Abstract
Objective:
To identify clinically useful parameters obtainable before treatment of predicting clinical outcomes in patients with esophageal carcinoma.
Summary Background Data:
Various factors regarding the biologic state of tumors or the nutritional state of patients have been individually reported to correlate with prognosis. Reliable estimates of life expectancy before treatment are important, and consideration needs to be given not only to tumor-related but also to host-related factors in patients with esophageal carcinoma.
Methods:
The following clinicopathological factors were retrospectively analyzed in 356 consecutive patients with surgical treatment: sex; age; serum C-reactive protein (CRP); proportion of lymphocytes; body weight changes; serum albumin; clinical TNM staging; tumor location; serum squamous cell-related antigen; serum carcinoembryonic antigen; and histology. Factors related to prognosis were evaluated by using univariate and multivariate analyses.
Results:
According to univariate analysis, significant differences in survival were found for sex, serum CRP, proportion of lymphocytes, body weight change, serum albumin, serum squamous cell-related antigen, and clinical TNM staging. Multivariate analysis demonstrated that CRP levels (P = 0.0285), body weight change (P = 0.0165), and clinical TNM staging (P = 0.0008) displayed independent correlations to prognosis. When serum CRP elevation, body weight loss, and clinical TNM staging III and IV were scored as a combined index, the total score (prognostic index for esophageal cancer, PIEC) demonstrated a good stratification value for prognosis. Moreover, PIEC was superior to the conventional clinical TNM staging by the likelihood ratio test.
Conclusions:
PIEC based on serum CRP, body weight change, and clinical TNM staging before treatment offers a very simple and informative method for predicting the prognosis of patients with esophageal carcinoma.
A new prognostic index based on serum CRP, proportion of bodyweight loss, and clinical TNM staging before treatment offers a simple and useful tool for predicting prognosis in patients with esophageal cancer.
The prognosis of patients with esophageal carcinoma remains unsatisfactory because esophageal cancer is one of the most aggressive carcinomas of the gastrointestinal tract. Reliable estimates of life expectancy are therefore important before initiating treatment in the patients with esophageal carcinoma. Various factors regarding the biologic state of tumors and the nutritional state of patients have been reported to correlate with prognosis.1-6 Pedersen et al reported that survival in patients with less than 10% loss of body weight was significantly longer than that in patients with greater weight loss.4 In addition, Onodera et al5 reported that a prognostic nutritional index using serum albumin level and total lymphocyte count of peripheral blood was useful in estimating the prognosis of patients with terminal stage cancer, including esophageal cancer. Moreover, recent reports by Nozoe et al3 have shown that preoperative serum elevation of C-reactive protein (CRP) could represent both a useful marker for malignant potential and an independent prognostic indicator in esophageal carcinoma. The aims of the present retrospective study were to verify the value of well-known prognostic factors and to identify a clinically useful prognostic index of host- and tumor-related factors, including TNM stage grouping,7 in esophageal cancer patients who received surgical treatment.
MATERIALS AND METHODS
Patients
Between 1991 and 2000, 446 consecutive patients with esophageal carcinoma were admitted to the First Department of Kagoshima University Hospital. Eighteen patients who died within a month after initial treatment were excluded. Surgical treatment was performed for 356 patients (83.2%), endoscopic mucosal resection (EMR) for 41 (9.6%), and nonsurgical therapies for 31 (7.2%). A total of 356 patients who underwent esophagectomy were enrolled in the present study. Esophagectomy by right thoracotomy was performed in 251 patients, left thoracotomy in 67, blunt dissection with lymphadenectomy in 28, and blunt dissection without lymphadenectomy in 10. Pre- and postoperative radiation and/or chemotherapy were administered to 93 and 96 patients, respectively. All patients were followed up after discharge as follows: radiographic examination every 1-3 months, computed tomography every 3-6 months, and ultrasonography every 6 months. Bronchoscopic and endoscopic examinations were performed when necessary. The clinical course of patients was monitored for at least 2 years after discharge until death. Follow-up data after surgery were available for all patients, and the median follow-up period was 33.6 months (range, 1-145 months). Recurrent disease was observed in 140 patients (39.3%) after surgery. Therapy for patients with recurrence comprised chemotherapy (32.9%), radiotherapy (26.4%), chemoradiotherapy (15.7%), or surgical therapy (6.4%).
