Abstract
Purpose:
The purpose of this report is to describe our experience in management of aortoesophageal fistulas (AEF) with special emphasis on the value of in situ aortic allograft replacement.
Patients:
Nine patients presenting with AEF were observed between May 1988 and April 2002. There were 4 men and 5 women with a mean age of 54.3 years (range, 32–77 years). Six patients presented secondary AEF after aortic repair. Two patients presented primary AEF after rupture of an atherosclerotic aneurysm into the esophagus. In the remaining patient, AEF was caused by swallowing a fishbone. In 6 cases involving true AEF with a direct communication between the aorta and esophagus, massive exsanguinating hematemesis occurred. It was usually preceded by minor sentinel bleeding. Two patients presented esophagoparaprosthetic fistula (EPPF). One patient presented primary AEF that was contained by a large thrombus in the communication. The clinical picture in these 3 patients involved severe sepsis without hemorrhage.
Results:
Two patients died as a result of massive hemorrhage before assessment and surgical treatment could be undertaken. One 77-year-old woman presenting EPPF refused to undergo surgery and died because of infection. The remaining 6 patients underwent surgical treatment with various outcomes. One man died during thoracotomy caused by exsanguinating hemorrhage. One woman presenting EPPF was treated by exclusion followed by ascending aorta to abdominal aorta bypass grafting, removal of the prosthesis, esophageal exclusion, and directed esophageal fistula. She died of infection. The other 4 patients were treated by in situ aortic allograft replacement. The damaged esophagus was repaired by using the Thal technique in 1 patient. In the remaining 3 cases subtotal esophagectomy was performed in association with cervical esophagostomy, ligation of the abdominal esophagus, gastrostomy, and jejunostomy. One patient died of sepsis during the first 24 hours after the operation. The other 3 patients underwent secondary esophagoplasty and survived with no further sign of infection. Mean duration of follow-up in the survivor group was 53 months (range, 15-95 months). Overall 6 patients, including 3 that did not undergo surgical treatment, died and 3 patients survived.
Conclusion:
Our experience confirms that AEF is a rare but catastrophic disorder. In situ allograft replacement usually in association with subtotal esophagectomy appears to be an excellent salvage modality whenever emergency surgery is feasible.
The clinical presentation and outcome of 9 aortoesophageal fistulas are presented. There were 3 survivors out of 6 operated patients, all of whom were treated with in situ aortic replacement by an allograft. This seems to be the method of choice, in combination with, in most cases, subtotal oesophagectomy.
Aortoesophageal fistula (AEF) is a rare but usually fatal disorder. The purpose of this report is to describe the experience of our vascular surgery department in a series of 9 patients presenting with AEF. Special emphasis is placed on the value of in situ aortic allograft replacement, usually in association with subtotal esophagectomy.
PATIENTS AND METHODS
Between May 1988 and April 2002, 9 patients presenting with AEF were observed in the Vascular Surgery Department of the Pitié-Salpêtrière University Hospital in Paris, France. Clinical findings, management modalities, and outcome for these 9 patients are listed in Table 1. There were 5 men and 4 women with a mean age of 57.8 years (range, 32–77 years). In 6 cases, AEF occurred secondary to operative treatment of aneurysms of the descending thoracic aorta, including 3 after conventional surgical repair (interval between repair and AEF, 3, 3, and 14 months) and 3 after endovascular repair (interval between repair and AEF, 6, 8, and 38 months). Two cases were the result of spontaneous rupture of an aneurysm of the descending thoracic aorta (n = 1) or thoracoabdominal aorta (n = 1) into the esophagus. The remaining case occurred after swallowing of a foreign body (fishbone). Eight cases of AEF were associated with aortic disease, ie, atherosclerotic aneurysm in 5 cases, aneurysm related to Behçet’s disease in 2, and dissecting aneurysm related to Marfan’s syndrome in 1. Only 1 case of AEF, involving ingestion of a fishbone, occurred in a patient with an otherwise-healthy aorta.
TABLE 1. Clinical Findings, Management Modalities, and Outcome in 9 Patients Observed in the Vascular Surgery Department of the Pitié-Salpêtrière University Hospital for Aortoesophageal Fistula
Six patients presented true AEF defined as the presence of a direct communication between the esophagus and aorta. Four of these 6 patients presented sentinel bleeding characterized by 1 or several minor episodes involving hematemesis of red blood within an interval ranging from a few hours to several days before occurrence of massive hematemesis. Two patients treated by conventional surgery presented esophagoparaprosthetic fistula (EPPF) caused by erosion by the prosthesis, causing a communication between the esophagus and the periprosthetic space without involvement of the adjacent anastomosis. The remaining patient presented primary AEF but bleeding was contained by the presence of a large thrombus in the communication. The main clinical finding in these 3 cases was severe sepsis.
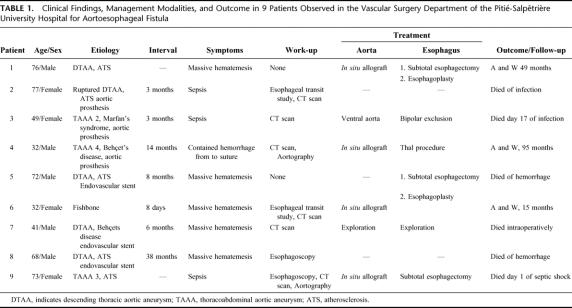
Preoperative assessment using various modalities was possible in 7 patients, including 4 during the interval between sentinel and massive hematemesis. Two patients underwent esophagoscopy before admission to our department. In both cases, a protruding throbbing mass was visualized under the wall of the esophagus that exhibited ulceration but not fistulization. Esophageal contrast studies were performed in 2 patients. In 1 case involving the patient presenting foreign body–related AEF, contrast study provided nonspecific evidence of esophageal perforation by visualizing extravasation of contrast material (Fig. 1). In the other case involving a patient with EPPF, contrast study showed extravasation of contrast material around the aortic prosthesis (Fig. 2). Aortography was performed in 2 patients. In both cases aortograms failed to visualize the fistula but were helpful for surgical planning. Computed tomography was performed in 6 patients. Images showed the presence of gas effusion either around the prosthesis (Fig. 3) or within aneurysmal thrombus (Fig. 4) in 3 cases, false aneurysm in 2 cases, including 1 containing the offending fishbone (Fig. 5), and adherence between the esophagus and the prosthesis with the presence of mural thrombus inside the prosthesis in 1 patient with EPPF (Fig. 6).
