Abstract
Objective:
To report the long-term results of our experience using cerebrospinal fluid drainage and distal aortic perfusion in descending thoracic and thoracoabdominal aortic repair.
Summary Background Data:
Repair of thoracoabdominal and thoracic aortic aneurysm by the traditional clamp-and-go technique results in a massive ischemic insult to several major organ systems. Ten years ago, we began to use distal aortic perfusion and cerebrospinal fluid drainage (adjunct) to reduce end-organ ischemia.
Methods:
Between January 1991 and February 2003, we performed 1004 thoracoabdominal or descending thoracic repairs. Adjunct was used in 741 (74%) of 1004. Multivariable data were analyzed by Cox regression. Number needed to treat was calculated as the reciprocal of the risk difference.
Results:
Immediate neurologic deficit was 18 (2.4%) of 741 with adjunct and 18 (6.8%) of 263 without (P < 0.0009). In high-risk extent II aneurysms, the numbers were 11 (6.6%) of 167 with adjunct, and 11 (29%) of 38 without. Long-term survival was improved with adjunct (P < 0.002). The long-term survival results persisted after adjustment for age, extent II aneurysm, and preoperative renal function.
Conclusion:
Use of adjunct over a long period of time has produced favorable results; approximately 1 neurologic deficit saved for every 20 uses of adjunct overall. In extent II aneurysms, where the effect is greatest, this increases to 1 saved per 5 uses. Adjunct is also associated with long-term survival, which is consistent with mitigation of ischemic end-organ injury. These long-term results indicate that cerebrospinal fluid drainage and distal aortic perfusion are safe and effective adjunct for reducing morbidity and mortality following thoracic and thoracoabdominal aortic repair.
Analysis of our 10-year experience of 1004 descending thoracic and thoracoabdominal aortic graft aneurysm repairs utilizing distal aortic perfusion and cerebrospinal fluid drainage demonstrated the effectiveness of these adjuncts and reinforced our previous findings of the benefits to survival and neurologic outcome.
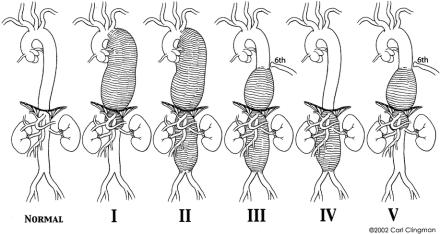
Thoracoabdominal aortic graft replacement has been plagued with the risk of postoperative neurologic deficits (paraplegia and paraparesis) since its inception. Before the early 1980s, the introduction of many surgical adjuncts aimed at spinal cord protection produced confusing results due to the lack of standardization in reporting criteria. The development of a meaningful system of aneurysm classification1 revealed that aneurysm extent has a significant influence in neurologic outcome (Fig. 1). Aneurysm classification also helped us to better analyze the success or failure of adjuncts. The simple cross-clamp technique was the mainstay of descending and thoracoabdominal aortic repair well into the 1980s, and the immediate survival rates and long-term results of that era were impressive. However, during that time, the rate of neurologic deficits, especially for extent II thoracoabdominal aortic aneurysm (TAAA), was persistently high. Neurologic deficits were clearly related to the duration of clamp time, aneurysm extent, rupture, patient age, proximal aortic aneurysm, and renal failure.
FIGURE 1. Normal thoracoabdominal aorta and aneurysm classification. Extent I, distal to the left subclavian artery to above the renal arteries. Extent II, distal to the left subclavian artery to below the renal arteries. Extent III, from the sixth intercostal space to below the renal arteries. Extent IV, from the twelfth intercostal space to below the renal arteries (total abdominal aortic aneurysm). Extent V, below the sixth intercostal space to just above the renal arteries.
In the last decade an abundance of adjuncts—regional or systemic cooling, cerebrospinal fluid (CSF) drainage delivered independently, intraoperatively or postoperatively, distal aortic perfusion, also delivered independently, medications, spinal cooling, and monitoring somatosensory-evoked potentials—have met with varying degrees of success.2-9 Among this array of adjuncts, following the results of both animal and clinical studies, we became particularly interested in the use of combined CSF drainage and distal aortic perfusion.
