Abstract
Objective:
This study sought to determine whether hypertonic saline (HTS) infusion modulates the host response to bacterial challenge.
Methods:
Sepsis was induced in 30 Balb-C mice by intraperitoneal injection of Escherichia coli (5 × 107 organisms per animal). In 10 mice, resuscitation was performed at 0 and 24 hours with a 4 mL/kg bolus of HTS (7.5% NaCl), 10 animals received 4 mL/kg of normal saline (0.9% NaCl), and the remaining animals received 30 mL/kg of normal saline. Samples of blood, spleen, and lung were cultured at 8 and 36 hours. Polymorphonucleocytes were incubated in isotonic or hypertonic medium before culture with E. coli. Phagocytosis was assessed by flow cytometry, whereas intracellular bacterial killing was measured after inhibition of phagocytosis with cytochalasin B. Intracellular formation of free radicals was assessed by the molecular probe CM-H2DCFDA. Mitogen-activated protein (MAP) kinase p38 and ERK-1 phosphorylation, and nuclear factor κ B (NFκB) activation were determined. Data are represented as means (SEM), and an analysis of variance test was performed to gauge statistical significance.
Results:
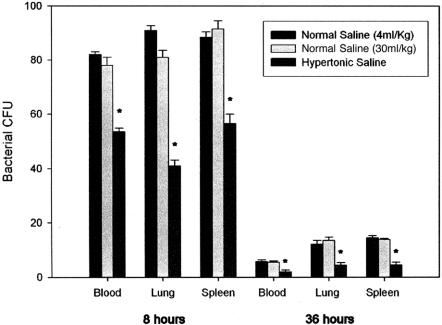
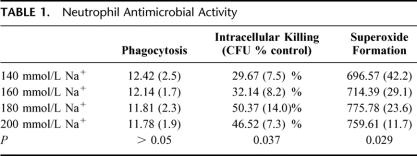
Significantly reduced bacterial culture was observed in the animals resuscitated with HTS when compared with their NS counterparts, in blood (51.8 ± 4.3 vs. 82.0 ± 3.3 and 78.4 ± 4.8, P = 0.005), lung (40.0 ± 4.1 vs. 93.2 ± 2.1 and 80.9 ± 4.7, P = 0.002), and spleen (56.4 ± 3.8 vs. 85.4 ± 4.2 and 90.1 ± 5.9, P = 0.05). Intracellular killing of bacteria increased markedly (P = 0.026) and superoxide generation was enhanced upon exposure to HTS (775.78 ± 23.6 vs. 696.57 ± 42.2, P = 0.017) despite inhibition of MAP kinase and NFκB activation.
Conclusions:
HTS significantly enhances intracellular killing of bacteria while attenuating receptor-mediated activation of proinflammatory cascades.
Hypertonic saline resuscitation significantly enhances neutrophil mediated intracellular killing of bacteria while attenuating receptor mediated activation of pro-inflammatory cascades.
Teleologically, neutrophil activation and transmigration equip the host to withstand and combat septic challenge, and are integral to the successful functioning of the innate immune system.1 The enticement of neutrophils to the site of microbial invasion by chemoattractants such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, is accompanied by an upregulation of integrin expression and enhanced adhesive potential, facilitating migration and extravasation.2 Subsequent phagocytosis of the offending micro organism is elicited by the interaction of membrane bound receptors with particulate ligands on the surface of the pathogen.3 Concomitant microbial killing results from neutrophil degranulation with release of proteolytic enzymes and generation of reactive oxygen species (ROS).4
Although the neutrophil is indispensable for antimicrobial host defense, overexuberant sequestration and entrapment of neutrophils, brimming with cytotoxic constituents, may result in unnecessary tissue damage.5,6 Regulation of the potent destructive faculties of polymorphonucleocytes (PMNs) is realized through complex intracellular signal transduction pathways triggered by tyrosine kinase activation,7 including the mitogen-activated protein (MAP) kinases p38, extracellular signal-related kinase (ERK), and Jun N-terminal kinase.8 Activation of p38 MAP kinase and local transcription factor nuclear factor kappa B (NFκB) results in a potentiation of the cytotoxic response with liberation of proinflammatory cytokines.9,10
The profound effects of hypertonic saline (HTS) on neutrophil function have rekindled interest in it as a resuscitative agent.11–15 Elevated sodium levels inhibit exaggerated neutrophil responses,16 resulting in moderated liberation of proteolytic enzymes, decreased receptor-mediated respiratory burst, shedding of adhesion molecules,17 and a manifest attenuation of end-organ injury. We have previously demonstrated significant attenuation of pulmonary injury attributed to a depressed neutrophilic response in an animal model of pancreatitis with both intravenous and aerosolized hypertonic resuscitation.11,12
Given the pre-eminent role of p38 MAP kinase, a homologue of the osmotically sensitive yeast kinase HOG-1, in determining neutrophil potency, the diminished neutrophil response observed with hypertonic therapy may be attributed to alterations in cell-signaling dynamics arising from fluctuations in extracellular tonicity. However, studies examining the effect of hypertonic exposure on MAP kinase activation have yielded apparently contradictory results; although high levels of hypertonicity prime neutrophils and induce phosphorylation of p38 MAP kinase,18,19 clinically relevant levels appear to attenuate p38 signaling.20,21
Although a number of previous studies have alluded to decreased septic complications accompanying hypertonic resuscitation,22–26 the effect of osmotic shock on the ability of neutrophils to neutralize invading pathogens remains ill-defined. It is conceivable that the potent immunodepressive effects of hypertonic therapy may impact adversely upon the host ability to withstand a septic insult, which would represent a significant impediment to the acceptance of hypertonic infusion as a valid therapeutic approach in states of immune dysfunction.