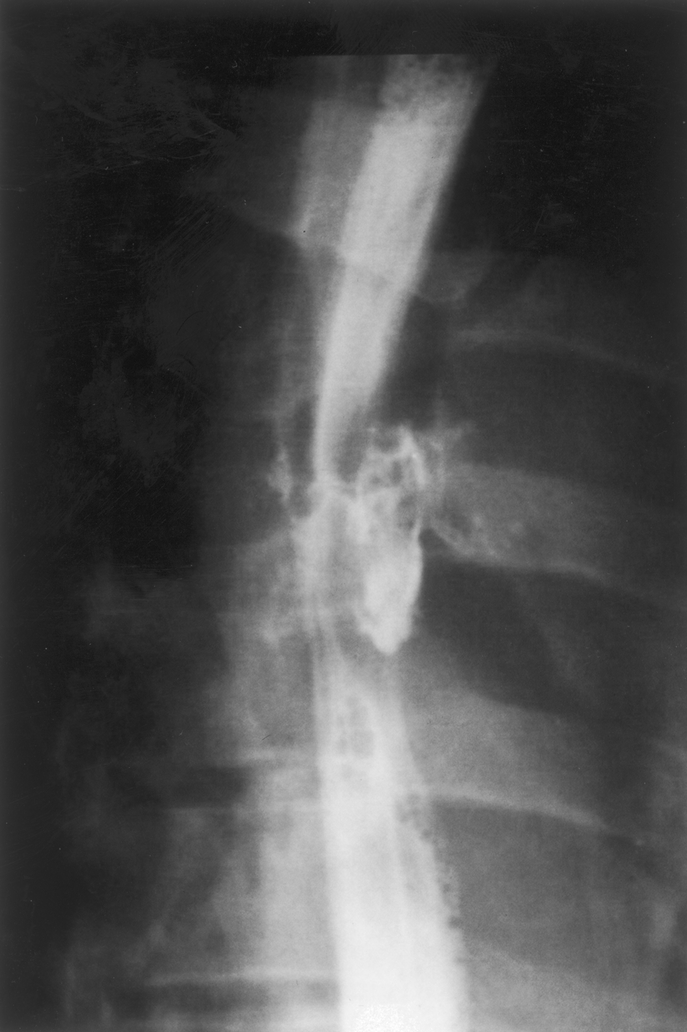
FIGURE 1. Esophageal contrast study showing esophageal narrowing associated with extravasation of contrast material in a patient presenting an aortoesophageal fistula induced by an ingested fishbone.
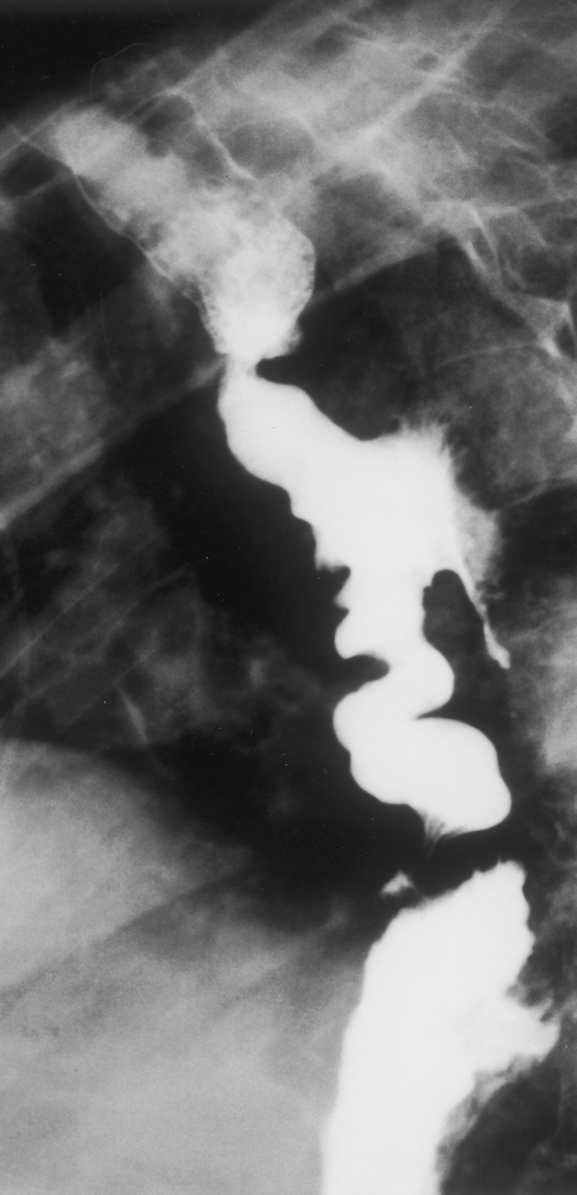
FIGURE 2. Esophageal contrast study showing extravasation of contrast material around the nearby prosthesis in a patient presenting an esophagoparaprosthetic fistula.
FIGURE 3. Computed tomography showing a large gas effusion around the prosthesis in a patient presenting esophagoparaprosthetic fistula.
