Abstract
Objective:
To evaluate the impact of spleen weight on operative and clinical outcome in a series of 108 consecutive laparoscopic splenectomies.
Background:
Laparoscopic splenectomy as an alternative to open splenectomy for splenomegaly is regarded as controversial.
Methods:
Patients underwent laparoscopic splenectomy for a range of hematological disorders between November 1992 and February 2000. Multiple linear and logistic regression analysis were used to assess the effect of massive splenomegaly (>1000 g) on perioperative mortality and morbidity, after adjusting for the joint effects of patient age, weight, pre- and postoperative full blood counts, operating time, estimated blood loss, conversion rate, reoperation rate, and duration of hospital stay.
Results:
Massive splenomegaly was recorded in 27 of 108 (25%) cases. In this group, splenic weight ranged from 1000 to 4750 g (median, 2500 g). Patients with splenic weight >1000 g had a significantly longer median operating time (170 vs. 102 minutes, P < 0.01), conversion rate (5/27 vs. 4/81, P < 0.05), postoperative morbidity (15/27 vs. 4/81, P < 0.01), and median postoperative stay (5 vs. 3 days, P < 0.01). Multivariate analysis found splenic weight to be the most powerful predictor of morbidity (P < 0.01). Patients with splenomegaly (>1000 g) were 14 times likely to have post operative complications. One patient died 3 days after surgery, following a pulmonary embolus (spleen weight 500 g, mortality 1/108, 0.9%).
Conclusions:
Laparoscopic splenectomy is feasible in patients with giant spleens. However, it is associated with greater morbidity, and the advantages of minimal access surgery in this subgroup of patients are not so clear.
Laparoscopic splenectomy, first performed in 1992, is becoming the method of choice as surgical expertise in advanced laparoscopic techniques has improved.1,2 Laparoscopic enthusiasts have shown that it is feasible to perform laparoscopic splenectomy for massive splenomegaly (splenic weight greater than 1000 g).3 However, difficulty in manipulating the large organ, problems in controlling bleeding, and retrieval of the specimen has meant that laparoscopic splenectomy in patients with massive splenomegaly should be approached with caution. Indeed, in some centers, estimated splenic weight greater than 1 kilogram, has even been regarded a contraindication to laparoscopic approach. The aim of this study was to assess the impact of splenic weight on outcome in patients undergoing laparoscopic splenectomy.
MATERIALS AND METHODS
Between November 1992 and February 2000, 108 patients underwent laparoscopic splenectomy for a range of hematologic disorders. During this period all splenectomies were attempted laparoscopically on the surgical unit. Detailed review of the medical records was conducted. Data collection included patient characteristics, hematologic diagnosis, operative details, postoperative morbidity, and mortality. Patients were divided into 2 groups according to splenic weight; less or greater than 1 kilogram. The splenic weight had been recorded prospectively.
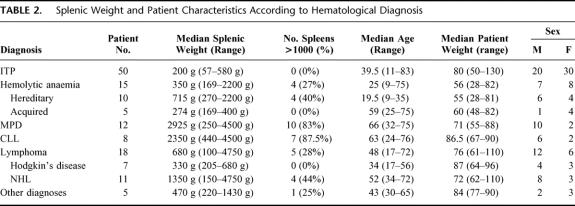
Patient characteristics are shown in Table 1. Age ranged from 9 to 83 years (median, 41) years and median weight was 76 kg (range, 28-130 kg). Fifty-one patients were women. Sixteen percent (n = 17) of patients had undergone previous abdominal surgery and 19% (n = 21) had coexisting medical conditions. The most frequent indication for splenectomy was steroid-refractory or steroid-dependent idiopathic thrombocytopenic purpura (n = 50, 46%). Other diagnoses included hemolytic anemias (hereditary n = 10, 9%; acquired n = 5, 5%) myeloproliferative disorders (n = 12, 11%), chronic lymphocytic leukemia (n = 8, 7%), lymphoma (n = 18, 17%), and miscellaneous hematologic disorders (n = 5, 5%; Table 2).