The present study sought to determine whether osmotic perturbation of neutrophils impairs the host response to bacterial challenge, and to test the hypothesis that the suppression of the inflammatory response may be attributed to osmotically induced alterations in cell signal transduction.
MATERIALS AND METHODS
Reagents and Salts
Sodium citrate, propidium iodide, Percoll, heparin, dextran, Triton X-100, ethylenediaminetetra-acetic acid (EDTA), tromethamine, and lipopolysaccharide (LPS) were purchased from Sigma Chemicals (St. Louis, MO). Ficoll-Paque was purchased from Pharmacia (Uppsala, Sweden). RPMI 1640, phosphate-buffered saline solution (PBS) without calcium and magnesium, fetal calf serum, penicillin, streptomycin sulfate, amphotericin, and glutamine were purchased from Gibco-BRL (Paisley, UK). Salts used in preparation of resuscitative solutions were purchased from Sigma Chemicals.
Preparation of Solutions
Balanced salt solutions contained 2 mM KCl, 1.5 mM K2HPO4, 1 mM MgSO4, 10 mM Hepes, 2 mM CaCl2, and 10 mM glucose, brought to pH 7.4. Isotonic saline solutions contained, in addition, 140 mM NaCl, while hypertonic saline solutions contained either 160 mM, 180 mM, or 200 mM NaCl. The osmolarity of all solutions was determined to be between 280 mosm/L and 400 mosm/L (Micro-osmometer 3M0, Advanced Instruments, Needham Heights, MA).
Preparation of Bacteria
E. coli was obtained from the Department of Microbiology, University College Cork. Bacteria were cultured in tryptic soy broth (Merck, Darmstadt, Germany) at 37°C, harvested at the logarithmic growth phase, washed twice, and resuspended in PBS. The concentration of resuspended bacteria was determined and adjusted spectrophotometrically at 550 nm.
Model of Sepsis
Thirty male Balb-C mice (8–10 weeks, 18–22 g; Harlan, Oxon, UK) were randomized into 3 groups (each n = 10). E. coli infection was initiated by intraperitoneal (ip) injection of 200 μL of PBS containing 5 × 107 colony-forming units (CFU) of viable bacteria per mouse.
Immediately after bacterial inoculation, 20 animals were resuscitated with either 4 mL/kg (n = 10) or 30 mL/kg (n = 10) normal saline (0.9% NaCl, NS) via tail vein cannulation. This was repeated at 24 hours. In an analogous manner, and at the same time points, the remaining 10 animals received HTS (4 mL/kg 7.5% NaCl). This experiment was conducted following approval by the Biologic Services Unit of the University, and the Department of Health and Children, Ireland.
Enumeration of Bacteria in Blood and Organs
Mice were killed at 8 and 36 hours after E. coli challenge. Spleens and lungs were dissected and homogenized. Serial 10-fold dilutions of whole blood and organ homogenates in sterile water containing 0.5% Triton X-100 (Sigma) were plated on tryptic soy agar (Merck) and cultured for 24 hours at 37°C for determination of bacterial CFU.
Isolation of Human Neutrophils
Whole venous blood was collected from healthy adult volunteers with lithium heparin used as an anticoagulant. The neutrophils were isolated with use of dextran sedimentation (6% dextran in 0.9% sodium chloride) followed by Ficoll-Paque gradient centrifugation, as previously described.6 Neutrophils were counted, and cell viability as determined by trypan blue exclusion was >98%. Neutrophil purity was >97% as determined by Rapi-Diff II (DiaCheM, Lancashire, UK) staining on cytocentrifuged samples.
Analysis of Bacterial Uptake, Ingestion, and Intracellular Killing
E. coli were heat-killed at 95°C for 20 minutes and labeled with 0.1% FITC (Sigma). Isolated human neutrophils from healthy adult volunteers were incubated in isotonic (140 mmol/L Na+) or hypertonic (160, 180, or 200 mmol/L Na+) medium and with 1 × 105, 1 × 106, and 1 × 107 CFU/mL of heat-killed, FITC-labeled bacteria at 37°C in a shaking water-bath for 15, 30, and 45 minutes. Bacterial uptake by neutrophils was assessed by fluorescence-activated cell sorter (FACScan) analysis on a flow cytometer. Bacterial ingestion was further determined by FACScan analysis after the external fluorescence of the bound, but noningested, bacteria was quenched with 0.025% crystal violet (Sigma) as described.27 Intracellular bacterial killing was determined as previously described.28 Briefly, neutrophils were incubated with live E. coli at ratios of 1:10, 1:20, and 1:50 (neutrophil:bacteria) at 37°C in a shaking water-bath for 30 and 60 minutes, in the presence or absence of 5 μg/mL cytochalasin B (Sigma), an inhibitor of bacterial phagocytosis, to assess extracellular bacterial killing. Cells were then lysed and incubated with serial dilutions on tryptone soya agar plates at 37°C for 24 hours. Intracellular bacterial killing was determined with the correction of extracellular bacterial killing.