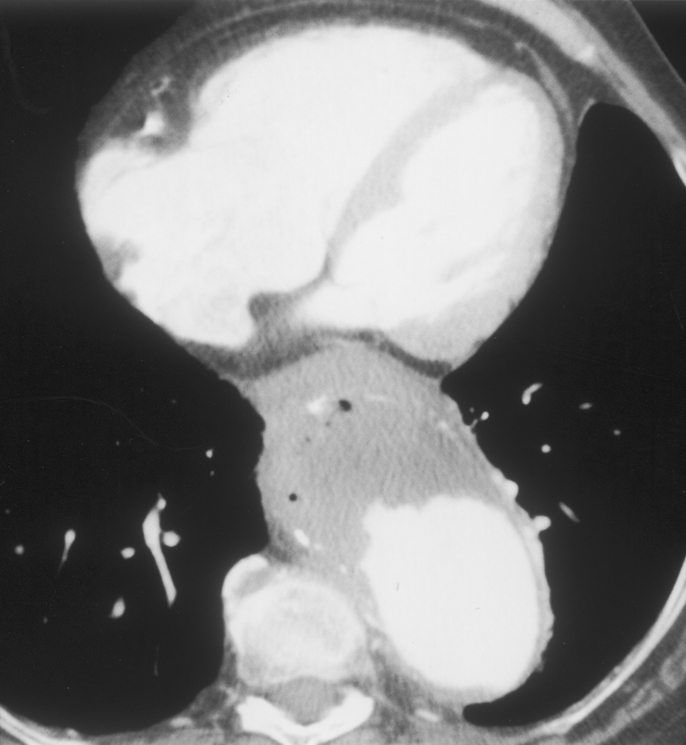
FIGURE 4. Computed tomography showing the presence of air bubbles in mural thrombus in an aneurysm of the thoracoabdominal aorta ruptured into the esophagus.
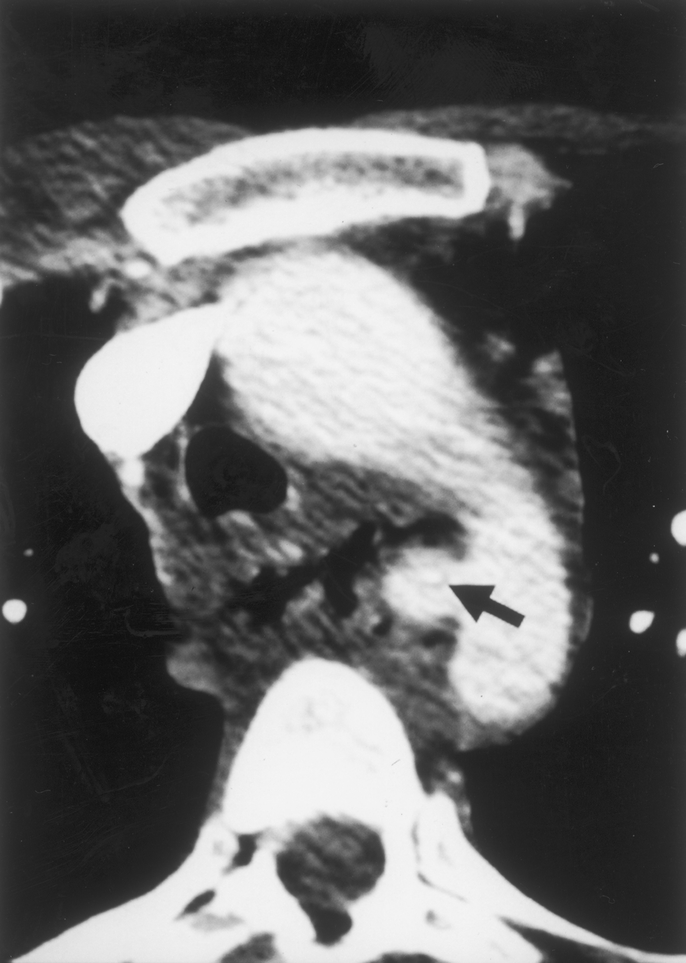
FIGURE 5. Computed tomography showing a small false aneurysm located in the distal segment of the aortic arch (same patient as in Fig. 1). The fishbone that caused the aortoesophageal fistula can be seen inside the aneurysm (arrow).
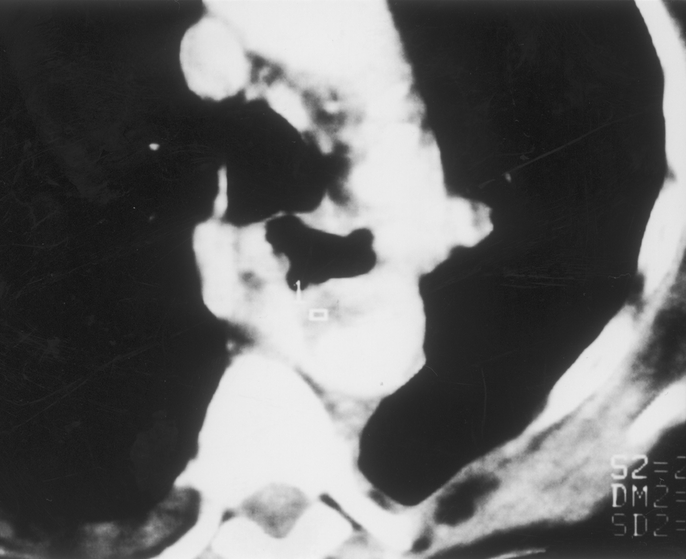
FIGURE 6. Computed tomography showing adherence of the esophagus to the prosthesis and presence of mural thrombus within the prosthesis (same patient as in Fig. 3).
RESULTS
Three patients did not undergo surgical treatment and died. One of these patients was a 77-year-old woman presenting EPPF who refused to undergo surgical treatment. She succumbed to infection 8 days after diagnosis. The other 2 patients presented true AEF after endovascular stent placement and died of massive hemorrhage before reaching the hospital. In 1 case, an autopsy confirmed perforation of the esophagus by the branches of the aortic stent. In the other case, autopsy was refused but clinical findings were fully consistent with diagnosis of AEF.
