Abstract
Objective
To describe our approach in the decision-making for taking the middle hepatic vein with the graft or leaving it with the remnant liver in right lobe live donor liver transplantation.
Summary Background Data
Right lobe living donor liver transplantation has been successfully performed. However, the extent of donor hepatectomy is still a subject of debate and the main considerations in the decision making are graft functional adequacy and donor safety.
Methods
An algorithm based on donor-recipient body weight ratio, right lobe-to-recipient standard liver volume estimate, and donor hepatic venous anatomy was used to decide the extent of donor hepatectomy. This algorithm was applied in 25 living donor liver transplant operations performed between January 1999 and January 2002. In grafts taken without the middle hepatic vein, anterior segment tributaries draining into it were not reconstructed. Outcomes between right lobe liver transplants with (Group I) and without (Group II) the middle hepatic vein were compared.
Results
Ten grafts included the middle hepatic vein and 15 did not. The mean graft to recipient standard liver volume ratio was 58% and 64% in Groups I and II, respectively, and the difference was not statistically significant. Donors from both groups had comparable recovery, with 2 complications, 1 from each group, requiring a percutaneous drainage procedure. The recipient outcomes were, likewise, comparable and there was 1 case of structural outflow obstruction in Group I, which required venoangioplasty and stenting. There were 2 recipient mortalities, 1 due to a biliary complication and the other to recurrent hepatitis C. Another patient required retransplantation for secondary biliary cirrhosis. The overall actuarial graft and patient survival rates are 84% and 96%, respectively, at a median follow-up of 16 months.
Conclusion
Based on certain preoperative criteria, a right lobe graft can be taken with or without the middle hepatic vein with equally successful outcomes in both the donors and recipients. The decision, therefore, of the extent of right lobe donor hepatectomy should be tailored to the particular conditions of each case.
This is a single center study comparing the outcomes of adult live donor liver transplantation using right lobe grafts with and without the MHV without reconstruction of the major tributaries. The approach to determine the extent of donor right lobectomy based on certain preoperative criteria is described.
Transplantation of the right hepatic lobe from a living donor is a major development in live donor liver transplantation (LDLT) that has brought about a significant, albeit insufficient, increase in graft supply. The main concerns in this type of complex operation are graft adequacy, in terms of size and function, and donor safety. Extremes of size mismatch in LDLT may be detrimental and adults are at greater risk for small-for-size syndrome,1,2 which brings to fore the question of the extent of right lobectomy a donor should undergo. The aim is to provide adequate functional mass to the recipient without compromising donor safety. Some centers choose to routinely take the middle hepatic vein (MHV) with the graft,3 or leave it with the remnant, with4 or without5 reconstruction of the major tributaries (segment V and VIII branches) in the recipient. Although these technical variants have been performed successfully, it is a fact that donor-recipient pairs will come in invariably different size matches and hepatic vascular configurations that need to be taken into consideration in deciding which type of graft would be most suitable. The issue, therefore, of whether the MHV should or should not be taken with the graft, or whether the segment V and VIII tributaries should be reconstructed in the recipient, is far from settled. We have opted for a selective approach using preoperative parameters to determine whether the MHV should be taken with the graft. This study, therefore, aims to determine the applicability of a selective approach based on preoperative parameters to determine whether the MHV should be taken with the graft or left with the donor, and to describe the outcomes after LDLT using 1 type of graft or the other.
PATIENTS AND METHODS
From June 1994 to January 2002 a total of 94 primary LDLT were performed at the Chang Gung Memorial Hospital, Kaohsiung, Taiwan. Twenty-five of these were with right lobe grafts and performed over a 3-year period from January 1999. The right lobe donors included parents (1 mother, 2 fathers), children (1 daughter, 7 sons), siblings (1 brother) and spouses (11 wives, 2 husbands). The indications for transplantation in the recipients were end-stage liver disease due to hepatitis B virus cirrhosis (n = 17), hepatitis C virus cirrhosis (n = 2), primary biliary cirrhosis (n = 4), Wilson’s disease (n = 1), and biliary atresia (n = 1). Ten of the patients with hepatitis B and 1 of the patients with hepatitis C also had hepatocellular carcinoma. No donor or recipient has been lost to follow-up ranging from 9 to 45 months (median, 16 months).
Donor Evaluation
Only consanguineous relatives up to the third degree and lawfully wedded spouses are considered legal donors in Taiwan. Spouses should have been married for at least 3 years or with children if less than this period.6 Donor volunteers underwent initial screening with hepatitis viral serology testing and blood typing.