Assessment of Intracellular Free Radical Formation
The intracellular formation of ROS in human neutrophils was detected by using the fluorescent probe CM-H2DCFDA (Molecular Probes, Eugene, OR), as described.29 Briefly, isolated neutrophils were incubated with live E. coli at ratios of 1:10, 1:20, and 1:50 (neutrophil:bacteria) at 37°C in a shaking water-bath for 30 and 60 minutes, in isotonic (140 mmol/L Na+) or hypertonic (160, 180, or 200 mmol/L Na+) medium. The neutrophils were then loaded with 20μM CM-H2DCFDA and incubated at 37°C for 10 minutes. Measurement of intracellular ROS was assessed by flow cytometry.
Assessment of MAP Kinase Activation
Isolated human neutrophils from healthy adult volunteers were treated with LPS (100 ng/mL) at 37°C in a humidified 5% carbon dioxide atmosphere before incubation in isotonic (140 mmol/L Na+) or hypertonic (180 mmol/L Na+) medium for 30 minutes. After being extensively washed with cold PBS, cells were lysed in lysis buffer (1% Triton X-100, 20 mM Tris, 137 mM NaCl, 1 mM phenylmethyl sulfonyl fluoride (PMSF), 2 mM Na3VO4, 10 g/mL leupeptin, 2 g/mL aprotinin). Protein concentrations were determined by using a micro bicin choninic acid (BCA) protein assay reagent kit (Pierce, Rockford, IL). The proteins were denatured at 95°C for 10 minutes in loading buffer (60 mM Tris, 2.5% SDS, 10% glycerol, 5% mercaptoethanol, 0.1% bromophenol blue). Aliquots containing equal amount of total proteins from each sample were separated in SDS-polyacrylamide gels and transblotted onto nitrocellulose membranes (Schleider & Schuell, Dassel, Germany). After blocking for 2 hours with TBS containing 0.1% Tween-20 and 6% nonfat milk, membranes were probed overnight at 4°C with anti pP-38 MAP kinase and ERK1 pABs (Santa Cruz Biotechnology). Blots were further incubated for 1 hour with secondary horseradish peroxidase-conjugated anti mouse IgG (Dako, Glostrup, Denmark), and developed by using an enhanced chemiluminescence detection system (Santa Cruz Biotechnology). Quantification of antibody binding was performed by using image analysis software (Adobe Systems Inc, CA).
Isolation of Nuclear Proteins and NFκB by Electrophoretic Mobility Gel-Shift Assay
Isolated human neutrophils from healthy adult volunteers were treated with LPS (100 ng/mL) at 37°C in a humidified 5% carbon dioxide atmosphere prior to incubation in isotonic (140 mmol/L Na+) or hypertonic (180 mmol/L Na+) medium for 30 minutes. After being extensively washed with cold PBS, cells were lysed in cold lysis buffer (1% Triton X-100, 20 mM Tris, 137 mM NaCl, 1 mM PMSF, 2 mM Na3VO4, 10μg/ml leupeptin, 2μg/ml aprotinin). After centrifugation at 1500 rpm (140 × g) for 10 minutes, the pellet was resuspended in 1 mL of cold lysis buffer A (10 mM Hepes-NaOH pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.5 mM PMSF) and centrifuged at 14,000 rpm (12183 × g) for 10 minutes. The supernatant was discarded and the pellet resuspended in 20 μL of cold lysis buffer A and 0.1% (vol/vol) Nonidet P-40 and incubated on ice for 10 minutes with gentle intermittent vortex. The sample was centrifuged at 14,000 rpm (12183 × g) for 10 minutes, supernatant discarded, and nuclear pellet resuspended in 15 μL of cold buffer B (20 mM Hepes-NaOH pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% [wt/vol]) glycerol, 0.5 mM DTT, 0.5 mM PMSF) on ice for 15 minutes with gentle intermittent vortex. After centrifugation at 14,000 rpm (12183 × g) for 10 minutes, the supernatant was collected, diluted with 75 μL of cold buffer C (20 mM Hepes-NaOH pH 7.9, 50 mM KCl, 0.2 Mm EDTA pH 8.0, 20% [wt/vol] glycerol, 0.5 mM DTT, 0.5 mM PMSF) and stored at −80°C. Protein concentrations were determined by using a micro BCA protein assay reagent kit (Pierce). NFκB consensus oligonucleotide (Double Stranded 5′-agttgaggggactttcccagg c-3′, Promega, Madison, WI) was 5′ end labeled with γ32 ATP with T4 polynucleotide kinase (Promega) by incubation for 1 hour at 37°C. Unincorporated oligonucleotide was removed by using Microspin G-25 centrifugation columns (Amersham Pharmacia Biotechnology, Germany) 6.5 μg of nuclear protein was incubated with labeled nucleotide (150,000–200,000 cpm) in binding buffer for 30 minutes at room temperature. Oligonucleotide labeled protein and unlabeled probe were separated by electrophoresis on a 3% native polyacrylamide gel in tris-glycine. Gels were dried and exposed to an intensifying screen and developed by using a Storm BSO Phosphoimaging System (Molecular Dynamics, Sunnyvale, CA). Quantification of nuclear factor activation was performed by using image analysis software (Adobe Systems).