Surgical repair was undertaken in 6 patients. In the patient presenting AEF caused by foreign body ingestion, midline sternotomy was used because attempts to control massive hemorrhage by using a Blakemore tube had been poorly successful and placement in lateral decubitus would probably have been fatal. After quickly starting femorofemoral cardiopulmonary bypass, repair was performed by sternolaparatomy using deep hypothermic circulatory arrest (DHCA). An in situ allograft was used for aortic replacement in association with subtotal esophagectomy, cervical esophagostomy, ligation of the abdominal esophagus, gastrostomy, and jejunostomy. Esophagocoloplasty was carried out in a second stage and the patient was doing well with no further sign of infection at 15 months.
One patient with EPPF underwent extra-anatomic bypass from ascending aorta to abdominal aorta (“ventral aorta”) via sternolaparotomy followed immediately during the same procedure by reopening of the left thoracotomy to allow bipolar exclusion of the esophagus with cervical esophagostomy, ligation of the abdominal esophagus, gastrotomy, and jejunostomy. The esophagus was left in place and 2 large thoracic drains were placed in the vicinity of the fistula. This patient died of unrelenting infection 17 days after the procedure.
In the remaining 4 cases repair was performed via left posterolateral thoracotomy. One patient presenting postoperative AEF after endograft placement died of exsanguination during aortic control. In 1 patient presenting primary AEF after rupture of an aneurysm of the descending thoracic aorta, simple crossclamping was used for 37 minutes. The procedure consisted of placement of an allograft followed by subtotal esophagectomy. Esophagocoloplasty was performed in a second stage and the patient survived for 49 months before death by myocardial infarction. Another patient presenting secondary AEF between a prosthesis used to treat a type IV thoracoabdominal aneurysm and the abdominal esophagus initially underwent suture of the fistula from inside the stomach at another institution. He was then transferred in stable condition to our department, where he underwent in situ allograft replacement and esophagoplasty (Thal procedure) using a left heart bypass between the inferior pulmonary vein and femoral artery. This patient is alive with a follow-up of 95 months. The last patient presenting a primary AEF secondary to rupture of a thoracoabdominal aortic aneurysm underwent aortic allograft replacement using DHCA followed by subtotal esophagectomy. The patient died from septic shock 24 hours after the procedure.
DISCUSSION
Albeit rare, development of a fistula between the aorta and esophagus has an extremely poor prognosis. Counting the 3 patients described in the present series, only 47 successfully managed cases have been reported in the literature1–43 (Table 1). In 1991 Hollander and Quick44 published a comprehensive review, including 500 cases of AEF gathered from the literature. The main etiological factor involved aortic disease with 54.2% of cases being secondary to rupture of an aneurysm of the descending thoracic aorta into the esophagus. The next most frequent causes were foreign body (19.2%) and advanced esophageal cancer (17.0%). Secondary AEF following operative treatment were rare (4.8%) with 50% occurring after aortic surgery and 50% after esophageal surgery.
Occurrence of AEF as a complication of surgical repair of the descending thoracic and thoracoabdominal aorta is still uncommon. Frequencies ranging from 0.5% to 1.7% have been reported.45–47 However, with more widespread use of surgical repair of thoracic aneurysms, the number of postoperative AEF cases has risen and they account for most AEF observed in a vascular surgery department. Another factor contributing to the higher frequency of AEF is expanding use of endovascular stent grafting in addition to conventional surgery with placement of a Dacron prosthesis.
Fistulization after conventional surgery without tissue interposition results from poor surgical technique if the suture needle is passed through the full thickness of the esophageal wall when the upper aortic anastomosis is performed by using the inclusion technique.49 The risk of postoperative AEF from this cause can be minimized by complete transection of the aorta and by performing suture under direct visual control.
The mechanism of AEF after endovascular repair involves progressive erosion through the aortic and esophageal walls by the rigid extremities located at either end of the stent. Perforation usually occurs in a curving segment of the aorta, eg, in the isthmic region or the middle region where the vessel may deviate before passing behind the esophagus.
The number of primary AEF resulting from aneurysm rupture seems to be currently decreasing. The most likely explanation for this decline is that descending or thoracoabdominal aortic aneurysms are being detected and treated at early stages. Primary AEF caused by ingested foreign bodies, such as bones (fish, chicken, and rabbit), nails, and needles, is uncommon. However, various frequencies have been reported. Nandi and Ong50 observed only 2 AEF in 2.394 cases of swallowed foreign body whereas El Barbery et al51 noted 5 cases in a 200-case pediatric series.
Diagnosis of AEF is rarely made before massive hematemesis. However, most cases are associated with characteristic Chiari’s triad features of aortoesophageal syndrome, including chest pain and sentinel hematemesis of red blood followed at a variable interval of time by rapidly fatal massive exsanguinating hematemesis. In the present series these symptoms were observed in 4 of the 6 patients with true AEF. Characteristic triad features were present in 45% of patients included in the review series of Hollander and Quick42 and 80% of patients described by Carter et al.52 In a few cases AEF can be suspected on the basis of isolated sepsis or septic embolism in a lower extremity.53 Other suggestive findings include dysphagia and/or chest pain and history of surgical treatment involving the thoracic aorta.