Eligible donors then proceeded with anthropometric measurements, thorough laboratory analysis, psychosocial evaluation, and detailed imaging studies including Doppler ultrasonography to check the quality of liver parenchyma and patency of blood vessels; and magnetic resonance (MR) venography, arteriography, and cholangiography to check hepatic and portal venous anatomy and hepatic artery and biliary tree branching patterns. Computed tomography (CT) venography and arteriography were used in the latter 14 cases in lieu of conventional percutaneous angiography, which was performed routinely in the early part of this series. Computed tomography volumetry was performed to measure the volumes of the right and left lobes of the liver with the line drawn along the MHV, based on a technique described by Higashiyama.7 Donors with fatty liver or other suspicious parenchymal pathology on imaging underwent a liver biopsy. The degree of steatosis was expressed as a percentage of combined macrovesicular and microvesicular fatty infiltration of liver tissue processed for light microscopy. All specimens were read by a single pathologist. Intraoperative cholangiography was performed routinely for right lobe donors.
Planning the Donor Hepatectomy
The donor-to-recipient body weight (DRBW) ratio was obtained. The prospective graft-to-recipient size ratio was obtained using the CT volume of the right lobe over the standard liver volume (SLV) of the recipient computed using a formula adapted to the Asian physique.8 The tributaries of the MHV were carefully studied by determining the size and number of segmental veins that join the MHV. The decision to take the MHV with the graft or leave it with the remnant was made following a schematic diagram shown in Figure 1. If the DRBW ratio was greater than 1, then the MHV was not taken with the graft. If the DRBW was less than or equal to 1, then the ratio of the size of the prospective graft, ie, volume of the donor’s right lobe to the recipient’s calculated standard liver volume (RLRSLV) was assessed and corrected for fatty change, if any. This correction was made by deducting the percentage of fatty change seen on liver biopsy from the RLRSLV, based on the assumption that 1% of fat replaced 1% of viable liver mass.9 If the RLRSLV was less than or equal to 50%, then the MHV was taken with the graft. If this ratio was greater than 50%, the decision was then based on the hepatic venous anatomy of the potential graft. If the segment V and VIII veins were relatively small (≤5 mm) or not detected on preoperative vascular imaging, and the right hepatic vein (RHV) was large, then the MHV was left with the remnant liver. However, if the segment V and VIII branches draining onto the MHV were greater than 5 mm or the RHV was relatively small or multiple in number, then a right lobe graft with the MHV was obtained.
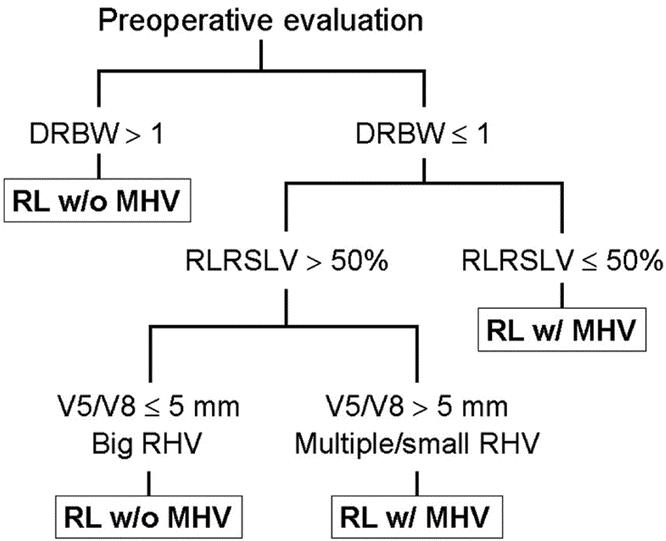
FIGURE 1. Algorithm for determining the extent of donor hepatectomy in right lobe living donor liver transplantation, with or without the middle hepatic vein (MHV). DRBW, donor-recipient body weight ratio; RLRSLV, right lobe-to-recipient standard liver volume estimate; V5, draining vein of segment V; V8, draining vein of segment VIII; RHV, right hepatic vein.
Surgical Technique in the Donor and Recipient
Donor hepatectomy aiming at minimal blood loss was carried out as described previously.10 Intraoperative ultrasonography was performed to mark the course of the MHV on the liver surface and to verify the hepatic venous configuration on the right side of the liver. The presence of right inferior hepatic veins was routinely checked. If the MHV was to be taken with the graft, the transection plane was along the left side of the MHV, often exposing part of this vessel. If the MHV was to remain with the remnant, the parenchyma was transected on the right of the MHV and the tributaries to the MHV were ligated. The distance of the transection plane from the MHV varied according to the surgeon’s gross estimate of what the final graft volume should be.