TABLE 1. Patient Characteristics
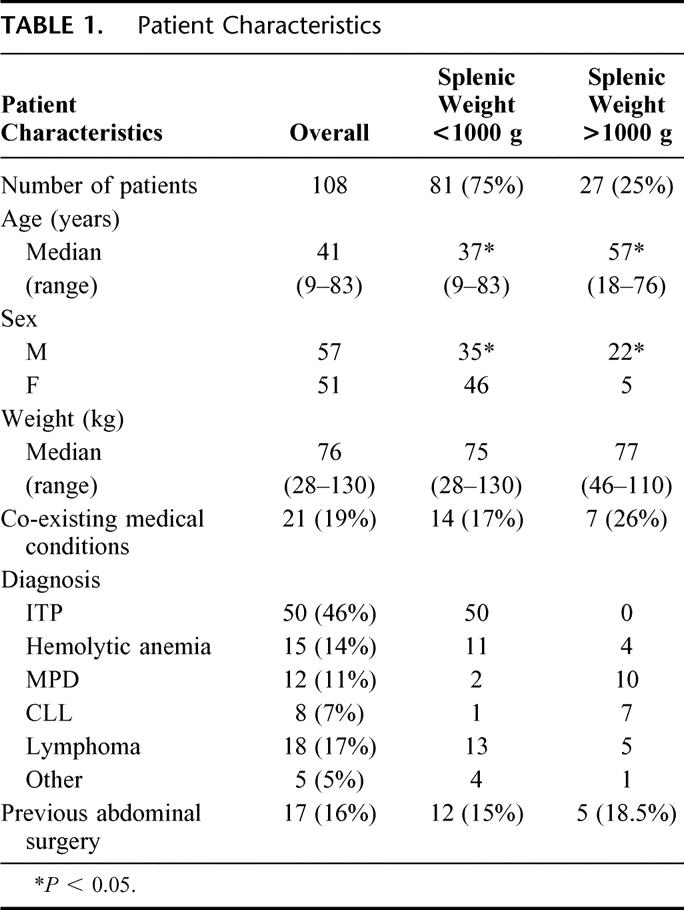
TABLE 2. Splenic Weight and Patient Characteristics According to Hematological Diagnosis
All patients received meningococcal and pneumococcal vaccine at least a week before surgery. Antibiotic prophylaxis was commenced on induction and continued postoperatively. Laparoscopic splenectomy was performed by using our technique, which has been previously described.4 Specifically, all patients were placed in the supine position. In those with nonpalpable spleens, a 3-L saline bag was placed below the left flank, thus tilting the patient toward (by approximately 60°) the right lateral decubitus position. However, in the massive splenomegaly group, this position becomes technically difficult because of the weight of the spleen. In these patients in the supine position, our approach was to mobilize the lower pole of the spleen as far as possible. The spleen was then rotated toward the left, allowing the splenic vessels to be more easily exposed. Although the majority of the mobilization was done with the harmonic scalpel, any medium-sized vessels were ligaclipped. The main splenic vessels were transected by using the EndoGIA vascular stapler (US Surgical, Norwalk, CT). In this series, the hand port was not used. The large spleens were divided intra-abdominally with scissors and placed into retrieval bags. After removal of the spleen a closed suction drain was placed through the lateral trocar site. The abdomen was irrigated with heparinized-saline and desufflated. After the trocars were taken out, the fascia at the larger than 5-mm sites was approximated with figure-of-eight sutures (Vivryl 1/0), and the skin closed in a subcuticular fashion (Monocryl 3/0) after injection of 0.5% Marcaine (Astrazeneca, Bedfordshire, England). Total operating time, blood loss, and splenic weight was noted at the end of each case. The latter was calculated as dry weight of the splenic pulp and the volume of blood leached from the spleen in the endocatch bag.
The nasogastric tube and the folly catheter were removed, and the patients were allowed to eat and drink as tolerated 6 hours after the procedure. Postoperative analgesia consisted of diclofenac sodium. The drain in the splenic bed, if present, was removed within 24 to 48 hours. Full blood count, coagulation screen, urea, and electrolytes were monitored postoperatively and at follow-up visit 6 weeks after the procedure.
Data Analysis
Statistical analysis was performed by using a χ2 analysis and Mann–Whitney U Test by using P < 0.05 to determine statistical significance. Multiple linear and logistic regression analysis were used to assess the effect of massive splenomegaly (>1000 g) on perioperative mortality and morbidity after adjusting for the joint effects of patient age, weight, pre- and postoperative full blood counts, operating time, estimated blood loss, conversion rate (patient requiring any incision over 5 cm), reoperation rate, and duration of hospital stay. Receiving operating characteristics analysis was used to find the optimal threshold values for any continuous variables that were retained in the model.