Except in patients presenting massive exsanguinating hemorrhage who require immediate emergency surgery, various investigative modalities can be used to confirm diagnosis and plan treatment. Esophageal contrast studies can be helpful either by showing of contrast material around the aortic prosthesis in patients with EPPF53 or by demonstrating esophageal perforation thus drawing attention to the esophagus. In the rare cases of EPPF, esophagoscopy may allow direct visualization of the prosthesis through esophageal defects of variable size depending on the amount of tissue loss.40 Esophagoscopy can also show the presence of a variable amount of submucosal hematoma indicating extravasation of blood into the esophageal wall.54,55 However even though some investigators advocate esophagoscopy as the diagnostic modality of choice for evaluation of AEF, others have advised against its use22 because several cases of fatal hemorrhage precipitated by flexible endoscopy have been reported.9,57,58 Although arteriograms rarely reveal the fistula site,59–63 they can provide useful data for surgical planning. Computed tomography scanning is the most valuable diagnostic method. Findings include visualization of a false aneurysm between the esophagus and aorta in patients presenting AEF induced by ingested foreign bodies and of adherences between the esophagus and prosthesis in patients with EPPF. In a few cases computed tomography scanning allows visualization of gas effusion either around the prosthesis62,66,67 or inside an aneurysm.68
Massive hemorrhage frequently mandates emergency surgery before confirmation of diagnosis. Laparotomy is often the initial procedure in patients with no surgical history suggesting involvement of the thoracic aorta.6,22,27,69 Gastrotomy is performed, demonstrating that hemorrhage stems from the esophagus. Insertion and inflation of a Blakemore tube can slow hemorrhage long enough to perform thoracotomy and aortic clamping proximal to the lesion.22,25,27,41,59,61,69 If involvement of the thoracic aorta is suspected preoperatively (as is usually the case in patients with postoperative AEF), a Blakemore tube should be introduced through the upper digestive tract to slow hemorrhage and allow exposure by the thoracic route.
Use of midline sternotomy is limited to 3 rare cases. The first is to expose AEF located on the aortic arch.12,70 The second is to perform extra-anatomic bypass in patients with no hemorrhage and presenting lesions located on the descending thoracic aorta. The third is to avoid positioning patients for left thoracotomy if massive hemorrhage from the aortic isthmus is poorly controlled by placement of a Blakemore tube. When used, midline sternotomy requires cardiopulmonary bypass to allow aortic repair (usually with DHCA) and to enable displacement of the heart for resection of the esophagus by the transpericardial route.
The most commonly used route for AEF repair is left thoracotomy. However control of the aorta proximal to the fistula may be difficult in patients with a history of thoracotomy. If the proximal anastomosis of the prosthesis is located on the aortic isthmus, cardiopulmonary bypass may be useful to allow DHCA. In most other cases, especially for AEF located more distally, various methods may be used including simple clamping2,18,27,71 or, optimally, circulatory assistance (either by a left heart bypass between the left atrium or inferior pulmonary vein and the femoral artery or by partial femorofemoral cardiopulmonary bypass).11,13,17,35,40,42 The main drawbacks of circulatory assistance are need for complete heparinization, time required for installation and, above all, unavailability at many centers admitting AEF patients. In emergency cases, cross-clamping should be unhesitatingly used if cardiopulmonary bypass is not unavailable.52
Aortic repair must be performed first. Extra-anatomic bypass is only possible for nonhemorrhaging lesions limited to the descending thoracic aorta. The procedure is performed via sternolaparotomy and consists of prosthetic bypass between the ascending aorta and either the supraceliac or abdominal aorta (“ventral aorta”).1,43,63 In addition to being frequently unfeasible because of hemorrhage, extra-anatomic bypass has 2 major drawbacks. First it requires aortic and esophageal repair either during the same procedure or shortly thereafter. Second it carries the risk of prosthetic and aortic stump infection, which can occur even as a long-term complication.63
In most cases direct exposure of the aorta is necessary. Aortic suture or patch angioplasty can only be used for small lesions such as those associated with AEF induced by swallowed foreign bodies.4,9,18,41,65 For larger lesions aortic resection must be performed into healthy tissue. Aortic replacement is usually required especially in cases involving postoperative AEF. Prosthetic replacement has long been used for aortic replacement with numerous successful case reports.2,5-7,11,12,14,17,22,23,26,28,31,33,35,39-41 However, because follow-up was short in most case reports, the question of how many patients developed late prosthetic infection with or without recurrence of AEF remains open. In this regard it should be pointed out that several reports have described failures salvaged by extra-anatomic bypass.13,37 Fatal cases involving late infection have probably not been reported.
In recent years, there has been a revival of interest in in situ aortic allografts for management of both primary and postoperative aortic infection.72 Vogt et al36 reported successful use of an in situ allograft for treatment of 7 patients with thoracic aortic infection including 1 patient presenting postoperative AEF. Our group recently reported successful use of in situ arterial allografts for treatment of infection following repair of the descending thoracic and thoracoabdominal aorta in 8 of 11 cases, including 1 of the patients described in this study.73 Despite two undeniable drawbacks, ie, susceptibility to infection especially by Pseudomonas aeruginosa and long-term risk for structural deterioration, aortic allografts have provided better middle term results than in situ prostheses. As a result, we consider aortic allografts as the material of choice for aortic replacement under septic conditions.
Endovascular graft placement is an attractive alternative to stop aortic hemorrhage.32,74 However it should only be considered as a stopgap measure to achieve short-term hemostasis as a bridge to definitive treatment using conventional surgery within a few hours or days.74
Treatment of the esophageal defect is mandatory. In the rare cases reported in the literature, neglect of the esophageal defect has invariably led to reoperation or death. Conservative treatment is seldom possible. Direct suture is feasible only for small lesions detected early without significant mediastinitis. The Thal procedure76 used in 1 of our patients is indicated only if the esophageal lesion is located at the lower end of the esophagus. Bipolar exclusion of the esophagus does not stop spreading of the infectious process from the defect.71 In our series the only patient in whom this treatment was used died of unremitting infection. Despite its highly invasive and debilitating nature, subtotal esophagectomy is the most effective technique. It is associated with cervical esophagostomy, ligation of the distal end of the esophagus, gastrostomy and jejunostomy. A second-stage procedure is necessary for esophagocolo or gastroplasty to re-establish digestive tract continuity.
Our experience demonstrates the effectiveness of in situ allograft replacement and subtotal esophagectomy for treatment of most cases of AEF. Direct repair by aortic and/or esophageal suture is feasible only under special circumstances. Early diagnosis based on recognition of clinical symptoms (Chiari’s triad) and history of descending thoracic aortic repair by conventional surgery or endovascular stent grafting is essential for successful management.