The graft was obtained with a RHV cuff from the inferior vena cava (IVC) to ensure a wide outflow orifice and perfused with cold UW solution through the right portal vein on the back table. In the recipient, reconstruction was performed with transient total occlusion of the IVC, except in the first case where the main hepatic vein stumps were clamped individually. The recipient RHV was made wider by excising part of the IVC along its distal border. The graft MHV, if present, was anastomosed to the recipient MHV or common trunk of the MHV and LHV, with or without a venoplasty for size adjustment. Right inferior hepatic veins larger than 5 mm were reconstructed on a separate venotomy on the IVC. The clamps across the IVC were released after reconstruction of the hepatic vein, if there was only 1, or after portal vein reconstruction, if there were multiple hepatic veins reconstructed.
The graft portal vein was reconstructed with any of the left, right, or main portal vein of the recipient, depending on which had better quality and size matching. All arterial anastomoses were performed under microsurgery. Biliary reconstruction was an end-to-side hepatodochojejunostomy in the first 10 cases and in the recipient with biliary atresia. In the other 14 cases, an end-to-end hepatodochocholedochostomy or hepatodochohepatodochostomy was performed with T-tube stenting in 10 cases and without T-tube stenting in 4 cases. Two separate graft bile ducts were anastomosed to a single recipient duct in 3 cases, and separately to the middle and left branches of the common hepatic duct in 1 case.
Data Analysis
Preoperative size ratio estimates and donor and recipient intraoperative and postoperative outcomes in patients receiving grafts with and without the MHV were compared using nonparametric statistical tests for comparison of independent means. Qualitative data were compared using Fisher’s exact test. Survival estimates were calculated using the Kaplan-Meier method. An SPSS commercial statistics software was used (SPSS 10.0 for Windows, Chicago, IL) and a P value less than 0.05 was considered significant. Data are given as mean ± SD.
RESULTS
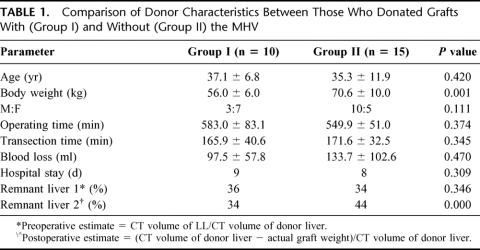
Based on our algorithm, 10 patients received grafts with the MHV (Group I) and 15 patients received grafts without the MHV (Group II). A comparison of the donor data including demographics, relevant intraoperative parameters, duration of hospital confinement, and remnant liver volume is shown in Table 1. The Group I donors were significantly smaller and there was an inverse ratio of males to females with more females in Group I. Operating time for a right lobectomy including the MHV was longer but not statistically significant from lobectomies without the MHV. Transection times were comparable. The blood loss in this series ranged from 30 to 360 mL and the mean loss for Group II was greater but not significantly different from Group I. The preoperative remnant liver expressed as a ratio of the CT volume of the left lobe volume to that of the whole liver ranged from 28% to 45% and the difference between means per group was not statistically significant. The actual remnant liver volume was, however, difficult to measure and was estimated using the following formula:
TABLE 1. Comparison of Donor Characteristics Between Those Who Donated Grafts With (Group I) and Without (Group II) the MHV
The mean values decreased slightly in Group I (34%) and increased in Group II (44%), as expected.
Postoperative donor recovery between the 2 groups was comparable, as shown by progressive improvement in liver function (Fig. 2 A to C). Total bilirubin was somewhat higher in donors who lost their MHV, although the difference was not statistically significant. There were 3 donor morbidities: 1 was a biloma on the cut surface of the remnant liver in a donor from Group II and a right pleural effusion and an exacerbation of a duodenal ulcer in 2 different donors from Group I. The fluid collections were successfully managed by percutaneous drainage and the duodenal ulcer was managed with medical treatment. All donors are alive and back to their predonation lifestyles. There have been no other donor complications at a median follow-up of 16 months.