Survival Study
A survival study was performed to validate the laboratory findings. Sepsis was induced in 30 male Balb-C mice by ip injection of 1 × 108 CFU of viable E. coli, a bacterial concentration previously determined to be lethal. Animals were randomized to receive either normal saline (4 mL/kg, n = 10, or 30 mL/kg, n = 10), or HTS (4 mL/kg, n = 10), on a daily basis via tail vein cannulation. Survival was monitored for 5 days.
Data Analysis
Data are presented as mean values with standard error of mean (SEM), with the exception of Western Blot antibody binding, presented as median luminosity values, as determined by image analysis. Statistical analysis was performed by using analysis of variance comparing both small and large volume NS resuscitation groups with HTS (in vivo) and increments of tonicity (in vitro). Mortality data were determined by employing Kaplan–Meier analysis. A P value < 0.05 was considered significant.
RESULTS
Bacterial Culture
Bacteria were cultured for 24 hours from homogenized and diluted lung and spleen, and diluted whole blood, with quantitative determination of bacterial CFUs. Significantly reduced bacterial culture was observed at both 8 and 36 hours in animals resuscitated with HTS when compared with their normal saline counterparts, regardless of volume (Fig. 1).
FIGURE 1. Effect of HTS on bacterial clearance from blood, lung, and spleen at 8 and 36 hours. Bacteria were cultured for 24 hours before quantitative determination of bacterial CFUs. Data expressed as mean (SEM). *, P < 0.05 vs. normal saline resuscitation.
Neutrophil Antimicrobial Activity
Uptake of FITC-labeled E. coli by neutrophils was assessed by flow cytometry. Osmotic perturbation of neutrophils had little impact on the capacity to engulf bacteria, despite the previously noted induction by HTS of actin cytoskeleton remodeling13 (Table 1). Intracellular killing of bacteria, assessed after inhibition of phagocytosis, was estimated at increments of ionic osmolarity (140, 160, 180, and 200 mmol/L Na+ [280 mosm/L to 400 mosm/L]); 180 and 200 mmol Na+ caused a significant potentiation in microbial killing (Table 1), contingent upon generation of ROS within the neutrophil. Although we observed that quiescent neutrophils evinced no significant change in superoxide production in response to osmotic shock, intracellular formation of ROS, determined by the introduction of a molecular probe, was enhanced with previous exposure to live bacteria. Although no significant difference was observed between neutrophils incubated at the higher levels of tonicity (180 and 200 mmol/L Na+), the trend toward potentiation of intracellular bacterial killing and superoxide formation with increasing osmolar strength appeared diminished above 180 mmol Na+ (Table 1).
TABLE 1. Neutrophil Antimicrobial Activity
MAPK Activation
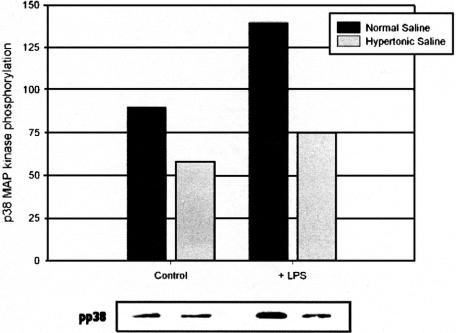
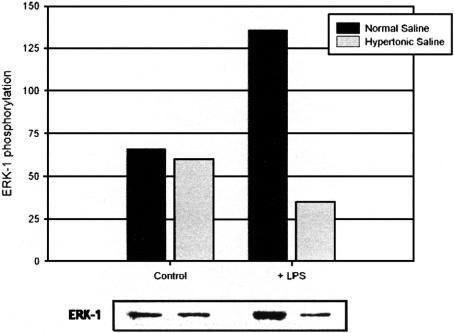
Activation of MAP kinases has been implicated in the cytotoxic response, including the liberation of ROS from activated neutrophils. To investigate the contribution of phosphorylated p38 and ERK-1 to neutrophil antimicrobial and cytotoxic potential, MAPK activation was determined with phosphospecific antibodies under both isotonic (140 mmol/L Na+) and hypertonic (180 mmol/L Na+) conditions in endotoxin-free conditions and in the presence of E. coli-derived LPS. Osmotic perturbation of quiescent neutrophils by high-sodium concentrations caused a decrease in constitutive p38 activation. Treatment with LPS resulted in p38 activation; however, this effect was attenuated by exposure to HTS (Fig. 2). Enhanced ERK-1 activity occasioned by LPS was similarly prevented by HTS (Fig. 3), though it exerted no significant effect on low-level constitutive ERK-1 activation.