Footnotes
Reprints: Edouard Kieffer, MD, Service de Chirurgie Vasculaire, Groupe Hospitalier Pitié-Salpêtrière, 47-83 boulevard de l’Hôopital, 75013 Paris, France. E-mail: edouard.kieffer@psl.ap-hop-paris.fr.
REFERENCES
- 1.BaulieuxJ, Durieux G. Anévrysme mycotique de l’aorte thoracique rompu dans l’œsophage: àa propos d’un cas opéré avec succèes. Chirurgie. 1982;108:72–77. [PubMed] [Google Scholar]
- 2.Bogey WM Jr, Thomas JH, Hermreck AS. Aortoesophageal fistula: report of a successfully managed case and review of the literature. J Vasc Surg. 1992;16:90–95. [PubMed] [Google Scholar]
- 3.Bruce CM, Paul VM, Ben EK. Successful salvage of an 8-month-old child with an aortoesophageal fistula. J Pediatr Surg. 1991;26:1394–1395. [DOI] [PubMed] [Google Scholar]
- 4.Bullaboy CA, Derkac WM, Johnson DH, et al. False aneurysm of the aorta secondary to an esophageal foreign body. Ann Thorac Surg. 1985;39:275–276. [DOI] [PubMed] [Google Scholar]
- 5.Cairols MA, Izquierdo LM, Barjau E, et al. Primary aorto-oesophageal fistula due to oesophageal carcinoma: report of a successfully managed case. Int Angiol. 2000;19:290–293. [PubMed] [Google Scholar]
- 6.Chughtai TS, Sheiner NM. Successful repair of aortoesophageal fistula secondary to traumatic pseudoaneurysm. Ann Thorac Surg. 1998;66:536–539. [DOI] [PubMed] [Google Scholar]
- 7.Coselli JS, Crawford ES. Primary aortoesophageal fistula from aortic aneurysm: successful surgical treatment by use of omental pedicle graft. J Vasc Surg. 1990;12:269–277. [PubMed] [Google Scholar]
- 8.Cronen P, Snow N, Nightingale D. Aortoesophageal fistula secondary to reflux esophagitis. Ann Thorac Surg. 1982;33:78–80. [DOI] [PubMed] [Google Scholar]
- 9.Ctercteko G, Mok CK. Aorta-esophageal fistula induced by a foreign body: the first recorded survival. J Thorac Cardiovasc Surg. 1980;80:233–235. [PubMed] [Google Scholar]
- 10.Debras B, Enon B, Kanane O, et al. Successful management of an aortoesophageal fistula using a cryopreserved arterial allograft. Ann Vasc Surg. 1996;10:292–296. [DOI] [PubMed] [Google Scholar]
- 11.Fuentes P, Ottomani R, Giudicelli R, et al. Hémorragie digestive de cause rare: rupture intra-oesophagienne d’un anévrysme aortique. Ann Gastro-entérol Hépatol. 1979;15:101–103. [Google Scholar]
- 12.Goto H, Utoh J, Hongoh H, et al. Successful treatment of aortoesophageal fistula resulting from aneurysm of the aortic arch. J Cardiovasc Surg. 1998;39:425–427. [PubMed] [Google Scholar]
- 13.Hageman JH, Nein AG, Davis JT. Primary aortoesophageal fistula caused by an atherosclerotic thoracoabdominal aortic aneurysm: a case report and review of the literature. Cardiovasc Surg. 1995;3:495–499. [DOI] [PubMed] [Google Scholar]
- 14.Hariya A, Makuughi H, Naruse Y, et al. Successful management of aorto-esophageal fistula due to rupture of a thoracic aortic aneurysm in an elderly patient. J Jpn Assn Thorac Surg. 1998;46:777–780. [DOI] [PubMed] [Google Scholar]
- 15.Hill JG, Munden MM. Aorto-oesophageal fistula associated with double aortic arch. Clin Radiol. 1999;54:847–850. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy FR, Cornwell EE, Camel J, et al. Aortoesophageal fistulae due to gunshot wounds: report of two cases with one survivor. J Trauma. 1995;38:971–974. [DOI] [PubMed] [Google Scholar]
- 17.Luketich JD, Sommers KE, Griffith BP, et al. Successful management of secondary aortoesophageal fistula. Ann Thorac Surg. 1996;62:1852–1854. [DOI] [PubMed] [Google Scholar]
- 18.McCornas BC, Miles PV, Katz BE. Successful salvage of an 8-month-old child with an aortoesophageal fistula. J Pediatr Surg. 1991;26:1394–1395. [DOI] [PubMed] [Google Scholar]
- 19.Magnusson I, Notander A, Rieger A, et al. Massive hematemesis due to an aorto-oesophageal fistula. Acta Chir Scand. 1987;153:317–319. [PubMed] [Google Scholar]
- 20.Maillard JN, Blanc L, Brucher Ch. Fistule aorto-oeso-gastrique aprèes résection d’un cancer de l’œsophage: cure opératoire-guérison. Ann Chir Thor Cardiovasc. 1967;6:103–109. [PubMed] [Google Scholar]
- 21.Marmuse JP, Hamdan M, Benhamou G. La fistule aorto-oesophagienne: cause rare d’hémorragie digestive. J Chir. 1992;129:433–435. [PubMed] [Google Scholar]
- 22.Marmuse JP, Servin F, Rcheid HA, et al. Les hémorragies digestives massives par fistule aorto-oesophagienne. J Chir. 1986;123:83–90. [PubMed] [Google Scholar]
- 23.Mehta VK, Lafaro RJ, De Vincenzo S. Successful management of an aneurysmal aortoesophageal fistula. J Cardiovasc Surg. 2000;41:721–723. [PubMed] [Google Scholar]
- 24.Montgomery ACV, Chilvers AS. Oesophago-aortic fistula. Postgraduate Med J. 1981;57:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel MA, Schmoker JD, Moses PL, et al. Mycotic arch aneurysm and aortoesophageal fistula in a patient with melioidosis. Ann Thorac Surg. 2001;71:1363–1365. [DOI] [PubMed] [Google Scholar]