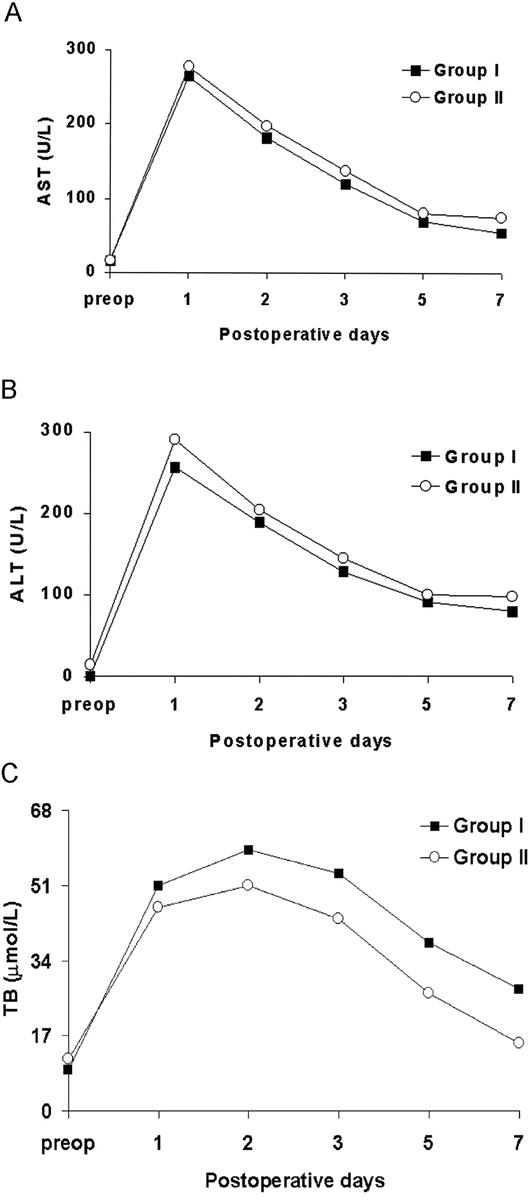
FIGURE 2. Comparison of serial postoperative (A) aspartate aminotransferase (AST), (B) alanine aminotransferase (ALT), and (C) total bilirubin (TB) between right lobe donors who donated (Group I) and retained (Group II) the MHV.
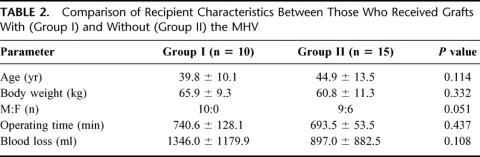
A comparison of recipient data in Table 2 showed that there was no significant difference between the 2 groups. All of the Group I recipients were males. Operating time for Group I recipients was longer because of the additional anastomosis of the MHV.
TABLE 2. Comparison of Recipient Characteristics Between Those Who Received Grafts With (Group I) and Without (Group II) the MHV
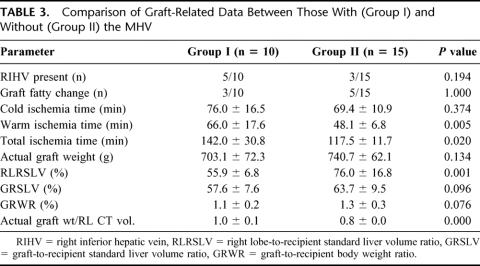
Graft-related data are shown in Table 3. The numbers of grafts with right inferior hepatic veins requiring reconstruction and with fatty change in the 2 groups were comparable. Warm ischemia time, which represented the time of hepatic and portal venous reconstruction, was significantly longer in Group I. The preoperative prospective graft-to-recipient volume estimates, expressed as RLRSLV, and the actual graft to recipient SLV ratio (GRSLV) were compared between groups. The actual ratios closely approximated preoperative estimates in Group I, but were smaller in Group II because the transection line was moved to the right of the MHV. There was, however, no statistically significant difference in the mean GRSLV between the 2 groups, which were 58% and 64%, respectively, because adequate volumes were provided for each patient. When graft proportion was expressed as graft-to-recipient body weight ratio (GRWR), there was likewise no difference between the 2 groups.
TABLE 3. Comparison of Graft-Related Data Between Those With (Group I) and Without (Group II) the MHV
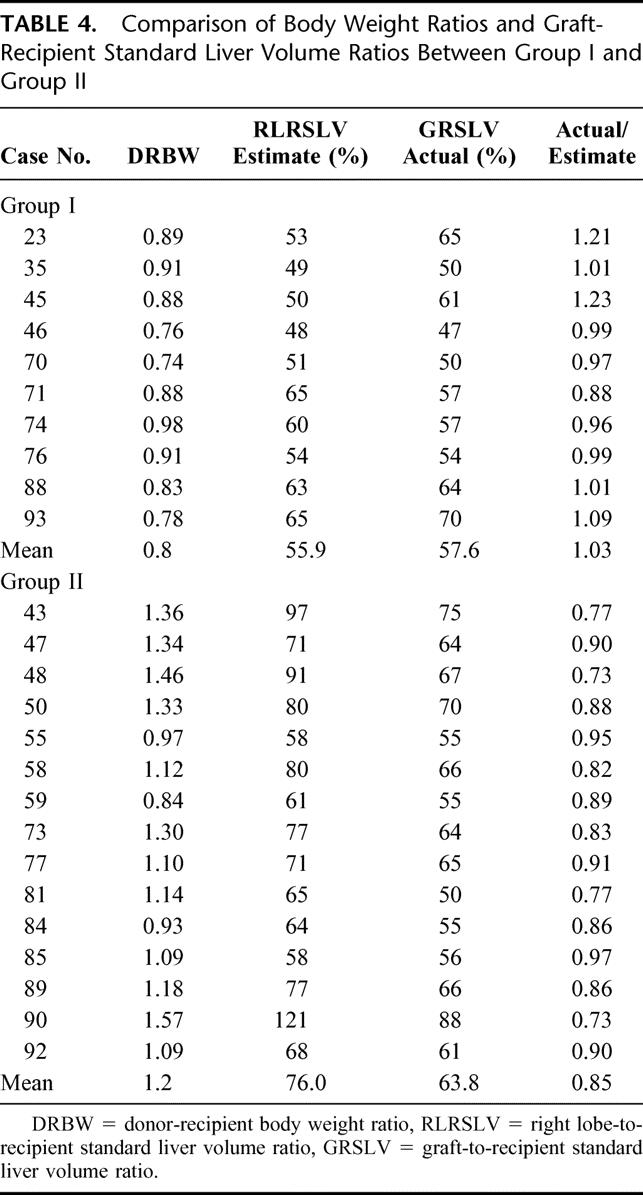