RESULTS
In the 108 patients who underwent laparoscopic splenectomy, median splenic weight was 300 g (range, 57–4750 g), with 27/108 (25%) weighing over 1000 g (Table 1). Massive splenomegaly was most common in myeloproliferative disorders (10/12, 83%), and chronic lymphocytic leukemia (7/8, 88%), whereas in idiopathic thrombocytopenic purpura, all spleens weighed <1000 g (Table 2). Patient age and ratio of males to females was significantly higher in the cohort with massive splenomegaly; this reflects variations in age ranges and sex distribution in the different disease categories (Table 2).
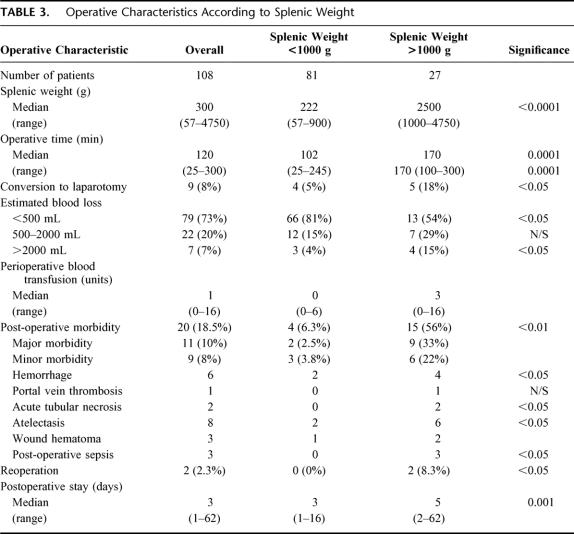
Overall, median operation duration was 120 minutes (range, 25–280). A correlation between operating time and splenic weight was observed (Spearman R = 0.45, P < 0.01); patients with massive splenomegaly having significantly longer operation (median 170 vs. 102 minutes, P < 0.01). Interoperative blood loss was minimal in the majority of patients with only 7 (7%) cases experiencing blood loss greater than 2 L. Major blood loss (>2 L) was significantly more common in patients with massive splenomegaly (4/27 vs. 3/81, P < 0.05). There was, however, no correlation between spleen size and perioperative transfusion requirements. Conversion to laparotomy occurred in 9/108 (8%) patients and was caused by excessive hemorrhage in all cases. Patients with massive splenomegaly were significantly more likely to undergo conversion than cases with splenic weight <1000 g (5/27 vs. 4/81, P < 0.05).
Postoperative complications arose in 20/108 (18.5%) patients, 11 cases (10%) experiencing major morbidity. Frequent complications included atelectasis (8/108, 7%) and secondary hemorrhage (6/108, 6%). In the latter group, 2 patients required reoperation, both of which had massive splenomegaly. Findings at reoperation included bleeding from 1 short gastric vessel in 1 patient. The other patient had bleeding from the staple line of the transected splenic vessels. Reoperation rate (2/27 vs. 0/81, P < 0.05) and postoperative morbidity (15/27 vs. 4/81, P < 0.01) was significantly higher in patients with splenic weight greater than 1000g (Table 3).