Parameters
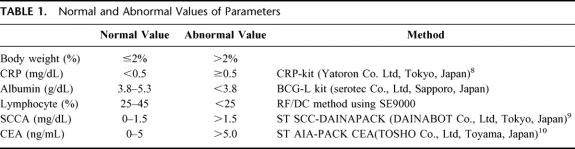
The clinicopathological factors analyzed in this study were as follows: sex; age; serum CRP level;8 proportion of lymphocytes; proportion of body weight loss; serum albumin level; clinical TNM staging; location of the tumor; serum squamous cell-related antigen (SCCA) level;9 serum carcinoembryonic antigen level;10 and histology (Table 1). Pretreatment body weight was compared with past body weight in healthy condition. Body weight loss was classified into 2 subdivisions; loss of 2% or less and loss of more than 2% compared with past body weight because changes in body weight within 2% were considered natural. In this study, loss of more than 2% was adopted before analysis.
TABLE 1. Normal and Abnormal Values of Parameters
Depth of tumor invasion, lymph node metastases, and distant metastasis were estimated using computed tomography, esophagoscope, barium studies, ultrasonography, and endoscopic ultrasonography. When comparing between clinical stage and pathologic stage according to the TNM classification of the International Union Against Cancer,7 the accuracy rate of preoperative diagnosis was 60.7% (216 of 356). When 2 groups were subdivided as groups A (stage I and II) and B (stage III and IV), the accuracy of preoperative diagnosis was 79.4% (283/356). In the present study, subdivision of clinical TNM staging was used because the discrepancy between clinical and pathologic stage was considered.
Statistical Analysis
Data were evaluated using the Stat View 5.0 software package (SAS Institute, Inc., Cary, NC). The χ2 test, Student’s t test, and Mann–Whitney U test were used to compare data. Survival data were analyzed using the Kaplan–Meier survival model. The log-rank test was used to assess statistical differences between groups. For multivariate analysis, the Cox proportional hazards model, including the likelihood-ratio (LR) test, was used to determine independent prognostic factors. Values of P < 0.05 were considered statistically significant.
RESULTS
Demographic Characteristics
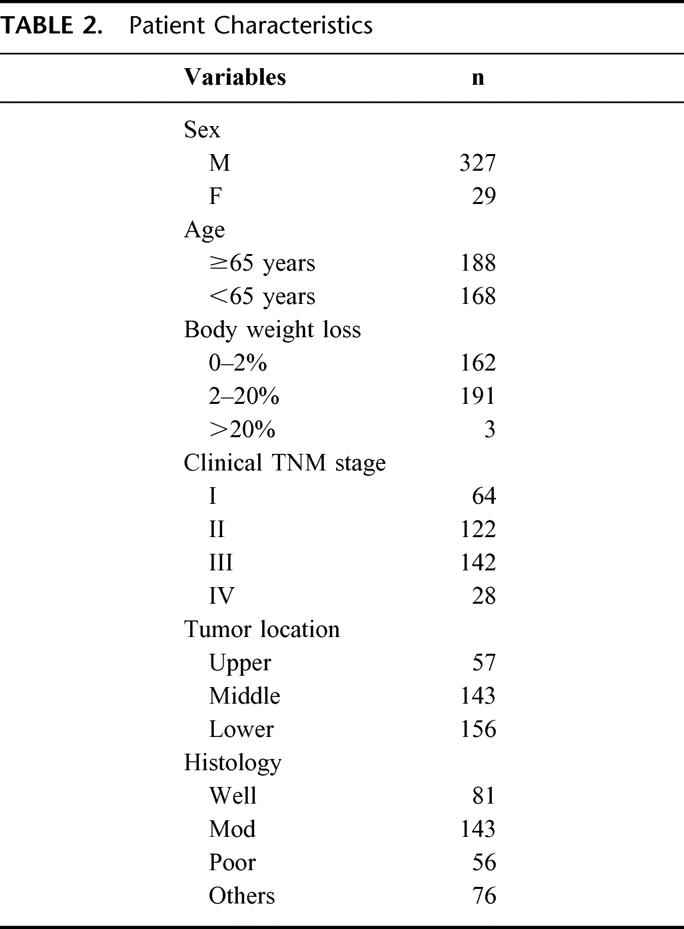
Table 2 shows patient characteristics. The subject group was composed of 327 men and 29 women, with a mean age of 65.0 years (range, 22–87 years). A total of 162 patients (45.5%) displayed 0–2% body weight loss and 194 (54.5%) displayed weight loss exceeding 2%. According to clinical TNM classification, the number of patients with stage I, II, III, and IV was 64 (18.0%), 122 (34.3%), 142 (39.9%), and 28 (7.9%), respectively. Lesions were located in the upper third of the esophagus in 57 patients; in the middle third in 143, and in the lower third in 156. Histologically, 280 tumors were classified as squamous cell carcinoma and the remaining 76 were miscellaneous types, such as carcinosarcoma, basaloid, and undifferentiated carcinoma.