Based on our algorithm, in 3 patients with DRBW less than 1, the MHV was not taken with the graft (cases 55, 59, and 84) (Table 4). This was because their donor right lobe preoperative estimates were still greater than 50% of the recipient standard liver volume and the segmental branches into the MHV were not big. On the other hand, in 6 of the Group I recipients, the MHV was taken with the graft even though the right lobe estimates were greater than 50% of the recipient SLV because of various anatomic indications as described in the algorithm. In 3 of the recipients, the decision was made intraoperatively because the right lobe actually looked smaller than the CT volume estimate so the MHV was taken instead.
TABLE 4. Comparison of Body Weight Ratios and Graft-Recipient Standard Liver Volume Ratios Between Group I and Group II
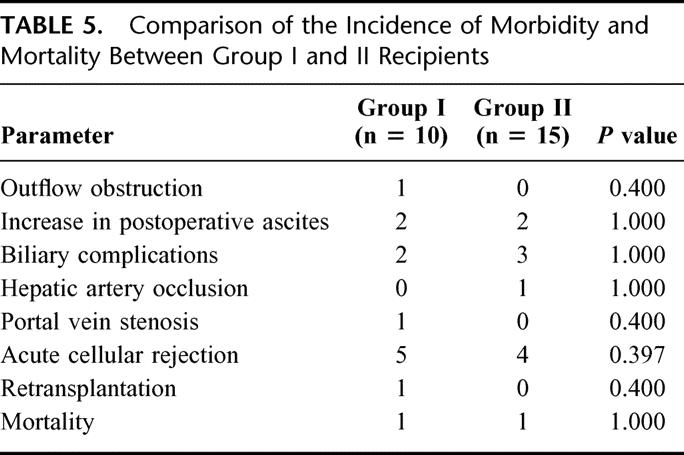
Significant morbidity in the recipients included an outflow obstruction due to a technical error (n = 1), transient increase in postoperative ascites (n = 4), biliary complications (n = 5), hepatic artery occlusion (n = 1), portal vein stenosis (n = 1), perforated peptic ulcer (n = 1), and acute cellular rejection (n = 9). A comparison of the incidence of morbidity and mortality showed that there was no significant difference between the 2 groups (Table 5). The patient who had outflow obstruction (case 23) required 3 hepatic vein anastomoses and the IVC was not cross-clamped. This might have led to malalignment of vessels during the anastomosis predisposing to hepatic vein narrowing. The problem was managed successfully by venoangioplasty and vascular stenting.11 One recipient presented with transient graft dysfunction secondary to outflow insufficiency in the early postoperative period (case 59). He was a 73-kg male who received a right lobe graft without the MHV from his 61-kg wife. The graft was 61% of his standard liver volume. She had prominent segment V and VIII veins that measured about 5 mm, which were ligated. He had increased ascites and hyperbilirubinemia in the early postoperative course and imaging studies showed congestion of the anterior segment of the liver which eventually resolved after 2 weeks accompanied by a decrease in the ascites and normalization of bilirubin. One donor in Group II had a large segment VIII vein that emptied at the base of the MHV near its point of entry into the IVC. This segmental vein was taken with the graft and reconstructed onto the MHV of the recipient.