We have successfully used perioperative CSF drainage with distal aortic perfusion for the past 10 years. We have found that moderate passive hypothermia, active visceral cooling with blood or crystalloid solution, and aggressive intercostal artery reattachment further augment the success of these adjuncts. This report describes the progress of our experience with adjuncts and the way that preoperative and postoperative factors influence their success in descending thoracic and thoracoabdominal aortic repair.
MATERIALS AND METHODS
We reviewed the data of 1004 patients operated between January 1991 and February 2003, for descending thoracic or thoracoabdominal aortic graft replacement. Six hundred thirty-one (63%) were male. The patient distribution of descending thoracic and TAAA, according to classification, was 213 (21%) extent I, 205 (20%) extent II, 84 (8%) extent III, 145 (14%) extent IV, 49 (5%) extent V, and 309 (30%) descending thoracic aortic aneurysms. The median age of all patients was 68 years, (range, 8 to 89 years). Three hundred eighteen (32%) were active smokers at the time of surgery. One hundred three (10%) suffered from cerebrovascular disease. Forty-one patients (4%) presented with acute dissection; 30 (3%) with free rupture. The adjuncts distal aortic perfusion and CSF drainage with moderate hypothermia were used in 741 (74%) of 1004 patients. Four hundred twenty-six (42.4%) of 1004 patients underwent intercostal artery reattachment.
Outcome Variables
Paraplegia or paraparesis upon awakening, regardless of the severity, defined immediate neurologic deficit. Paraplegia or paraparesis after a period of normal neurologic function was termed delayed neurologic deficit. Surgery performed in fewer than 14 days after the onset of related pain defined acute dissection; after 14 days, chronic. A history of stroke, transient ischemic attack, or intervention for carotid artery disease characterized cerebrovascular disease. Chronic obstructive pulmonary disease was identified by a history of chronic bronchitis and emphysema or < 60% of predicted forced expired volume in 1 second (FEV1). A serum creatinine level of > 2.0 mg/dl or the need for dialysis characterized renal dysfunction. Emergency repair patients were symptomatic or had acute dissection or free rupture.
Surgical Technique
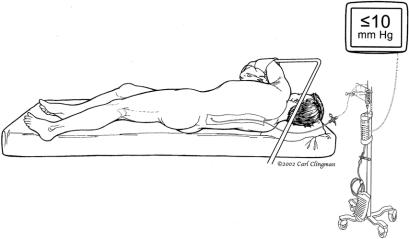
The patient is placed on the operating table in the supine position, anesthetized, and intubated using a double lumen endotracheal tube. A balloon-tip catheter is inserted into the right side of the heart for thermal dilution and a transesophageal echo (TEE) probe is inserted into the esophagus. Electrodes attached to the scalp for electroencephalogram (EEG) and along the spinal cord for somatosensory-evoked potential (SSEP) assess cerebral and spinal cord function. The anesthesiologist inserts and secures a spinal catheter into the third or fourth lumbar space (Fig. 2). CSF pressure is kept at 10 mm Hg intraoperatively, and the drain remains in place for 3 days postoperatively. The patient is positioned in the right lateral decubitus position with the hips flexed 60 degrees for accessibility of the left and right groins. With the patient’s shoulder at 90 degrees and the hips tilted at 60 degrees, an incision is made from the symphysis pubis, midline to the umbilicus straight to the costal cartilage and then along the 6th rib contour and between the vertebral border of the scapula and the spine. The 6th rib is excised, and the left lung is collapsed. The muscular portion of the diaphragm is incised, and the abdominal organs are rotated medially and extraperitoneally. A self-retaining retractor secures exposure of the aorta from the left subclavian artery to the iliac bifurcation. The pericardium posterior to the left phrenic nerve is opened, exposing the left atrium and the left lower pulmonary vein. A BioMedicus pump (Minneapolis, MN) and in-line heat exchanger is attached to the cannula for distal aortic perfusion and postoperative rewarming (Fig. 3). The left femoral artery is dissected out, isolated, and looped with umbilical tape. A cannula is inserted in the left common femoral artery for perfusion inflow. If cannulation of the femoral artery is not feasible, the distal thoracic aorta is cannulated instead. Perfusion is initiated just before applying the aortic clamp.
FIGURE 2. Cerebrospinal fluid drainage.