TABLE 2. Patient Characteristics
Prognostic Significance of Various Parameters
According to univariate analysis, significant differences in cumulative survival were observed for sex, serum CRP level, proportion of lymphocytes, body weight change, serum albumin level, serum SCCA level, and clinical TNM staging, (Table 3 and Fig. 1). Tumor location and histologic type did not display significant differences.
TABLE 3. Univariate Analysis of Various Factors
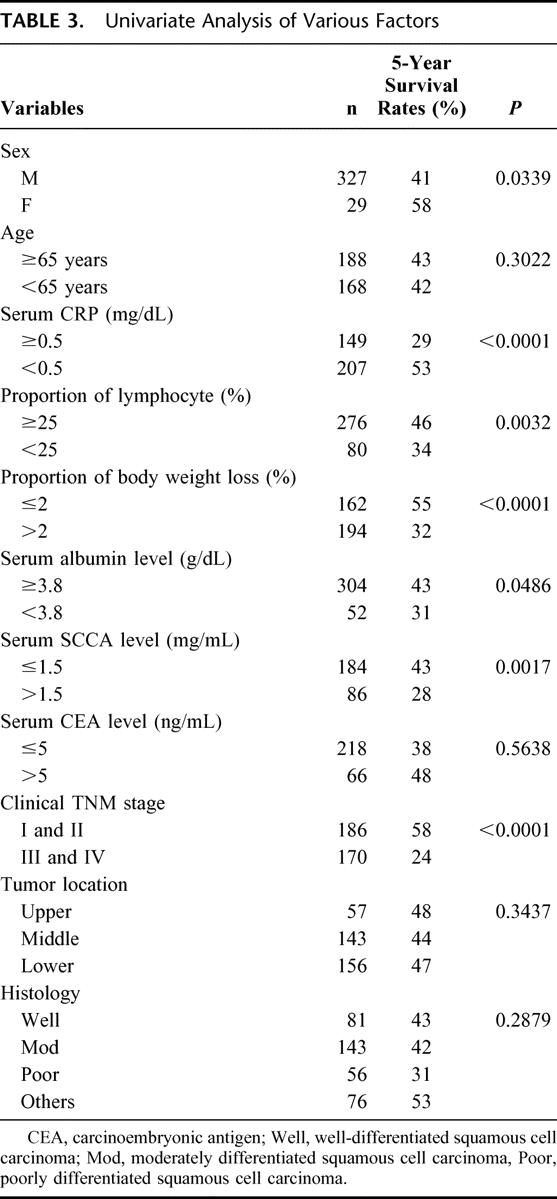
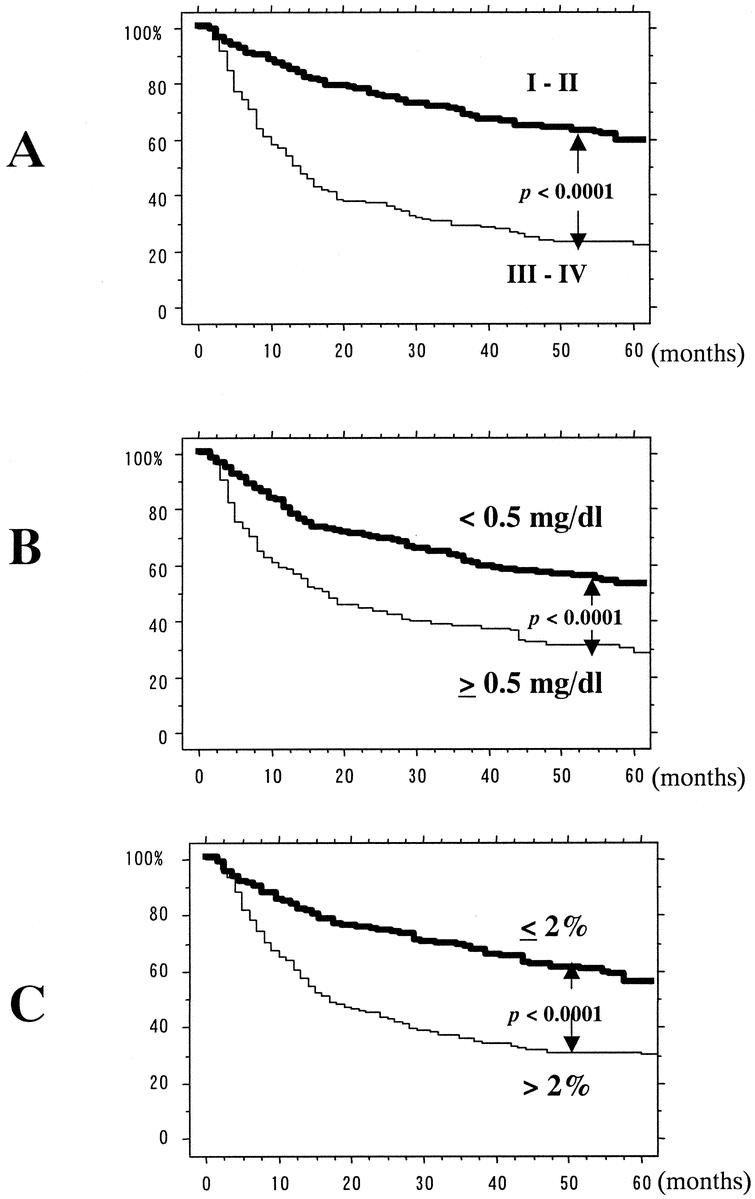
FIGURE 1. Five-year survival curves after univariate analysis using log-rank test. Significant differences were found in clinical TNM staging (a), serum CRP level (b), and proportion of body weight loss (c).
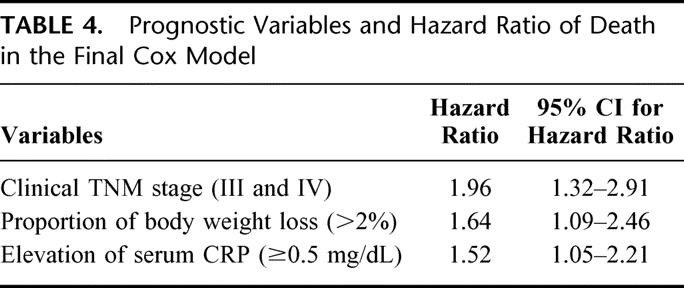
Multivariate analysis demonstrated that elevation of serum CRP levels (P = 0.0285), body weight loss (P = 0.0165), and clinical TNM staging (P = 0.0008) were all independently correlated with prognosis in patients with esophageal carcinoma (Table 4).