TABLE 5. Comparison of the Incidence of Morbidity and Mortality Between Group I and II Recipients
One female patient developed hepatic artery occlusion (case 58) as a result of intimal dissection in the native artery 1 week posttransplant. The problem was solved by revision of the arterial anastomosis using the recipient gastroepiploic artery, after which she recovered fully. Portal vein narrowing was detected 5 months post-LDLT in a male recipient (case 76) who required a thrombectomy for subintimal thrombosis during the transplant operation. He presented with mild derangement of liver function and CT angiography revealed portal vein stenosis with thrombosis. The thrombus was successfully lysed with systemic recombinant tissue plasminogen activator (Actilyse, Boehringer Ingelheim, Ingelheim/Rhein, Germany) although there was residual mild stenosis at the anastomotic site. There were no side effects to the treatment and his liver function has normalized since then.
Five (20%) of 25 recipients developed biliary complications. Among them were anastomotic bile leaks that progressed to biliary strictures in 2 patients and recurrent cholangitis due to a retained short indwelling stent in 1 patient, which resolved after surgical removal of the stent. These 3 complications occurred in the early part of this series in cases with double small hepatic ducts with difficult bilioenteric reconstruction. Of the 2 patients who developed biliary strictures, 1 patient expired of sepsis after an interventional biliary drainage procedure 4 months after LDLT, and the other developed secondary biliary cirrhosis that required retransplantation. This patient (case 45) received a second graft (cadaveric) 22 months after LDLT and has since remained well with good liver function. The 2 other patients with biliary complications were a leak and a stricture associated with placement of T-tubes after duct-to-duct reconstruction. The leak resolved after percutaneous drainage of a biloma, whereas the patient with a stricture remains with a percutaneous transhepatic biliary drainage 14 months posttransplant.
There were 2 mortalities in this series (Table 5). One patient died of a biliary complication (case 48), as mentioned above, whereas the other of recurrent hepatitis C (case 35) at 31 months after LDLT. There were no differences in survival rates between the 2 groups and, excluding recurrence of original disease as a cause of death, the overall graft and patient survival were 84% and 96%, respectively.
DISCUSSION
Adult-to-adult LDLT using extended right lobe grafts was initially conceptualized including the MHV to provide adequate functional mass, especially in cases in which the donor was smaller than the recipient.12 Although this technique was proven feasible, other researchers believed that it was too extensive an operation for the donor and opted to do right donor lobectomies without the MHV.5,13,14 However, right lobe grafts without the MHV places the anterior segment at risk for congestion and the drainage problem can indeed lead to dismal outcomes.4 Hence, reconstruction of the major anterior segment veins draining into the MHV using interposition vascular grafts was deemed as the logical solution. However, reconstruction of the segment V and VIII branches with jump grafts would mean a more complex operation and a longer operating time, especially when autologous vein grafts are used. Besides the relatively long segment of an interposition graft bridging a low pressure hepatic venous system would be more prone to thrombosis and long-term patency is not assured.15 If drainage of the anterior segment is required, taking the MHV instead of reconstruction of its lesser tributaries would be a more simple and straightforward approach and the risk of thrombosis is reduced, if not eliminated. On the other hand, if the functional mass of the graft was adequate without the MHV, some degree of congestion may be tolerated in the early postoperative period until the graft would have regenerated16 and/or the anterior sector drainage rerouted. It has been demonstrated that obliterated tributaries of the MHV recanalize in a retrograde direction and eventually drain into the RHV through intrahepatic channels.17,18 One of our patients from Group II who had a congested right anterior sector had transient graft dysfunction that resolved in 2-3 weeks, at which time the graft mass would have increased and intrahepatic collaterals opened. Therefore, a higher RLRSLV ratio for grafts without the MHV is preferable to assure adequate function and recovery from the insult of congestion.
If graft volume was adequate with or without the MHV, we then based our decision on the size and configuration of the veins draining into the MHV, because drainage patterns of the right lobe of the liver can vary considerably.19 We believe that there was a need to assure their patency only if they were relatively big (caliber >5 mm), in which case, it would be a more simple procedure to take the MHV instead of reconstructing the tributaries individually. Reconstruction of the tributaries with interposition grafts would probably be necessary only if the graft would inevitably be small-for-size and the volume of the remnant liver without the MHV would be less than the recommended minimum of 30%.20
Because right lobe grafting either with or without the MHV has been performed successfully, the issue to settle, therefore, is when to use 1 type or the other, considering that donor-recipient pairs will present in a variety of size matches, donor liver qualities and venous branching patterns. A simple parameter to use is body weight ratios because the liver volume is proportional to an individual’s body weight. In our experience, donors who weighed at least 10% more than the recipient would likely have a right lobe that could provide sufficient liver mass without the MHV. Moreover, in this series all donor-recipient pairs with DRBW > 1 had preoperative graft estimates that were >50% of the RSLV (Table 4).