FIGURE 2. HTS causes a decrease in constitutive p38 activation and attenuates the response to LPS. Data expressed as median luminosity values, and are representative of 3 separate experiments.
FIGURE 3. Effect of hypertonicity on ERK-1 activation. HTS prevents ERK-1 phosphorylation subsequent to LPS exposure. Data expressed as median luminosity values and are representative of 3 separate experiments.
NFκB Activation
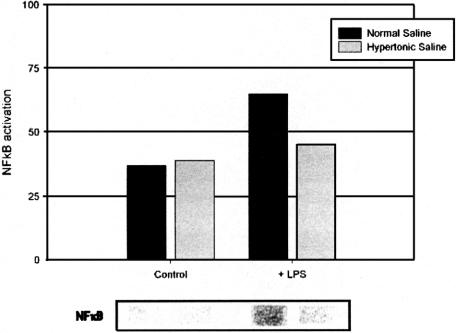
Induction of local transcription factor NFκB and the consequent expression of multiple rapid response inflammatory genes has been described in a variety of inflammatory conditions30–32 and contributes to neutrophil cytotoxic capacity. Activation of NFκB subsequent to LPS exposure was abrogated by increased extracellular sodium concentrations (Fig. 4).
FIGURE 4. Induction of local transcription factor NFκB after LPS treatment is abrogated by increased extracellular sodium concentrations. Data expressed as median luminosity value, and are representative of 3 separate experiments.
Survival Study
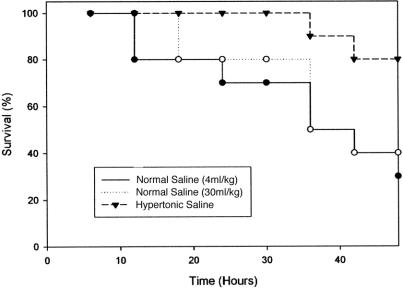
A survival study was performed by using a model of sepsis previously determined to be lethal. At 72 hours, survival in the normal saline groups was only 30% (small volume resuscitation) and 40% (large volume resuscitation), whereas animals resuscitated with HTS exhibited 80% survival, a significant increase in survival (Fig. 5).
FIGURE 5. Improved survival after resuscitation with HTS. HTS (4 mL/kg) or normal saline (4 mL/kg or 30 mL/kg) was administered daily after induction of sepsis. P < 0.05 vs. normal saline groups.
DISCUSSION
This study describes the benefit accruing to the host from hypertonic infusion in the setting of overwhelming sepsis. The host response to septic challenge normally comprises both adaptive and innate immune responses, the former consisting of T and B lymphocytes, whereas the latter is mediated through cytokines and complement and effected by phagocytic cells and cytotoxic natural killer cells.1
Although a key effector cell of the innate immune system, the neutrophil has in addition been identified as an agent of host tissue destruction because of its considerable cytotoxic potential.5,6 Unrestrained neutrophil activation may engender acute lung injury and acute respiratory distress syndrome, the most pertinent manifestations of inappropriate neutrophil-mediated tissue insult in states of immunologic disarray.33,34 Neutrophil degranulation occasions the release of the potent vasoregulatory mediator nitric oxide and arachidonic acid metabolites35,36; however, it is the simultaneous unleashing of proteases, such as elastase and matrix metalloproteinases, that results in the stigmata of lung injury.4,37
The recognition that HTS may moderate PMN cytotoxicity and may serve to dampen an overexuberant inflammatory response, provides a potential strategy for attenuating inappropriate neutrophil activity. The benefits of transient hyperosmolar resuscitation extend to the attenuation of receptor-mediated neutrophil functions, including the down-regulation of oxidative burst activity and adhesion molecule expression, and the suppression of neutrophil activation.16,38–40
The present study demonstrates that hypertonic resuscitation enhances clearance of bacteria from lung and spleen and is associated with diminished bacteremia. The failure of isotonic resuscitation, irrespective of volume, to attenuate the septic insult, further confirms the profound effects of osmotic shock on host immune response. The correlation with survival suggests that HTS confers increased ability to withstand a significant septic insult. Although previous studies of bacterial killing in the urinary tract have concluded that hyperosmolarity impedes neutrophil activity,41,42 a number of recent studies have affirmed HTS-induced prevention of bacterial translocation following hemorrhagic shock,22–26 with enhanced containment of infection.22
Cytoskeletal integrity is a prerequisite for the PMN cytotoxic response and plays a pivotal role in determining neutrophil interaction with the local environment, facilitating appropriate migratory responses, phagocytosis of pathogens, and ultimately degranulation.43 Alterations in cytoskeletal conformation can result from the induction of F-actin polymerization by HTS, with extravasation of osmotic water.13 Previous studies have demonstrated that infusion of 7.5% saline results in a rapid, though transient, rise in plasma sodium levels to 40mmol/L above isotonic levels,19 which remain elevated for up to 4 hours.44 It was at this level of tonicity that we observed maximal neutrophilic potentiation, which declined as the osmolarity climbed higher. Although some authors have alluded to a deficiency in phagocytic ability at extremes of osmotic stress,42 we were unable to demonstrate impaired phagocytosis in our model system, implying that clinically relevant levels of hypertonicity (ie, 180 mmol/L Na+) do not impede the engulfment of opsonized pathogens.