TABLE 3. Operative Characteristics According to Splenic Weight
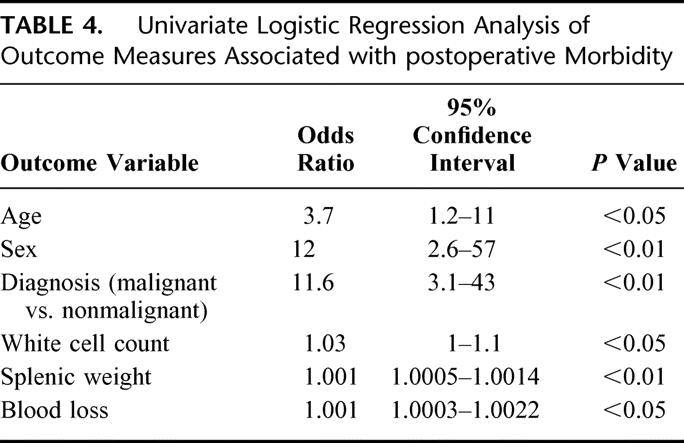
The presence of morbidity was one of the main outcome variables investigated. On univariate logistic regression, outcome measures that showed a significant association with morbidity were splenic weight, sex, age, diagnosis (malignant vs. nonmalignant), white cell count, and blood loss (Table 4). However, splenic weight correlated highly with white cell count (r = 0.5, P < 0.01), blood loss (r = 0.31, P < 0.05), and hematological malignancy (r = 0.61, P < 0.01). On a multiple logistic regression model, the most powerful predictor of morbidity was splenic weight (odds ratio [OR] = 1.001, 95% confidence interval [CI] 1.0003–1.0013, P < 0.01). Sex presented a borderline significance (OR = 5.5, 95% CI 1.1-28, P = 0.05) but was retained in the model. The odds of morbidity increased by 0.1% for each unit increase in splenic weight above 1000 g and 5-fold for men in relation to women. By using receiving operating characteristics analysis techniques, the optimal threshold value for splenic weight was approximately 1000 g (95% CI 3.6-53, P < 0.01). After adjusting for the effect of gender, the odds of morbidity were increased 14-fold for patients with a splenic weight above 1000 g. This classification has a sensitivity of 89%, a specificity of 74%, and a misclassification rate of 13%. One patient with known severe ischemic heart disease died 16 days postoperatively after pulmonary embolism (perioperative mortality 0.9%).
TABLE 4. Univariate Logistic Regression Analysis of Outcome Measures Associated with postoperative Morbidity
Time to discharge after surgery ranged from 1 to 62 days (median 3 days), with 64/108 (59%) patients being discharged by day 3. Discharge was significantly longer in patients with massive splenomegaly (5 vs. 3 days, P < 0.01) and those experiencing postoperative complications (median 8 days) compared with no complications (median 3 days; P < 0.01).
DISCUSSION
Splenomegaly has been defined by Goldstone as a spleen weighing over 1.5 kg or 10 times normal weight.5 However, a number of authors have suggested that for the purposes of splenectomy a threshold of 1 kg is more realistic.6,7 In our series, 27 patients had splenic weight over 1 kg with 21 having spleens weighing over 1.5 kg. Analysis of the data indicates that the optimal threshold at which morbidity associated with laparoscopic splenectomy increases is 1 kg. Estimation of splenic weight can be difficult. The splenic hilum (artery and vein) in all our cases was divided with firing of the endoGIA vascular stapler and therefore unlikely to cause large changes in splenic weight. However, even small capsular tears will cause underestimation of splenic weight. A more accurate assessment of splenic size by volumetric assessment of the spleen by computed tomography scan or ultrasound would have been more objective but was not performed as part of our preoperative assessment. Ultrasound measurement of the length of a spleen would also have been useful, as a 15-cm span spleen is approximately equal to 1 kg and fits into a 1 15-mm endocatch (autosuture; unpublished data).
The laparoscopic approach has over the last decade become the gold standard technique for performing splenectomies for small spleens.8 Baccarani et al reviewed the literature and suggest that laparoscopic splenectomy for hematological diseases is as safe and effective as open splenectomy and offers the advantages of short hospital stay, decreased complications, more rapid return to normal activity, and better cosmetic result.9 This is well demonstrated by our data; 81/108 patients with normal or moderately enlarged spleens (<1000 g) had a short operating time (median 102 minutes), low transfusion rate (median = 0 units), conversion rate (5%), morbidity (6%), and a median hospital stay of 3 days.