Another factor that is well recognized as important in predicting the adequacy of the estimated graft volume is steatosis in the donor liver. Functional mass is decreased by the amount of fatty change in the liver and adjustments in the preoperative estimates need to be made for this.9 Ideally, deducting the percentage of fatty liver from the estimated graft-to-recipient liver volume estimate should still yield an acceptable functional mass of at least 40%.21 Grafts from 2 of our donors (cases 55 and 77) had fatty content of 10% and 15% and subtracting them from the preoperative estimates yielded 48% and 56%, respectively, which were still acceptable. Because they belonged to Group II, the actual graft mass was even less than the estimate, but still acceptable. If, in such conditions, the estimate would be markedly reduced, then it would be better to take a bigger graft and include the MHV.
Congestion, likewise, affects liver function and theoretically the proportion of congested segment could also be deducted from the preoperative RLRSLV for a more accurate estimation of functional graft mass. However, it would be difficult to predict, and more so quantify, congestion preoperatively, and it can probably be as high as 20-25% of the right lobe volume if the MHV is not taken with the graft and the segmental tributaries are not reconstructed. The deleterious effect of segmental congestion on hepatocyte function in a liver graft is still poorly understood.22 Although there is considerable evidence that damage is not permanent and recovery is possible, the exact determinants of the outcome of segmental congestion in a liver graft would yet have to be identified.
In this study the proposed algorithm proved to be useful in assuring that an adequate functional mass was provided for every recipient. The recommended minimum of 40% graft-to-recipient standard liver weight ratio is based on actual graft weights,21 but what is available preoperatively are only estimates and dependent on the accuracy of CT volumetry. We have chosen a preoperative minimum of 50% to allow for error margin, considering that the radiologic measurement is not always equivalent to the actual graft weight.23 A 50% cut-off, likewise, provides a safe allowance in cases in which we opt not to take the MHV. As shown in Table 4, all actual graft measurements were less than the estimates in Group II in which the transection of the liver was on a plane to the right of the MHV. Conversely, the actual graft weights closely approximated the preoperative estimates in majority of the cases in Group I because the CT volume of the prospective graft was calculated with the line drawn on the MHV.
Recovery was good in all the donors regardless of whether the MHV was taken. There were no complications related to inadequate remnant liver mass or drainage. The serial postoperative bilirubin was higher in Group I compared with Group II donors, although the difference was not statistically significant. This could probably be attributed to a relatively smaller liver remnant or to the division of a greater number of vascular and biliary radicles from the caudate. Although females tended to be smaller and lose their MHV when donating to male recipients, this does not have to be always the case because volumes of the right and left lobes also vary in proportion.24 Donors who are smaller than the recipient may actually have bigger right lobes that can be taken without the MHV. In this series of donors, the proportion of the right lobe to the entire liver by CT volumetry ranged from 56% to 74% (median, 65.7%).
Biliary complications occurred in 5 of 25 recipients and led to mortality in 1 case and graft loss in another. These occurred during our learning period and were attributed to technical mishaps related to multiple bilioenteric reconstructions and stenting rather than inappropriate graft size or drainage problems. These 5 patients had GRSLVs that were adequate, ranging from 54% to 67%, and the incidence of biliary complications did not correlate with presence or absence of the MHV in the graft. Neither were the other morbidities or mortalities related to graft functional inadequacy. Barring death from recurrent disease, which is a cause independent of graft size, overall patient survival was 96%. Similar to the experience of other researchers, our approach to biliary reconstruction has evolved according to patient outcomes and our current preference in adult LDLT is duct-to-duct anastomosis without stenting.
In conclusion, we believe that the extent of the donor hepatectomy in right lobe LDLT should be based on certain criteria to provide for an adequate functional mass in the recipient while keeping the risk in the donor to a minimum. The algorithm described herein proved useful in the preoperative decision making of the extent of donor hepatectomy and allowed provision of adequate functional liver graft mass in every case. When the donor-recipient body weight ratio is greater than 1, the potential graft is estimated to be greater than 50% of the recipient’s calculated standard liver volume, and the segment V and VIII branches are not present or ≤5 mm in size, then the right lobe graft may be taken without the MHV. These conditions apply provided that adjustments for fat content and possible congestion in the prospective graft have been considered. A graft that is large enough to provide an adequate functional mass could stand the insult of segmental or subsegmental congestion, which is expected to be transient. If drainage of the anterior segment would have to be assured or the graft would be small-for-size, then taking the MHV is a simpler approach compared with reconstruction of the major tributaries, provided that the liver remnant would be adequate. The decision then of whether to take the MHV with the graft or retain it in the donor needs to be tailored to the particular conditions of each donor-recipient pair.
Footnotes
Reprints: Chao-Long Chen, MD, Department of Surgery, Chang Gung Memorial Hospital, Kaohsiung Medical Center, 123 Ta-Pei Road, Niao-Sung, Kaohsiung 83305, Taiwan. E-mail: clchen@adm.cgmh.org.tw.