- 26.Pipinos II, Reddy DJ. Secondary aortoesophageal fistula. J Vasc Surg. 1997;26:144–149. [DOI] [PubMed] [Google Scholar]
- 27.Quandalle P, Pruvot FR, Latreille JP. Fistule aorto-oesophagienne secondaire àa une perforation de l’œsophage par corps étranger. Ann Chir Thorac Cardiovasc. 1984;38:159–161. [PubMed] [Google Scholar]
- 28.Reardon MJ, Brewer RJ, LeMaire SA, et al. Surgical management of primary aortoesophageal fistula secondary to thoracic aneurysm. Ann Thorac Surg. 2000;69:967–970. [DOI] [PubMed] [Google Scholar]
- 29.Schiessel R, Rath T, Kretschmer G. Giant ulcer of the oesophagus with erosion of the aorta: successful treatment with aortic suture secured by an omental graft and oesophagectomy. Br J Surg. 1985;72:650. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher KJ, Weaver DL, Knight MR, et al. Aortic pseudoaneurysm due to ingested foreign body. South Med J. 1986;79:246–248. [DOI] [PubMed] [Google Scholar]
- 31.Simao da Silva E, Lambertini Tozzi F, Pinhatta Otochi J, et al. Aortoesophageal fistula caused by aneurysm of the thoracic aorta: successful surgical treatment, case report, and literature review. J Vasc Surg 1999;30:1150–1157. [DOI] [PubMed] [Google Scholar]
- 32.Sato O, Miyata T, Matsubara T, et al. Successful surgical treatment of aortogastric fistula after an esophagectomy and subsequent endovascular graft placement. Jpn J Surg. 1999;29:431–434. [DOI] [PubMed] [Google Scholar]
- 33.Snyder DM, Crawford ES. Successful treatment of primary aorta-esophageal fistula resulting from aortic aneurysm. J Thorac Cardiovasc Surg. 1983;85:457–463. [PubMed] [Google Scholar]
- 34.Sugawara Y, Tada Y, Sato O, et al. Aortoesophageal fistula: report of an unusual case. Jpn J Surg. 1998;28:843–845. [DOI] [PubMed] [Google Scholar]
- 35.Tkebuchava T, von Segesser LK, Turina MI. Successful repair of primary concomitant aortobronchial and aortoesophageal fistulas. Ann Thorac Surg. 1997;63:1779–1781. [DOI] [PubMed] [Google Scholar]
- 36.Vogt PR, Pfammatter T, Schlumpf R, et al. In situ repair of aortobronchial, aortoesophageal, and aortoenteric fistulae with cryopreserved aortic homografts. J Vasc Surg. 1997;26:11–17. [DOI] [PubMed] [Google Scholar]
- 37.Von der Emde D. In discussion of Von Segesser LK, Tkebuchava T, Niederhauser U et al. Aortobronchial and aortoesophageal fistulae as risk factors in surgery of descending thoracic aortic aneurysms. Eur J Cardiothorac Surg. 1997;12:195–201. [DOI] [PubMed]
- 38.Von Oppell UO, De Groot M, Thierfelder C, et al. Successful management of aortoesophageal fistula due to thoracic aortic aneurysm. Ann Thorac Surg. 1991;52:1168–1170. [DOI] [PubMed] [Google Scholar]
- 39.Wang N, Sparks SR, Bailey LL. Staged repair using omentum for posttraumatic aortoesophageal fistula. Ann Thorac Surg. 1994;58:557–559. [DOI] [PubMed] [Google Scholar]
- 40.Wickstrom PH, Streitz JM Jr, Erickson RV, et al. Repair of aortoesophageal fistula after aortic grafting. Ann Thorac Surg. 1997;64:253–255. [DOI] [PubMed] [Google Scholar]
- 41.Wu MH, Lai WW. Aortoesophageal fistula induced by foreign bodies. Ann Thorac Surg. 1992;54:155–156. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda F, Shimono T, Tonouchi H, et al. Successful repair of an aortoesophageal fistula with aneurysm from esophageal diverticulum. Ann Thorac Surg. 2002;73:637–639. [DOI] [PubMed] [Google Scholar]
- 43.Yonago RH, Iben AB, Mark JBD. Aortic bypass in the management of aortoesophageal fistula. Ann Thorac Surg. 1969;7:253–257. [DOI] [PubMed] [Google Scholar]
- 44.Hollander JE, Quick G. Aortoesophageal fistula: a comprehensive review of the literature. Am J Med. 1991;91:279–287. [DOI] [PubMed] [Google Scholar]
- 45.Lawrie GM, Earle N, DeBakey ME. Evolution of surgical techniques for aneurysms of the descending thoracic aorta: twenty-nine years experience with 659 patients. J Card Surg. 1994;9:648–661. [DOI] [PubMed] [Google Scholar]
- 46.Svensson LG, Crawford ES, Hess KR et coll. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–370. [PubMed] [Google Scholar]
- 47.Svensson LG, Crawford ES, Hess KR, et al. Variables predictive of outcome in 832 patients undergoing repairs of the descending thoracic aorta. Chest. 1993;104:1248–1253. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell RS, Miller DC, Dake MD, et al. Thoracic aortic aneurysm repair with an endovascular stent graft: the “first generation”. Ann Thorac Surg. 1999;67:1971–1974. [DOI] [PubMed] [Google Scholar]
- 49.Svensson LG. Thoracoabdominal graft infections. In: Calligaro KD, Veith FJ, eds. Management of Infected Arterial Grafts. St Louis: Quality Medical Publishing; 1994:65–81.
