hepatology. 1994;20:1418–1425. [DOI] [PubMed] [Google Scholar]
- 45.Osada T, Sakamoto M, Ino Y, et al. E-cadherin is involved in the intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 1996;24:1460–1467. [DOI] [PubMed] [Google Scholar]
- 46.Naor D, Vogt Sionou R, Ish Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997;241–319. [DOI] [PubMed]
- 47.Tanaka Y, Mimori K, Shiraishi T, et al. Alpha-6 integrin expression in esophageal carcinoma. Int J Oncol. 2000;16:725–729. [DOI] [PubMed] [Google Scholar]