Supported in part by program project grant DOH-87-HR-702 from the National Health Research Institutes, Taiwan.
REFERENCES
- 1.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–327. [DOI] [PubMed] [Google Scholar]
- 2.Sugawara Y, Makuuchi M, Takayama T, et al. Small-for-size grafts in living related liver transplantation. J Am Coll Surg. 2001;192:510–513. [DOI] [PubMed] [Google Scholar]
- 3.Lo CM, Fan ST, Liu CL, et al. Adult-to-adult living donor liver transplantation using extended right lobe grafts. Ann Surg. 1997;226:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee S, Park K, Hwang S, et al. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812–814. [DOI] [PubMed] [Google Scholar]
- 5.Inomata Y, Uemoto S, Asonuma K, et al. Right lobe graft in living donor liver transplantation. Transplantation. 2000;69:258–264. [DOI] [PubMed] [Google Scholar]
- 6.Human Organ Transplant Act of the Republic of China. June 19, 1987. Ratified May 21, 1993.
- 7.Higashiyama T, Yamagichi T, Mori K, et al. Graft size assessment by computed tomography in living related partial liver transplantation. Br J Surg. 1993;80:489–492. [DOI] [PubMed] [Google Scholar]
- 8.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 9.Marcos A, Fisher RA, Ham JM, et al. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000;69:1375–1379. [DOI] [PubMed] [Google Scholar]
- 10.Chen CL, Chen YS, De Villa V, et al. Minimal blood loss living donor hepatectomy. Transplantation. 2000;69:2580–2586. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YF, Chen YS, Huang TL, et al. Interventional radiologic procedures in living donor liver transplantation. Transplant Int. 2001;14:223–229. [DOI] [PubMed] [Google Scholar]
- 12.Lo CM, Fan ST, Liu CL, et al. Extending the limit on the size of adult recipient in living donor liver transplantation using extended right lobe graft. Transplantation. 1997;63:1524–1528. [DOI] [PubMed] [Google Scholar]
- 13.Marcos A, Fisher RA, Ham JM, et al. Right lobe living donor liver transplantation. Transplantation. 1999;68:798–803. [DOI] [PubMed] [Google Scholar]
- 14.Wachs ME, Bak TE, Karrer FM, et al. Adult living donor liver transplantation using a right hepatic lobe. Transplantation. 1998;66:1313–1316. [DOI] [PubMed] [Google Scholar]
- 15.Miller CM, Gondolesi GE, Florman S, et al. One hundred nine living donor liver transplantation in adults and children: a single center experience. Ann Surg. 2001;234:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishizaki T, Ikegami T, Hirosige S, et al. Small graft for living donor liver transplantation. Ann Surg. 2001;233:575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneko T, Kaneko K, Sugimoto H, et al. Intrahepatic anastomosis formation between the hepatic veins in the graft liver of the living related liver transplantation: observation by Doppler ultrasonography. Transplantation. 2000;70:982–985. [DOI] [PubMed] [Google Scholar]
- 18.Cescon M, Sugawara Y, Sano K, et al. Right liver graft without middle hepatic vein reconstruction from a living donor. Transplantation. 2002;73:1164–1166. [DOI] [PubMed] [Google Scholar]
- 19.Hata F, Murakami G, Hirata K, et al. Configuration of hepatic veins in the right surgical lobe of the human liver with special reference to their complementary territorial relationships: morphometric analysis of controlled specimens with clearly defined portal segmentation. Okajimas Folia Anat Jpn. 1999;76:1–16. [DOI] [PubMed] [Google Scholar]
- 20.Fan ST, Lo CM, Liu CL, et al. Safety of donors in live donor liver transplantation using right love grafts. Arch Surg. 2000;135:336–340. [DOI] [PubMed] [Google Scholar]
- 21.Lo CM, Fan ST, Liu CL, et al. Minimum graft size for successful living donor liver transplantation. Transplantation. 1999;68:1112–1116. [DOI] [PubMed] [Google Scholar]
- 22.Cui D, Kiuchi T, Egawa H, et al. Microcirculatory changes in right lobe grafts in living donor liver transplantation: a near-infrared spectrometry study. Transplantation. 2001;72:291–295. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto S, Uemoto S, Uryuhara K, et al. Graft size assessment and analysis of donors for living donor liver transplantation using right lobe. Transplantation. 2001;71:1407–1413. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki S, Makuuchi M, Matsunami H, et al. Preoperative measurement of segmental liver volume of donors for living related liver transplantation. Hepatology. 1993;18:1115–1120. [PubMed] [Google Scholar]