- 50.NandI P, Ong GB. Foreign body in the oesophagus: review of 2394 cases. Br J Surg. 1978;65:5–9. [DOI] [PubMed] [Google Scholar]
- 51.El Barbary AS, Foad H, Fathi A. Oesophageal fistulae caused by swallowed foreign bodies. J Laryngol Otol. 1969;83:251–259. [DOI] [PubMed] [Google Scholar]
- 52.Carter R, Mulder GA, Snyder EN Jr, et al. Aortoesophageal fistula. Am J Surg. 1978;136:26–30. [DOI] [PubMed] [Google Scholar]
- 53.Seymour EQ. Aortoesophageal fistula as a complication of aortic prosthetic graft. AJR. 1978;131:160–161. [DOI] [PubMed] [Google Scholar]
- 54.Maher MM, Murphy J, Dervan P, et al. Aorto-oesophageal fistula presenting as a submucosal oesophageal haematoma. Br J Radiol. 1998;71:972–974. [DOI] [PubMed] [Google Scholar]
- 55.Myers HS, Silber W. Oesophageal bleeding from aorto-oesophageal fistula due to aortic aneurysm: case reports and a review of the literature. S Afr Med J. 1983;63:124–127. [PubMed] [Google Scholar]
- 56.Sosnowik D, Greenberg R, Bank S, et al. Aortoesophageal fistula: early and late endoscopic features. Am J Gastroenterol. 1988;83:1401–1404. [PubMed] [Google Scholar]
- 57.Baron RL, Koehler RE, Gutierrez FR, et al. Clinical and radiographic manifestations of aortoesophageal fistulas. Radiology. 1981;141:599–605. [DOI] [PubMed] [Google Scholar]
- 58.Benson MJ, Rouse D, van Someren N, et al. Fatal hemorrhage from an aorto-esophageal fistula precipitated by flexible endoscopy. Gastrointest Endosc. 1991;37:193–196. [DOI] [PubMed] [Google Scholar]
- 59.Gomez-Alonso A, Lozano F, Cuadrado F, et al. Traumatic aorta-oesophageal fistula. J Thorac Cardiovasc Surg. 1984;87:148–149. [PubMed] [Google Scholar]
- 60.Han SY, Jander HP, Ho KJ. Aortoesophageal fistula. South Med J. 1981;74:1260–1262. [DOI] [PubMed] [Google Scholar]
- 61.Strug BS, Saltzman DA, Feldman MI, et al. Aorto-esophageal fistula. Cardiovasc Res Center Bull. 1979;18:34–38. [PubMed] [Google Scholar]
- 62.Tierney LM, Wall SD, Jacobs RA. Aortoesophageal fistula after perigraft abscess with characteristic CT findings. J Clin Gastroenterol. 1984;6:535–537. [DOI] [PubMed] [Google Scholar]
- 63.Wong RS, Champlin A, Temes RT, et al. Aortoesophageal fistula after repair of descending aortic dissection. Ann Thorac Surg. 1996;62:588–590. [PubMed] [Google Scholar]
- 64.Lim CCT, Cheah FK, Tan JCH. Spiral computed tomography demonstration of aorto-oesophageal fistula from fish-bone. Clin Radiol. 2000;55:976–977. [DOI] [PubMed] [Google Scholar]
- 65.Longo JM, Lopez-Rasines G, Ortega E, et al. CT demonstration of an aortoesophageal fistula. Cardiovasc Intervent Radiol. 1987;10:84–85. [DOI] [PubMed] [Google Scholar]
- 66.Kay D, Kalmar JA. Computerized tomographic evaluation of aortic prosthetic graft complications. South Med J. 1985;78:296–298. [DOI] [PubMed] [Google Scholar]
- 67.Wareing TH, Merrill WH. Aortoesophageal fistula: unusual complication. South Med J. 1989;82:1306–1308. [DOI] [PubMed] [Google Scholar]
- 68.Rose BS, Gordon DM, Geihsler JD. Primary aortoesophageal fistula after chronic aortic dissection. AJR. 1990;155:989–990. [DOI] [PubMed] [Google Scholar]
- 69.McFaddin DM, Dang C. Management of aortoesophageal fistula: a case report. Am Surg. 1985;51:548–550. [PubMed] [Google Scholar]
- 70.Burack JH, Lamelas J, Sabado MF, et al. Mycotic aneurysm of the aortic arch with aortoesophageal fistula. J Card Surg. 1991;6:334–337. [DOI] [PubMed] [Google Scholar]
- 71.Amin S, Luketich J, Wald A. Aortoesophageal fistula: case report and review of the literature. Dig Dis Sci. 1998;43:1665–1671. [DOI] [PubMed] [Google Scholar]
- 72.Kieffer E, Bahnini A, Koskas F, et al. In situ allograft replacement of infected infrarenal aortic prosthetic grafts: results in forty-three patients. J Vasc Surg. 1993;17:349–356. [PubMed] [Google Scholar]
- 73.Kieffer E, Sabatier J, Plissonnier D, et al. Prosthetic graft infection after descending thoracic/thoracoabdominal aneurysmectomy: management with in situ arterial allografts. J Vasc Surg. 2001;33:671–678. [DOI] [PubMed] [Google Scholar]
- 74.Burks JA Jr, Faries PL, Gravereaux EC, et al. Endovascular repair of bleeding aortoenteric fistulas: a 5-year experience. J Vasc Surg. 2001;34:1055–1059. [DOI] [PubMed] [Google Scholar]
- 75.Alcantara M, Carrobles JM, Potenciano JLM, et al. Hemorrhage in the upper digestive tract caused by an aorto-esophageal fistula. Endoscopy. 1990;22:51–53. [DOI] [PubMed] [Google Scholar]
- 76.Thal AP. A unified approach to surgical problems of the esophagogastric junction. Ann Surg. 1968;168:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]