A putative mechanism for the potentiating effects of HTS on bacterial clearance is enhanced intracellular killing, attributed in this study to augmented intracellular ROS generation. However, the additive direct effects of osmotic perturbation on bacteria cannot be discounted. Epithelial adherence of E. coli is compromised under hypertonic conditions,45 and one may surmise that elevated extracellular tonicity renders the systemic circulation an inhospitable environment for a rapidly dividing organism.
The seemingly uniform cellular response to a heterogeneous group of cell stressors, such as bacterial products, hypoxia, growth factors and cytokines, arises from the convergence of these stimuli at specific cell-signaling cascades, causing activation of the tyrosine kinases phosphatidyl inositol 3-kinase,46 protein kinase C,47 Src-kinases, and MAP kinases.8 MAP kinase p38 activation is recognized as a major determinant of the functional response of immunocompetent cells to noxious stimuli. The activation of p38 by, among others, LPS,48 augments neutrophil cytotoxic potential by causing an escalation in the liberation of free radicals, enhancing integrin adhesive qualities,9 and regulating the synthesis of pro-inflammatory cytokines.49
Despite osmotic down-regulation of p38 and ERK-1, this study demonstrates enhanced superoxide generation in response to bacteria, suggesting that neither kinase plays a prominent role in receptor-independent ROS generation, despite integral involvement in cell signaling responsible for neutrophil degranulation. This reveals an evident paradox in osmoregulation of neutrophil activity. Although hypertonicity results in attenuation of end-organ injury by compromising neutrophil cytotoxic capacity, it also appears to enhance the response to E. coli. This suggests that HTS interrupts the propagation of the inflammatory cascade at the level of cell signal transduction, rather than disrupting the nicotinamide adenine dinucleotide phosphate oxidase complex responsible for ROS generation.4
The propensity of increased extracellular osmolarity to decrease postinjury inflammation may also be attributed to the osmotic inhibition of NFκB demonstrated in this study. A temporal correlation has been observed between NFκB activation in lung, and the expression of cytokine mRNA.50 Consequently, disruption of NFκB activation is immunosuppressive, reducing pulmonary inflammation and mortality in experimental models of pancreatitis.51,52
This study provides further evidence of the beneficial effects of hypertonic resuscitation. HTS attenuates the receptor-mediated activation of proinflammatory cascades, while enhancing the host response to significant bacterial challenge. Regulators of cellular signal transduction pathways, such as p38 MAP kinase and NFκB inhibitors, provide potential pharmacologic targets for suppressing unchecked and unrestrained inflammation. Reversible and transient inhibition of these pathways may be achieved through hypertonic resuscitation, obviating exposure to the potential toxicity of kinase inhibitors. A clinical trial is warranted.
Footnotes
Reprints: H. P. Redmond, Department of Academic Surgery, Cork University Hospital, Wilton, Cork, Ireland. E-mail: redmondhp@shb.ie.
REFERENCES
- 1.Mollinedo F, Borregaard N, Boxer LA. Novel trends in neutrophil structure, function and development. Immunol Today. 1999;20:535–537. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway C Jr. The Toll receptor family and microbial recognition. Trends Microbiol. 2000;8:452–456. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz AH, Williams RE, Liu PS, et al. Bactericidal/permeability-increasing protein inhibits growth of a strain of Acholeplasma laidlawii and L forms of the gram-positive bacteria Staphylococcus aureus and Streptococcus pyogenes. Antimicrob Agents Chemother. 1999;43:2314–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krump E, Sanghera JS, Pelech SL, et al. Chemotactic peptide N-formyl-met-leu-phe activation of p38 mitogen- activated protein kinase (MAPK) and MAPK-activated protein kinase-2 in human neutrophils. J Biol Chem. 1997;272:937–944. [DOI] [PubMed] [Google Scholar]
- 5.Kawabata K, Hagio T, Matsumoto S, et al. Delayed neutrophil elastase inhibition prevents subsequent progression of acute lung injury induced by endotoxin inhalation in hamsters. Am J Respir Crit Care Med. 2000;161:2013–2018. [DOI] [PubMed] [Google Scholar]
- 6.Sookhai S, Wang JH, McCourt M, et al. Dopamine induces neutrophil apoptosis through a dopamine D-1 receptor- independent mechanism. Surgery. 1999;126:314–322. [PubMed] [Google Scholar]