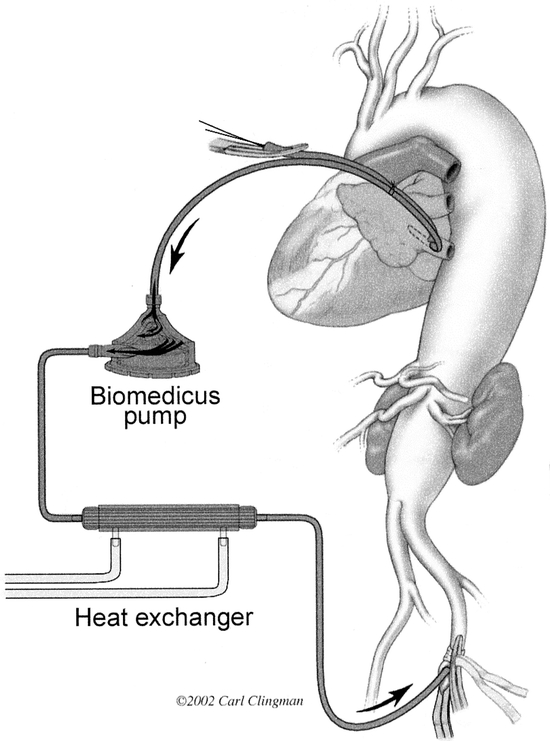
FIGURE 3. Illustration of distal aortic perfusion circuit.
To expose the proximal thoracic aorta, we begin the dissection at the hilum of the left lung and proceed cephalad, exposing the phrenic, vagus, and left recurrent nerves. The thoracic aorta is clamped sequentially, beginning distal or proximal to the left subclavian artery and at the mid-descending thoracic aorta. The aorta between the clamps is opened, and the proximal descending thoracic aorta is completely transected and lifted off the esophagus to prevent esophageal graft fistula. A Dacron graft is sutured to the proximal descending thoracic aorta, using 3-0 polypropylene suture. This anastomosis is checked for bleeding and, if necessary, sutured with 3-0 pledgeted polypropylene. We reattach all patent lower intercostal arteries from T8 to T12, either individually or together as a patch, to an elliptical incision made in the Dacron graft. When lower intercostal arteries are occluded, we reattach upper intercostal arteries instead. The proximal clamp is moved down on the graft, restoring flow to the intercostal arteries.
The infrarenal abdominal aorta is clamped, and the remainder of the aorta is opened. The graft is passed through the aortic hiatus into the abdominal cavity. The walls of the aorta are retracted laterally, using #2 silk retraction sutures. The visceral vessels are identified and perfused with cold blood via #9 or #12 Pruitt catheters. The rate of flow depends on the proximal aortic pressure and is maintained between 300 and 600 mL/min. The temperature of the left kidney is kept below 20°C, preferably around 15°C. Since cold visceral perfusion may cause hypothermia, core body temperature is kept between 32°C and 33°C by warming the lower extremities. A side hole is cut into the graft opposite the visceral vessels, and the vessels are reattached as a patch using running 3-0 polypropylene suture. If the left renal artery is located too far from the other visceral arteries, it is then reattached as a button patch or by using a short interposition bypass graft. If there is significant atherosclerotic occlusive disease of the left or right renal arteries, we perform endarterectomy. In that setting, the left kidney can be perfused retrograde through the left renal vein to keep the renal temperature below 20°C. Once the visceral anastomosis is completed, we move the descending thoracic clamp down on the graft and restore the flow to the viscera and kidneys. Indigo carmine dye is infused after reperfusion of the kidneys, and the dye clearance time is used to estimate renal function.
A clamp is placed above the iliac bifurcation or on the left common iliac artery. The remainder of the aneurysm is opened longitudinally. We stretch, cut, and suture the Dacron graft to the distal infrarenal abdominal aorta above the aortic bifurcation, using 3-0 polypropylene suture. Before the completion of this anastomosis, we flush the aorta distally and proximally, remove the clamps, and restore pulsatile flow to the legs. If due to technical reasons we cannot use a distal clamp and distal circulatory warming cannot be performed, cold visceral perfusion is avoided to prevent core body cooling that may result in cardiac dysrhythmias. The distal aortic graft anastomosis can be performed first, the graft clamped, the pump restarted, and the visceral anastomosis is completed after. We rewarm the patient until the nasopharyngeal temperature reaches 36°C. Once this is accomplished, we remove the cannulae from the lower left pulmonary vein and femoral artery, and the femoral artery is repaired. After achieving good hemostasis the thoracoabdominal incision is closed. The patient is again placed in the supine position. The anesthesiologist replaces the double endotracheal tube with a single tube, and the patient is taken to the intensive care unit. Figures 4A and 4B show the preoperative computed tomography scans and illustrations of a representative TAAA repair in a Marfan patient.