TABLE 4. Prognostic Variables and Hazard Ratio of Death in the Final Cox Model
Clinical Outcome Based on New Scoring System
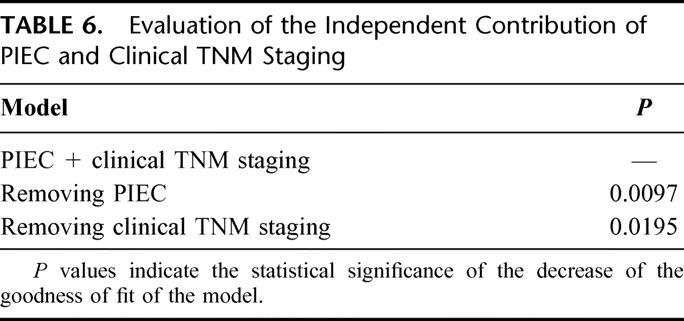
Based on these results, we established new prognostic index for esophageal cancer (PIEC) to reliably estimate survival of patients with esophageal carcinoma. Because relative risk ratios for clinical TNM staging, body weight loss, and serum CRP were basically the same (1.96, 1.64, and 1.52, respectively), these 3 parameters were used to create the new prognostic index. TNM stage I or II, body weight loss 0-2%, and pretreatment serum CRP <0.5 mg/dL were scored as 0. Conversely, TNM stage III or IV, bodyweight loss >2%, and pretreatment serum CRP ≥0.5 mg/dL was scored as 1. According to the total score, prognosis of patients with total score 2 and 3 was almost similar (P = 0.4746) and therefore patients were divided into 3 groups, score 0, 1, and ≥2. The number of patients with scores of 0, 1, and ≥2 were 88, 101, and 167, respectively. According to these scores, 1-, 3-, and 5-year survival rates were 90%, 81%, and 67% for score 0, 83%, 61%; 51% for score 1; and 56%, 30%, and 24% for score ≥2, respectively (P < 0.0001; Fig. 2). The distribution of patients between PIEC and clinical TNM staging is shown in Table 5. Although PIEC correlated with TNM staging (P < 0.01), some discrepancies was found, especially in patients with stage I or II tumors. Finally, independent contribution of the 2 prognostic indices was evaluated by the LR test comparing the full model with the 2 models (PIEC and clinical TNM staging) with each index alone as a covariate. As shown in Table 6, removal of the PIEC from a model containing both the PIEC and the TNM staging did significantly reduce the “fit” of the model more than the removal of the TNM staging.

FIGURE 2. Five-year survival curves according to score 0-3. Significant difference was not found between score 2 and 3 (a). Five-year survival curves according to score 0, 1, and 2 or more. Significant differences were identified between each group (b).
TABLE 5. Distribution of PIEC and Clinical TNM Staging
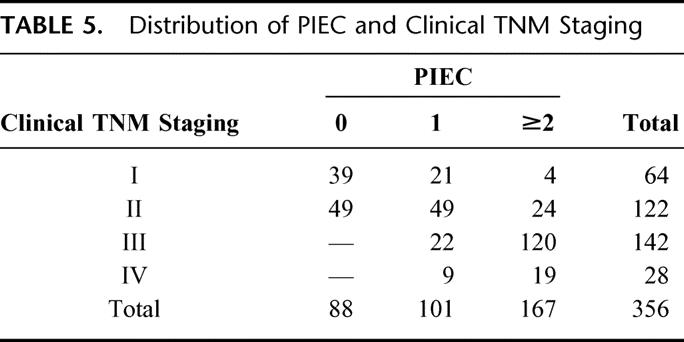
TABLE 6. Evaluation of the Independent Contribution of PIEC and Clinical TNM Staging
DISCUSSION
Identification of a simple and useful indicator for predicting the prognosis of patients with esophageal carcinoma before treatment is an important goal.2–5,11—16 Of course, pathologic TNM classification is valuable for predicting prognosis of esophageal cancer patients. However, this is difficult to determine accurately before treatment. Moreover, when predicting the prognosis of the patients, not only tumor-related but also host-related factors need to be considered.
In the present study, univariate analysis revealed that 5 host-related factors (sex, serum CRP level, proportion of lymphocytes, proportion of body weight loss, and serum albumin level) and 2 tumor-related factors (clinical TNM staging and serum SCCA level) correlated well with patient survival. Moreover, multivariate analysis identified 3 independent prognostic indicators: serum CRP level, proportion of body weight loss, and clinical TNM staging. Pretreatment elevation of serum CRP and body weight loss affected the prognosis of esophageal cancer patients more strongly than other host-related factors.