- 7.Yan SR, Berton G. Antibody-induced engagement of beta2 integrins in human neutrophils causes a rapid redistribution of cytoskeletal proteins, Src-family tyrosine kinases, and p72syk that precedes de novo actin polymerization. J Leukoc Biol. 1998;64:401–408. [DOI] [PubMed] [Google Scholar]
- 8.Scherle PA, Jones EA, Favata MF, et al. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J Immunol. 1998;161:5681–5686. [PubMed] [Google Scholar]
- 9.Nick JA, Avdi NJ, Young SK, et al. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J Clin Invest. 1997;99:975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffray C, Yang J, Carter G, et al. Pancreatic elastase activates pulmonary nuclear factor kappa B and inhibitory kappa B, mimicking pancreatitis-associated adult respiratory distress syndrome. Surgery. 2000;128:225–231. [DOI] [PubMed] [Google Scholar]
- 11.Shields CJ, Winter DC, Sookhai S, et al. Hypertonic saline attenuates end-organ damage in an experimental model of acute pancreatitis. Br J Surg. 2000;87:1336–1340. [DOI] [PubMed] [Google Scholar]
- 12.Shields CJ, Sookhai S, Winter DC, et al. Attenuation of pancreatitis-induced pulmonary injury by aerosolized hypertonic saline. Surg Infect. 2001;2:215–224. [DOI] [PubMed] [Google Scholar]
- 13.Rizoli SB, Rotstein OD, Parodo J, et al. Hypertonic inhibition of exocytosis in neutrophils: central role for osmotic actin skeleton remodeling. Am J Physiol Cell Physiol. 2000;279:C619–C633. [DOI] [PubMed] [Google Scholar]
- 14.Murao Y, Hoyt DB, Loomis W, et al. Does the timing of hypertonic saline resuscitation affect its potential to prevent lung damage? Shock. 2000;14:18–23. [DOI] [PubMed] [Google Scholar]
- 15.Mattox KL, Maningas PA, Moore EE, et al. Prehospital hypertonic saline/dextran infusion for post-traumatic hypotension. The U. S. A. Multicenter Trial. Ann Surg. 1991;213:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junger WG, Coimbra R, Liu FC, et al. Hypertonic saline resuscitation: a tool to modulate immune function in trauma patients? Shock. 1997;8:235–241. [PubMed] [Google Scholar]
- 17.Rizoli SB, Rotstein OD, Kapus A. Cell volume-dependent regulation of L-selectin shedding in neutrophils. A role for p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:22072–22080. [DOI] [PubMed] [Google Scholar]
- 18.Ciesla DJ, Moore EE, Biffl WL, et al. Hypertonic saline activation of p38 MAPK primes the PMN respiratory burst. Shock. 2001;16:285–289. [DOI] [PubMed] [Google Scholar]
- 19.Junger WG, Hoyt DB, Davis RE, et al. Hypertonicity regulates the function of human neutrophils by modulating chemoattractant receptor signaling and activating mitogen-activated protein kinase p38. J Clin Invest. 1998;101:2768–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciesla DJ, Moore EE, Gonzalez RJ, et al. Hypertonic saline inhibits neutrophil (PMN) priming via attenuation of p38 MAPK signaling. Shock 2000;14:265–270. [DOI] [PubMed] [Google Scholar]
- 21.Rizoli SB, Kapus A, Parodo J, et al. Hypertonicity prevents lipopolysaccharide-stimulated CD11b/CD18 expression in human neutrophils in vitro: role for p38 inhibition. J Trauma. 1999;46:794–799. [DOI] [PubMed] [Google Scholar]
- 22.Coimbra R, Hoyt DB, Junger WG, et al. Hypertonic saline resuscitation decreases susceptibility to sepsis after hemorrhagic shock. J Trauma. 1997;42:602–607. [DOI] [PubMed] [Google Scholar]
- 23.Topaloglu U, Yilmazcan A, Guloglu R, et al. Hypertonic saline prevents early bacterial translocation in hemorrhagic shock. Surg Today. 1999;29:47–50. [DOI] [PubMed] [Google Scholar]
- 24.Yada-Langui MM, Coimbra R, Lancellotti C, et al. Hypertonic saline and pentoxifylline prevent lung injury and bacterial translocation after hemorrhagic shock. Shock. 2000;14:594–598. [DOI] [PubMed] [Google Scholar]
- 25.Assalia A, Bitterman H, Hirsh TM, Krausz MM. Influence of hypertonic saline on bacterial translocation in controlled hemorrhagic shock. Shock. 2001;15:307–311. [DOI] [PubMed] [Google Scholar]
- 26.Reed LL, Manglano R, Martin M, et al. The effect of hypertonic saline resuscitation on bacterial translocation after hemorrhagic shock in rats. Surgery. 1991;110:685–690. [PubMed] [Google Scholar]
- 27.Albanyan EA, Vallejo JG, Smith CW, et al. Nonopsonic binding of type III Group B Streptococci to human neutrophils induces interleukin-8 release mediated by the p38 mitogen- activated protein kinase pathway. Infect Immunol. 2000;68:2053–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy JE, Redmond PH, Duggan SM, et al. Characterization of the defects in murine peritoneal macrophage function in the early postsplenectomy period. J Immunol. 1995;155:387–396. [PubMed] [Google Scholar]