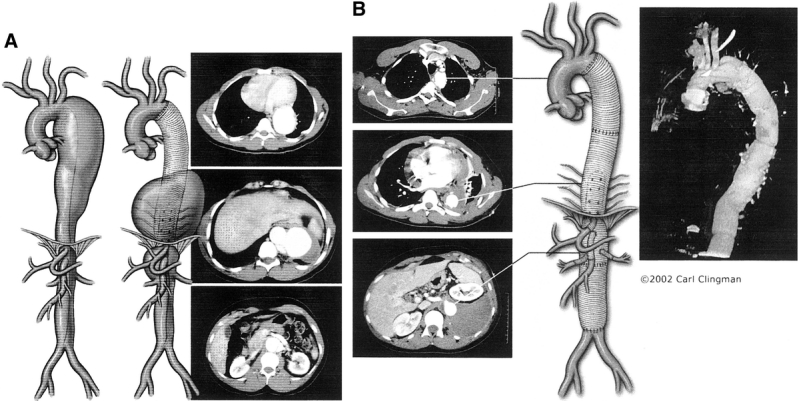
FIGURE 4. A, 4B: Artist’s illustration and computed tomography scans of a descending thoracic aortic aneurysm that developed into a extent II thoracoabdominal aortic aneurysm after first graft replacement (A) and second graft replacement (B) with reattachment of lower intercostal arteries and reimplantation of the celiac axis, superior mesenteric, right and left renal arteries via separate interposition bypass grafts in a Marfan patient.
Postoperatively, the CSF drainage catheter is kept in place and the patient’s CSF pressure monitored for 3 days. CSF pressure is maintained below 10 mm Hg. Because of the association of arterial blood pressure and oxygen delivery to delayed neurologic deficit, we maintain MAP between 90 mm Hg and 100 mm Hg, hemoglobin above 10 mg/dl, and cardiac index above 2.0 L/min. Urine output is closely monitored. After 3 days, the CSF drainage catheter is removed, and the patient is transferred to the regular floor. If delayed neurologic deficit occurs after removal of the CSF drain, the patient is placed supine, CSF is drained freely for 72 hours, and the CSF pressure is kept below 10 mm Hg.
Statistical Analysis
Data were collected from chart reviews done by a trained nurse abstractor, and were entered into a dedicated Microsoft Access database. Data were exported to SAS for analysis, and all computations were performed using SAS version 8.02 running under Windows 2000. Univariate categorical data were analyzed using contingency table analyses. For 2 × 2 tables, common odds ratios with test-based confidence intervals were computed, and χ2 statistics are reported for hypothesis tests. For tables greater than 2 × 2, χ2 statistics were computed for hypothesis tests, and univariate logistic regression estimates were also computed keeping data in their native continuous distribution. P values and confidence intervals for continuous data are based on maximum-likelihood estimates. Multivariable logistic regression analysis was performed for adjusted analysis of risk factors for neurologic deficit. Univariate long-term survival analysis was computed by Kaplan-Meier method, and adjusted analysis was performed by multiple Cox regression. Number needed to treat was computed as the reciprocal of the risk difference for neurologic deficit. The null hypothesis was rejected at P < 0.05.