Elevation of serum CRP level is thought to reflect host reactions to the biologic behavior of a tumor.3 Reactive inflammation caused by tumor invasion to other organs may also influence the elevation of serum CRP. Interleukin-6 is well known to be capable of enhancing CRP production, and some esophageal cancer cells could produce cytokines, such as interleukin-6 and/or cytokine receptors.17–19 Patients with advanced esophageal carcinoma reportedly often display decreased bodyweight, serum albumin level, proportion of lymphocytes, and immunologic function, due to poor nutritional conditions resulting from dysphagia.4,5,20,21 Moreover, the presence of advanced cancer appears to be related to abnormal lipid metabolism leading to an increased rate of lipid oxidation. Patients with prominent bodyweight loss might therefore correlate well with the development of cancer cachexia.22 Regarding other host-related factors, females generally display better survival rates than males for esophageal carcinoma. Because some esophageal carcinoma cells possess androgen receptors, tumor growth can be enhanced by testosterone.23,24 However, sex did not represent an important prognostic factor in Cox multivariate analysis in the present study. Adjuvant radiation and/or chemotherapy were excluded in this statistical analysis, because those therapies were performed only in patients with far advanced or recurrent disease.
Clinical TNM staging is considered to affect the prognosis of esophageal carcinoma most strongly of all pretreatment factors. Determination of clinical TNM staging as accurately as possible before treatment is important. To improve the accuracy of pretreatment diagnosis, we performed ultrasonography and endoscopic ultrasonography. Through these studies, we discovered that the number of lymph node metastases determined by those modalities was consistently predictive of patient survival.2,25–27
In the present study, we devised a new prognostic index using preinterventional serum CRP level, body weight loss, and clinical TNM staging. Levels of serum CRP ≥ 0.5 mg/dL, body weight loss >2%, and clinical TNM staging of stage III or IV were scored as 1 because the relative risk ratios for these 3 parameters were almost the same. When displaying survival curves using the Kaplan–Meier method, good stratification values were shown according to total score. Moreover, in the group with total score ≥ 2, nearly 50% of patients died of esophageal carcinoma within 1 year, even if they had been categorized with clinical TNM stage I or II. These results emphasize the importance of the 2 host-related variables, as shown by the LR test that the PIEC was superior to the conventional clinical TNM staging. Although TNM staging system is surely an important classification, at present, clinical TNM staging before treatment is not completely in accord with pathologic TNM staging. This simple PIEC, therefore, seems particularly effective for providing information regarding the prognosis of patients with esophageal carcinoma.
In conclusion, the following factors are associated with prognosis in patients with esophageal cancer: serum CRP level, proportion of body weight loss, and clinical TNM staging. Our prognostic index using these 3 parameters offers a method of reliably predicting patient survival before treatment.
Footnotes
Reprints: Masanori Ikeda, MD, First Department of Surgery, Kagoshima University School of Medicine, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan. E-mail: masap@m3.kufm.kagoshima-u.ac.jp.
REFERENCES
- 1.Baba M, Aikou T, Yoshinaka H, et al. Long-term results of subtotal esophagectomy with three-field lymphadenectomy for carcinoma of the thoracic esophagus. Ann Surg. 1994;219:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natsugoe S, Yoshinaka H, Shimada M, et al. Number of lymph node metastases determined by presurgical ultrasound and endoscopic ultrasound is related to prognosis in patients with esophageal carcinoma. Ann Surg. 2001;234:613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nozoe T, Saeki H, Sugimachi K, et al. Significance of preoperative elevation of serum C-reactive protein as an indicator of prognosis in esophageal carcinoma. Am J Surg. 2001;182:197–201. [DOI] [PubMed] [Google Scholar]
- 4.Pederson H, Hansen HS, Cederqvist C, et al. The prognostic significance of weight loss and its integration in stage-grouping of oesophageal cancer. Acta Chir Scand. 1982;148:363–366. [PubMed] [Google Scholar]
- 5.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nippon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 6.Gotoda T, Matsumura Y, Kondo H, et al. Expression of CD44 variants and prognosis in oesophageal squamous cell carcinoma. Gut. 2000;46:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.International Union Against Cancer. TNM Classification of Malignant Tumors. 5th ed. New York: Wiley–Liss; 1997.