- 29.Wu QD, Wang JH, Fennessy F, et al. Taurine prevents high-glucose-induced human vascular endothelial cell apoptosis. Am J Physiol. 1999;277:C1229–C1238. [DOI] [PubMed] [Google Scholar]
- 30.Ardite E, Panes J, Miranda M, et al. Effects of steroid treatment on activation of nuclear factor kappaB in patients with inflammatory bowel disease. Br J Pharmacol. 1998;124:431–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sioud M, Mellbye O, Forre O. Analysis of the NF-kappa B p65 subunit, Fas antigen, Fas ligand and Bcl-2-related proteins in the synovium of RA and polyarticular JRA. Clin Exp Rheumatol. 1998;16:125–134. [PubMed] [Google Scholar]
- 32.Blackwell TS, Holden EP, Blackwell TR, et al. Cytokine-induced neutrophil chemoattractant mediates neutrophilic alveolitis in rats: association with nuclear factor kappa B activation. Am J Respir Cell Mol Biol. 1994;11:464–472. [DOI] [PubMed] [Google Scholar]
- 33.Rabinovici R, Bugelski PJ, Esser KM, et al. ARDS-like lung injury produced by endotoxin in platelet-activating factor-primed rats. J Appl Physiol. 1993;74:1791–1802. [DOI] [PubMed] [Google Scholar]
- 34.Moore FA, Moore EE, Read RA. Postinjury multiple organ failure: role of extrathoracic injury and sepsis in adult respiratory distress syndrome. New Horiz. 1993;1:538–549. [PubMed] [Google Scholar]
- 35.Tsukahara Y, Horita Y, Anan K, et al. Role of nitric oxide derived from alveolar macrophages in the early phase of acute pancreatitis. J Surg Res. 1996;66:43–50. [DOI] [PubMed] [Google Scholar]
- 36.Tsukahara Y, Morisaki T, Horita Y, et al. Phospholipase A2 mediates nitric oxide production by alveolar macrophages and acute lung injury in pancreatitis. Ann Surg. 1999;229:385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tkalcevic J, Novelli M, Phylactides M, et al. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–210. [DOI] [PubMed] [Google Scholar]
- 38.Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic saline enhances cellular immune function. Circ Shock. 1994;42:190–196. [PubMed] [Google Scholar]
- 39.Rizoli SB, Kapus A, Parodo J, et al. Hypertonic immunomodulation is reversible and accompanied by changes in CD11b expression. J Surg Res. 1999;83:130–135. [DOI] [PubMed] [Google Scholar]
- 40.Angle N, Hoyt DB, Coimbra R, et al. Hypertonic saline resuscitation diminishes lung injury by suppressing neutrophil activation after hemorrhagic shock. Shock. 1998;9:164–170. [DOI] [PubMed] [Google Scholar]
- 41.Hampton MB, Chambers ST, Vissers MC, Winterbourn CC. Bacterial killing by neutrophils in hypertonic environments. J Infect Dis. 1994;169:839–846. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto T, van der Auwera P, Watanabe Y, et al. Neutrophil function in hyperosmotic NaCl is preserved by phosphoenol pyruvate. Urol Res. 1991;19:223–227. [DOI] [PubMed] [Google Scholar]
- 43.Howard TH, Watts RG. Actin polymerization and leukocyte function. Curr Opin Hematol. 1994;1:61–68. [PubMed] [Google Scholar]
- 44.Ciesla DJ, Moore EE, Zallen G, et al. Hypertonic saline attenuation of polymorphonuclear neutrophil cytotoxicity: timing is everything. J Trauma. 2000;48:388–395. [DOI] [PubMed] [Google Scholar]
- 45.Smith JW. Microbial and host factors that influence adherence of Escherichia coli to kidney epithelium. Am J Kidney Dis. 1986;7:368–374. [DOI] [PubMed] [Google Scholar]
- 46.Capodici C, Hanft S, Feoktistov M, et al. Phosphatidylinositol 3-kinase mediates chemoattractant-stimulated, CD11b/CD18-dependent cell-cell adhesion of human neutrophils: evidence for an ERK-independent pathway. J Immunol. 1998;160:1901–1909. [PubMed] [Google Scholar]
- 47.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. [DOI] [PubMed] [Google Scholar]
- 48.St-Denis A, Chano F, Tremblay P, et al. Protein kinase C-alpha modulates lipopolysaccharide-induced functions in a murine macrophage cell line. J Biol Chem. 1998;273:32787–32792. [DOI] [PubMed] [Google Scholar]
- 49.Denham W, Yang J, Wang H, et al. Inhibition of p38 mitogen activate kinase attenuates the severity of pancreatitis-induced adult respiratory distress syndrome. Crit Care Med. 2000;28:2567–2572. [DOI] [PubMed] [Google Scholar]
- 50.Blackwell TS, Blackwell TR, Holden EP, et al. In vivo antioxidant treatment suppresses nuclear factor-kappa B activation and neutrophilic lung inflammation. J Immunol. 1996;157:1630–1637. [PubMed] [Google Scholar]
- 51.Ethridge RT, Hashimoto K, Rajarman S, et al. Selective inhibition of NF-kB results in attenuation of acute pancreatitis and pancreatitis-associated lung injury. Surg Forum. 2001;LII:25–27. [Google Scholar]
- 52.Satoh A, Shimosegawa T, Fujita M, et al. Inhibition of nuclear factor-kappaB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]