RESULTS
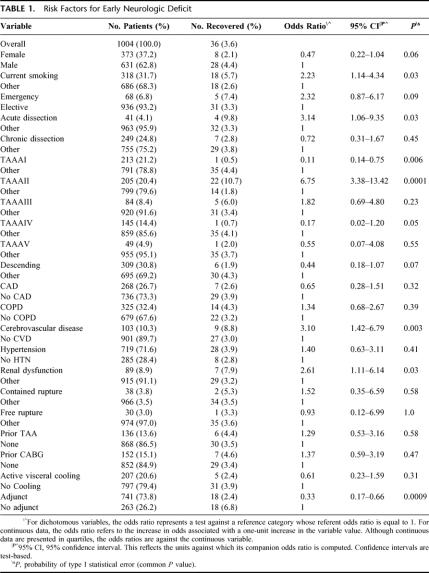
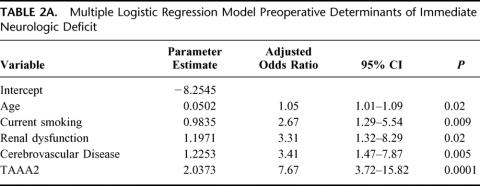
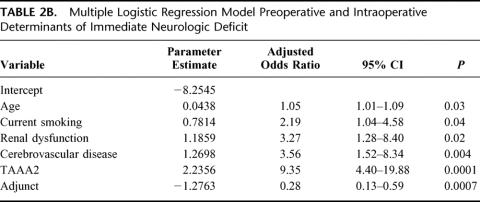
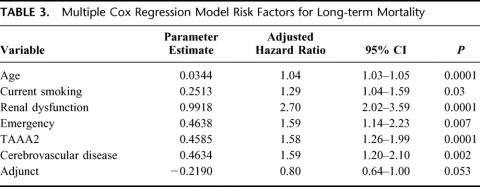
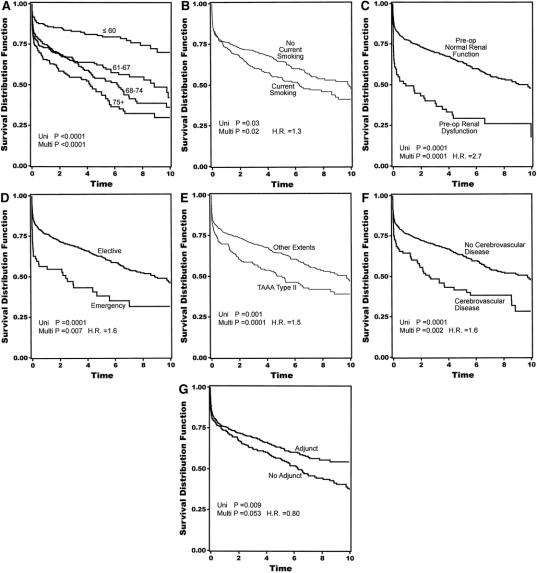
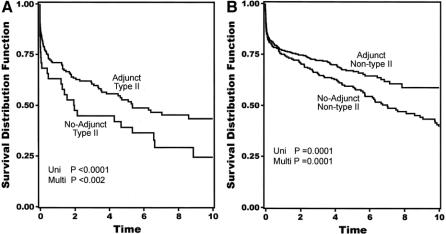
Overall 30-day mortality was 141 (14%) of 1004. Univariate analysis revealed current smoking, acute dissection, extent II TAAA, cerebrovascular disease, renal dysfunction, and the absence of adjuncts as risk factors in the development of neurologic deficits (Table 1). Extent I and extent IV TAAA also emerged as significant, but in the absence rather than development of neurologic deficits. Adjuncts played the most significant role overall (P < 0.0009), preventing 1 in 20 cases of neurologic deficit for all patients and 1 in 5 for extent II TAAA. Immediate neurologic deficit occurred in 36 (3.6%) of 1004 patients overall, and in 18 (2.4%) of 741 with adjuncts and 18 (6.8%) of 263 without adjuncts (P < 0.0009). Overall number needed to treat with adjunct to prevent a single paraplegia was 23. In high-risk extent II aneurysms, the numbers were 11 (6.6%) of 167 with adjunct, and 11 (29%) of 38 without. Number needed to treat with adjunct among extent II aneurysms was 5. In the multiple logistic regression models, divided between preoperative and intraoperative factors in Tables 2A and 2B, all factors except acute dissection remained significant. Age in this analysis emerged as significant. As for long-term mortality (Table 3), age, current smoking, renal dysfunction, emergency presentation, extent II, and cerebrovascular disease arose as significant predictors of death. Figures 5A to 5G show the effect of these same factors on long-term survival. As exhibited in the prevention of neurologic deficits, adjuncts also played a significant role in survival outcome (P < 0.002). Figures 6A and 6B show the effect of adjuncts on long-term survival for all extent II TAAA patients, and all patients other than extent II. The long-term survival results persisted after adjustment for age, extent II TAAA, and preoperative renal function.