Open splenectomy for massive splenomegaly is associated with a high mortality and morbidity (between 20% and 60%).5,6,10–15 Letoquart et al reported a mortality of 2% and morbidity of 26% in a series of 47 patients with splenomegaly undergoing open splenectomy.16 This is not dissimilar to our series of massive splenomegaly performed laparoscopically, with a 0% mortality, 33% major morbidity, and minor morbidity of 22%. McAneny et al showed in a meta-analysis that patients with massive spleens (>1.5 kg) have a higher mortality and morbidity than normal and moderately enlarged spleens (<1.5 kg), although this difference was not apparent when comparing patients with the same diagnosis. In 223 patients undergoing elective open splenectomy, they concluded that after adjusting for age and diagnosis using multivariate analysis, spleen size was not a risk factor.17 In contrary, in our series of laparoscopic splenectomies, splenic weight (thus splenic size) was a powerful predictor of morbidity. This maybe explained by the fact that during laparoscopic splenectomy for splenomegaly the space available in the abdominal cavity after the creation of the pneumoperitoneum is diminished, the organ is more difficult to manipulate and technically demanding.18 During open surgery, small increments in wound size to aid removal of a large spleen is not likely to effect outcome. Targarona and colleagues divided 69 patients undergoing laparoscopic splenectomy into 3 groups according to splenic weight (I = <400 g; II = 400-1000 g; III = >1000 g). They showed that there was no significant difference in morbidity between patients with different splenic weights (I = 12%, II = 33%, III = 30%).3 However, small patient numbers with massive splenomegaly (group III, n = 10) made statistical analysis meaningless. In a follow-up series of 105 patients, morbidity in the cohort with massive splenomegaly (n = 21) was significantly higher than those with normal or moderately enlarged spleens.18 They also compared morbidity in patients with massive splenomegaly undergoing laparoscopic splenectomy (27%) with open splenectomy (55%), and showed no significant difference (6/21 vs. 11/20, P = 0.08).
Laparoscopic splenectomy in the large spleens was associated with a conversion rate of 18% in our group of patients; this is not dissimilar to other series reported in the literature.3 In the series of Targarona et al, all spleens weighing over 3.2 kg required conversion to open.18 Similarly, Schlachta et al demonstrated that in 14 patients with hematological malignancies, all spleens having a diameter greater than 27 cm required conversion.19 In our experience, 7 patients had splenic weight over 3.2 kg (range, 3.25–4.75 kg), none of which were converted. This may reflect the difference in our operative technique. Targarona et al use a hanging spleen technique with the use of 2 additional endoretractors to raise the spleen and allow transection of the splenic hilum.3 An anterior approach with the patients supine is used in our patients with splenomegaly. This avoids having to counteract the weight of the spleen in the lateral approach. All conversions in the group with massive splenomegaly group (18%) were secondary to bleeding. Moreover, a correlation between splenic weight and blood loss (r = 0.31, P < 0.05) was observed. To minimize the risk of intraoperative bleeding in patients with splenomegaly, Poulin and colleagues, performed splenic artery embolization in 8 patients with spleens longer than 20 cm before laparoscopic splenectomy and achieved a conversion rate of 17%.20 We did not perform preoperative splenic artery embolization in this series. Instead our approach, similar to Nicholson and colleagues, has been to adopt a policy of early hilar devascularization.21 Other measures have included the use of a powerful motorized suction irrigation system available, and the addition of a 1000 IU of heparin to the irrigation fluid (1 L of normal saline) to prevent clotting of blood and blockage of the sucker in the event of bleeding. Another potential solution to reduce bleeding is to apply direct pressure using a hand port to assist the laparoscopic splenectomy. Berman and colleagues reviewed 22 patients undergoing laparoscopic splenectomy for hematological malignancies. The conversion rate was significantly (P < 0.01) less with the use of the hand port 10% (1/10) compared with 75% (8/12) without the use of the hand port.22 The hand port also aids retrieval of the organ.
Operating time correlated with splenic weight (r = 0.45, P < 0.01). A major influence on operating time is the retrieval of the spleen once it is free in the abdominal cavity. To minimize the time required with retrieval of the large spleens an accessory incision is made in some patients in the left upper quadrant. Other methods used to facilitate retrieval include liposuction of the splenic pulp using the 10-mm Stryker Suction Irrigation System (Stryker Europe, Switzerland) and then dividing the spleen into 2 or more pieces intraperitoneally; providing that there are no hematological contraindications.
Preoperative assessment of splenic size with ultrasound gives the potential for identification of high risk patients and therefore allow other approaches to be adopted. In our view splenomegaly should not be considered a contraindication for laparoscopic splenectomy.3 Patients with massive splenomegaly (>1000 g), however, have a high morbidity, conversion rate, and longer hospital stay. The benefits of a laparoscopic approach in massive splenomegaly is not as clear cut as for normal or moderately enlarged spleens; this subgroup requires further evaluation with the open procedure.
Footnotes
Reprints: Ameet G. Patel, Consultant Surgeon, Department of Surgery, King’s College Hospital, Denmark Hill, London SE5 9RS, United Kingdom. E-mail: agp@ameet.dircon.co.uk.