- 8.Senju O, Takagi Y, Uzawa R, et al. A new immuno quantitative method by latex agglutination—application for the determination of serum C-reactive protein (CRP) and its clinical significance. J Clin Lab Immunol. 1986;19:99–103. [PubMed] [Google Scholar]
- 9.Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;40:1621–1628. [DOI] [PubMed] [Google Scholar]
- 10.Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinoma by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siewert JR, Stein HJ, Feith M, et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234:360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ireland AP, Shibata DK, Chandrasoma P, et al. Clinical significance of p53 mutations in adenocarcinoma of the esophagus and cardia. Ann Surg. 2000;231:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uchida S, Shimada Y, Watanabe G, et al. In oesophageal squamous cell carcinoma vascular endothelial growth factor is associated with p53 mutation, advanced stage and poor prognosis. Br J Cancer. 1998;77:1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iihara K, Shiosaki H, Tahara H, et al. Prognostic significance of transforming growth factor-α in human esophageal carcinoma. Implication for the autocrine proliferation. Cancer. 1993;71:2902–2909. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda Y, Ozawa S, Ando N, et al. Meanings of c-erbB and int-2 amplification in superficial esophageal squamous cell carcinomas. Ann Thorac Surg. 1996;62:835–838. [DOI] [PubMed] [Google Scholar]
- 16.Shinozaki H, Ozawa S, Ando N, et al. Cyclin D1 accumulation as a new predictive classification for squamous cell carcinoma of the esophagus, adding gene information. Clin Cancer Res. 1996;2:1155–1161. [PubMed] [Google Scholar]
- 17.Oka M, Hirose K, Iizuka N, et al. Cytokine mRNA expression patterns in human esophageal cancer cell lines. J Interferon Cytokine Res. 1995;15:1005–1009. [DOI] [PubMed] [Google Scholar]
- 18.Oka M, Yamamoto K, Takahashi M, et al. Relationship between serum levels of interleukin 6, various disease parameters and malnutrition in patients with esophageal squamous cell carcinoma. Cancer Res. 1996;56:2776–2780. [PubMed] [Google Scholar]
- 19.Wang LS, Chow KC, Wu CW, et al. Expression and up-regulation of interleukin-6 in oesophageal carcinoma cells by n-sodium butyrate. Br J Cancer. 1999;80:1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Gorman P, McMillan DC, McArdle CS. Longitudinal study of weight, appetite, performance status, and inflammation in advanced gastrointestinal cancer. Nutr Cancer. 1999;35:127–129. [DOI] [PubMed] [Google Scholar]
- 21.Guo SZ, Shen Q, Zhan HB. Expression and significance of immunosuppressive acidic protein (IAP) in human esophageal squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi. 1994;16:125–127. [PubMed] [Google Scholar]
- 22.Hansell DT, Davis JW, Shenkin A, et al. The oxidation of body fuel stores in cancer patients. Ann Surg. 1986;204:637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita Y, Hirai T, Mukaida H, et al. Detection of androgen receptors in human esophageal cancer. Jpn J Surg. 1989;19:195–202. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka S, Ueno H, Mafune K, et al. A novel isoform of human fibroblast growth factor 8 is induced by androgens and associated with progression of esophageal carcinoma. Dig Dis Sci. 2001;46:1016–1021. [DOI] [PubMed] [Google Scholar]
- 25.Yoshinaka H, Nishi M, Kajisa T, et al. Ultrasonic detection of lymph node metastases in the regional around the celiac axis in esophageal and gastric cancer. J Clin Ultrasound. 1985;13:153–160. [DOI] [PubMed] [Google Scholar]
- 26.Natsugoe S, Yoshinka H, Morinaga T, et al. Ultrasonographic detection of lymph node metastases in superficial carcinoma of the esophagus. Endoscopy. 1996;28:674–679. [DOI] [PubMed] [Google Scholar]
- 27.Natsugoe S, Yohinaka H, Shimada M, et al. Assessment of cervical lymph node metastasis in esophageal carcinoma using ultrasonography. Ann Surg. 1999;229:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]