TABLE 1. Risk Factors for Early Neurologic Deficit
TABLE 2A. Multiple Logistic Regression Model Preoperative Determinants of Immediate Neurologic Deficit
TABLE 2B. Multiple Logistic Regression Model Preoperative and Intraoperative Determinants of Immediate Neurologic Deficit
TABLE 3. Multiple Cox Regression Model Risk Factors for Long-term Mortality
FIGURE 5. (A-G) Kaplan-Meier actuarial plots showing the effect of selected risk factors on 10-year survival. Time in years on y-axis. H.R. = hazard ratio.
FIGURE 6. Kaplan-Meier actuarial plots showing the effect of adjuncts on 10-year survival in extent II (A) and nonextent II patients (B). Time in years on y-axis.
DISCUSSION
The last decade has witnessed an explosion in the number of available surgical adjuncts for protection of the spinal cord during descending thoracic and TAAA repair. In 1992, we settled on the combination of cerebrospinal fluid drainage and distal aortic perfusion and have used it ever since (74% of all cases). We were immediately impressed with the improvements in morbidity and mortality10 and suspected early on that these adjuncts had made a particularly striking difference in the rate of neurologic deficit for extent II TAAA.11
In the current study, we found that, as in our past studies, acute dissection, extent II TAAA, renal dysfunction, and the absence of adjuncts were the significant risk factors in the development of neurologic deficits. Although it is unclear why current smoking and cerebrovascular disease also emerged as significant. Aortic cross-clamp time and extent I TAAA, risk factors in the era of cross-clamp and go, were no longer significant to outcome. In our large patient group, the longstanding use of adjuncts has reduced the effect of clamp time to the degree that it is no longer an independent predictor of neurologic deficit risk. As far as statistical modeling, long clamp times are highly correlated with extent II TAAA and adjunct use, but produce such a high degree of multicollinearity in logistic models that the terms cannot all be modeled together. When the population was restricted to the adjunct group, clamp time was not a significant predictor of neurologic deficit risk.
Currently, the overall rate of neurologic deficit for all our patients operated with adjuncts is 2.4%, compared with 16% for patients operated without adjuncts during the era of cross-clamp and go.12 The reduced incidence of neurologic deficit for extent II TAAA has in fact been the most noticeable result of adjuncts with a current rate of 6.6% compared with a previous incidence of 31% for cross-clamp and go.12 Before the use of adjuncts, extent I was also considered high risk12, with a 15% incidence of neurologic deficit.
Our analysis of long-term survival emphasized the importance of elective operative repair, as rupture and acute dissection took their toll. Age was also important in long-term survival, with patients over 75 years of age most affected. Because adjuncts have eliminated all aneurysm types except extent II TAAA as risk factors in the development of neurologic deficit, we will in the future for the purpose of analysis look at the feasibility of simply dividing patients by extent II versus nonextent II. The appearance of cerebrovascular disease (stroke, transient ischemic attack, or previous intervention for carotid artery disease) as a risk factor for lowered long-term survival will also need to be addressed in the future. In the past decade, the adjuncts cerebrospinal fluid drainage and distal aortic perfusion have made a major impact on the incidence of neurologic deficit following thoracoabdominal aortic repair. In the next decade we will concentrate on developing strategies to improve organ protection and patient survival.
ACKNOWLEDGMENTS
The authors thank Amy Wirtz Newland for her editorial assistance and Carl Clingman for his illustrations.
Discussions
Dr. Richard P. Cambria (Boston, Massachusetts): Congratulations, Dr. Safi, on a great series. I am sure the members of the Association recognize that this is 1 of the largest, most carefully studied series and productive of some of the best results in the literature. We have talked about the problems in these patients many times, and I have a few questions about your cohort.
First, were the patients who were not managed without adjunct simply a function of time of operation, time sequence, or were there other factors such as urgency of operation that might have led against the use of adjuncts?