REFERENCES
- 1.Cuschieri A, Shimi S, Banting S, et al. Technical aspects of laparoscopic splenectomy: Hilar segmental devascularization and instrumentation. J R Coll Surg Edinburgh. 1992;37:414–416. [PubMed] [Google Scholar]
- 2.Delaitre B, Maignien B, Icard PH. Laparoscopic splenectomy. Br J Surg. 1992;79:1334. [DOI] [PubMed] [Google Scholar]
- 3.Targarona EM, Espert JJ, Balague C, et al. Splenomegaly should not be considered a contraindication for laparoscopic splenectomy. Ann Surg. 1998;228:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhodes M, Rudd M, O’Rourke N, et al. Laparoscopic splenectomy and lymph node biopsy for hematologic disorders. Ann Surg. 1995;222:43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstone J. Splenectomy for massive splenomegaly. Am J Surg. 1978;135:385–388. [DOI] [PubMed] [Google Scholar]
- 6.Johnson HA, Deterling RA. Massive splenomegaly. Surg Gynecol Obstet. 1989;168:131–137. [PubMed] [Google Scholar]
- 7.Ly B, Albrechtsen D. Therapeutic splenectomy in hematologic disorders: effects and complications in 221 adult patients. Acta Medica Scandinavica. 1981;209:21–29. [PubMed] [Google Scholar]
- 8.Friedman RL, Fallas MJ, Carroll BJ, et al. Laparoscopic splenectomy for ITP. The gold standard. Surgical Endoscopy. 1996;10:991–995. [DOI] [PubMed] [Google Scholar]
- 9.Baccarani U, Donini A, Terrosu G, et al. Laparoscopic splenectomy for haematological diseases: review of current concepts and opinions. Eur J Surg. 1999;165:917–923. [DOI] [PubMed] [Google Scholar]
- 10.Bickerstaff KI, Morris PJ. Splenectomy for massive splenomegaly. Br J Surg. 1987;74:346–349. [DOI] [PubMed] [Google Scholar]
- 11.King DJ, Dawson AA, Thompson WD. Splenectomy in patients with malignant lymphoma presenting with massive splenomegaly. Eur J Haematol. 1987;38:162–165. [DOI] [PubMed] [Google Scholar]
- 12.Shaw JHF, Clark M. Splenectomy for massive splenomegaly. Br J Surg. 1989;76:395–397. [DOI] [PubMed] [Google Scholar]
- 13.Fabri PJ, Metz EN, Nick WV, et al. A quarter century with splenectomy: changing concepts. Arch Surg. 1974;108:569–575. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell A, Morris PJ. Surgery of the spleen. Clin Haematol. 1983;12:565–590. [PubMed] [Google Scholar]
- 15.Musser G, Lazar G, Hocking W, et al. Splenectomy for hematologic disease: the UCLA experience with 306 patients. Am Surg. 1984;200:40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letoquart JP, La Gamma A, Kunin N, et al. Splenectomy for splenomegaly exceeding 1000 grams: analysis of 47 patients. Br J Surg. 1993;80:334–335. [DOI] [PubMed] [Google Scholar]
- 17.McAneny D, LaMorte WW, Scott TE, et al. Is splenectomy more dangerous for massive spleens? Am J Surg. 1998;175:102–107. [DOI] [PubMed] [Google Scholar]
- 18.Targarona EM, Espert JJ, Cerdan G, et al. Effect of spleen size on splenectomy outcome. A comparison of open and laparoscopic surgery. Surg Endosc. 1999;13:559–562. [DOI] [PubMed] [Google Scholar]
- 19.Schlachta CM, Poulin EC, Mamazza J. Laparoscopic splenectomy for hematologic malignancies. Surg Endosc. 1999;13:865–868. [DOI] [PubMed] [Google Scholar]
- 20.Poulin EC, Mamazza J, Schlachta CM. Splenic artery embolization before laparoscopic splenectomy. An update. Surg Endosc. 1998;12:870–875. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson IA, Falk GL, Mulligan SC. Laparoscopically assisted massive splenectomy. A preliminary report of the technique of early hilar devascularization. Surg Endosc. 1998;12:73–75. [DOI] [PubMed] [Google Scholar]
- 22.Berman RS, Yahanda AM, Mansfield PF, et al. Laparoscopic splenectomy in patients with hematologic malignancies. Am J Surg. 1999;178:530–536. [DOI] [PubMed] [Google Scholar]