The second question is, I notice that you pointed out the powerful impact of emergency presentation on late survival. And, in fact, your survival curve showed that most of that difference was right up front. Yet it did not appear to come out as a predictor for early death or early neurologic deficit. Our own series, which we reviewed at this forum last year, and the other Houston series have repeatedly shown the powerful negative impact of urgent operation or something other than elective operation in the early results.
Dr. Hazim J. Safi (Houston, Texas): Thank you, Dr. Cambria.
With regard to whether we use the adjunct or not is a function of time - before September 1992 we used the clamp-and-go technique - as well as the emergency nature of the patient. The patient who presents to us as hemodynamically unstable, we can’t put a catheter in to drain the spinal cord fluid or use perfusion.
With regard to the emergency, and it didn’t come out as a risk factor for immunologic deficit but it is a risk factor for death. So most of the people are in a pine box, so you don’t have paraplegia. And our bias, that this patient told me they really impacted the long-term results in that way.
I didn’t know you were going to discuss or I would have sent you the transcript. I am sorry.
Dr. Larry H. Hollier (New York, New York): Dr. Safi, this is an excellent body of work and I think you are to be congratulated for documenting the efficacy of CFS drainage and visceral perfusion. I have several questions.
In the manuscript you talk about the incidence of neurologic deficit as being an immediate neurologic deficit rate of 3.6%. In my experience and certainly in that of most other authors who have presented their data, at least 50% of the neurologic deficits occur in a delayed fashion; that is, beyond 24 hours or so. Could you give us the incidence of late onset deficit in your series?
Secondly, you noted a reduced neurologic deficit with adjuncts versus no adjuncts and presumably without visceral perfusion. You also noted, however, that there was no difference in neurologic deficit if active visceral cooling was used or not used. I am assuming that they did not get any cooling at all except whatever might happen from the ambient temperature of the room. This would suggest to me that it is not the cooling aspect that is important in reducing morbidity but the visceral perfusion itself. Some of our own work has documented that simply the occurrence of visceral ischemia dramatically increases the incidence of neurologic deficit. You might want to comment on that.
Finally, you noted that late survival was reduced significantly, but you didn’t mention the impact of postoperative complications on late survival. For example, preoperative renal dysfunction might likely be associated with an increased incidence of postoperative dialysis. Paraplegia as a postoperative complication might change postoperative survival. Maybe you can put that into perspective for us.
Thank you very much. I enjoyed the paper.
Dr. Hazim J. Safi (Houston, Texas): Thank you, Dr. Hollier. We are grateful to you. You are the first 1 to ring the bell that the clamp-and-go technique is not the way to go, and you suffered a little bit about that.
With regard to the neurologic - said 3.6%, dropped from 16% at the LLF clamp-and-go, our bias position was adamant we should not put the delayed paraplegia on the paper that coming in The Journal of Vascular Surgery and also in The Journal of Cardiovascular and Thoracic Surgery. The delayed paraplegia in our hand is 2.4, where the patient would wake up moving everything a day, two, or three, and then they are paralyzed. And that is correlated to low hemoglobin, low blood pressure mean less than 60, as well as complication of CFF drainage catheter. So this is an important question.
With regard to the visceral perfusion, I think it did prevent renal failure. But it prevented liver dysfunction and it preserved the function of the small bowel. And you are absolutely right, visceral perfusion may be a factor in preventing neurologic deficit. We are prospectively analyzing, studying that matter with Dr. Fred Moore regarding the impact of visceral cooling on everything on the liver function, small bowel function and kidney function, as well as pulmonary function.
With late complication, you are absolutely right, people with a creatinine above two, the odds ratio of developing renal failure requiring hemodialysis over 70 times and their survival rate is not that great. When we talked about paraplegia and asked the question, the number was so small that we are making up a slide for you and for myself - refused to make me a graph for the survival because we have 36 cases and you can make it anyway you want. I think people with paraplegia don’t live long. The average life-span, I think, is 2 years.
Dr. Gregorio A. Sicard (St. Louis, Missouri): Dr. Safi, this is a great contribution. We always look to you for the large series and to sort out the problems and show us the way to improve the treatment of this complex vascular pathology.
I notice that in your drawings, and I have actually watched you do this operation, where you continue to use a fairly large patch, including on the type II thoracoabdominal aneurysms in which the area of the visceral and renal vessels are fairly far apart. We have gone to making the patches as small as possible. These patients live longer, and then you can have complications related to the patch - which obviously makes a secondary procedure very high risk. Will you comment on your experience with that and how many of these patients you have had to go back because of patch complications?
Dr. Hazim J. Safi (Houston, Texas): Thank you, Dr. Sicard. This is an excellent question.
Actually, Dr. Arkus, I brought him into the operating room, and he liked to make it big. I don’t know why. Your anastomosis has to be in the orifices of the viscera. And if the patient has, as you describe, they are wide apart, we use the ready-made graft with the side arm. They call it the octopus graft, commercially available. We no longer use the patch in patients with connective tissue disorder like - syndrome. We like to use the side arm grafts. Because the incidence of false aneurysm or this patch will get big is about 25% in our hands. So that is an important point.
Footnotes
Reprints: Hazim J. Safi, M.D., Professor and Chairman, Department of Cardiothoracic and Vascular Surgery, The University of Texas Health Science Center at Houston Medical School, 6410 Fannin Street, Suite 450, Houston, TX 77030. E-mail: Hazim.J.Safi@uth.tmc.edu
REFERENCES
- 1.Crawford ES, Crawford JL, Safi HJ, et al. Thoracoabdominal aortic aneurysms: preoperative and intraoperative factors determining immediate and long-term results of operations in 605 patients. J Vasc Surg. 1986;3:389-404. [DOI] [PubMed] [Google Scholar]
- 2.Cambria R, Davison J, Zannetti S, et al. Clinical experience with epidural cooling for spinal cord protection during thoracic and thoracoabdominal aneurysm repair. J Vasc Surg. 1997;25:241-243. [DOI] [PubMed] [Google Scholar]
- 3.Coselli JS, LeMaire SA. Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 1999;67:1931-1934. [DOI] [PubMed] [Google Scholar]
- 4.de Haan P, Kalkman CJ, Meylaerts SA, et al. Development of spinal cord ischemia after clamping of noncritical segmental arteries in the pig. Ann Thorac Surg. 1999;68:1278-1284. [DOI] [PubMed] [Google Scholar]
- 5.Griepp RB, Ergin MA, Galla JD, et al. Minimizing spinal cord injury during repair of descending thoracic and thoracoabdominal aneurysms: the Mount Sinai approach. Semin Thorac Cardiovasc Surg. 1998;10:25-28. [DOI] [PubMed] [Google Scholar]
- 6.Hollier LH, Money SR, Naslund TC, et al. Risk of spinal cord dysfunction in patients undergoing thoracoabdominal aortic replacement. Am J Surg. 1992;164:210-213. [DOI] [PubMed] [Google Scholar]
- 7.Schepens MA, Defauw JJ, Hamerlijnck RP, et al. Use of left heart bypass in the surgical repair of thoracoabdominal aortic aneurysms. Ann Vasc Surg. 1995;9:327-338. [DOI] [PubMed] [Google Scholar]
- 8.Kouchoukos NT, Daily BB, Rokkas CK, et al. Hypothermic bypass and circulatory arrest for operations on the descending thoracic and thoracoabdominal aorta. Ann Thorac Surg. 1995;60:67-76. [PubMed] [Google Scholar]
- 9.Acher CW, Wynn MM, Hoch JR, et al. Combined use of cerebral spinal fluid drainage and naloxone reduces the risk of paraplegia in thoracoabdominal aneurysm repair. J Vasc Surg. 1994;19:236-246. [DOI] [PubMed] [Google Scholar]
- 10.Safi HJ, Bartoli S, Hess KR, et al. Neurologic deficit in patients at high risk with thoracoabdominal aortic aneurysms: the role of cerebral spinal fluid drainage and distal aortic perfusion. J Vasc Surg. 1994;20:434-444. [DOI] [PubMed] [Google Scholar]
- 11.Safi HJ, Campbell MP, Miller CC 3rd, et al. Cerebral spinal fluid drainage and distal aortic perfusion decrease the incidence of neurological deficit: the results of 343 descending and thoracoabdominal aortic aneurysm repairs. Eur J Vasc Endovasc Surg. 1997;14:118-124. [DOI] [PubMed] [Google Scholar]
- 12.Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357-368. [PubMed] [Google Scholar]