Abstract
Objective:
To determine the percentages of major T lymphocyte subsets in the circulating peripheral blood mononuclear cell population in patients with major traumatic injury at early and late time points and to determine the expression of coreceptors and cytokine production by these T cell subsets.
Summary Background Data:
Prior studies suggest that serious injury in humans suppresses the adaptive immune system as revealed by diminished proliferation and altered cytokine production in response to polyclonal T cell activation. However, the contribution of individual cell types to this immune dysfunction has not been well characterized.
Methods:
The percentage of circulating CD4+ and CD8+ T cells and the relative density of CD4 and CD8 coreceptor expression was determined by flow cytometry in 17 consecutive trauma patients (injury severity score > 20) within 24 hours of injury and at day 7. Intracellular expression of the cytokines interleukin 2 (IL-2), interferon gamma (IFNγ), IL-4, and IL-10 were also studied after stimulation with bacterial superantigen (SEB). Patients were compared with age- and sex-matched controls and to themselves for differences between early and late cytokine expression.
Results:
The percentage of circulating CD4+ and CD8+ T cells was decreased versus controls at day 1 and further decreased by day 7 following injury. CD4 and CD8 cell surface expression was also decreased at days 1 and 7. CD4+ T cells in injured patients responded to SEB activation with decreased expression of IFNγ and IL-2 on day 1 versus controls (P < 0.05) and of all 4 cytokines by day 7 (P < 0.05), while CD8+ T cells showed diminished expression of IFNγ and IL-2 only at both time points. When day 1 and day 7 cytokine expression results were compared in the same patients, CD4+ T cells showed diminished expression of IFNγ, IL-2, and IL-4 by day 7 (P < 0.05), but maintained expression of IL-10. CD8 T cells showed diminished expression of IFNγ only.
Conclusions:
Severe injury induces a loss of circulating CD4+ and CD8+ T lymphocytes and diminished coreceptor expression by these cells. Both T cell subsets show progressive loss of immunostimulatory cytokine production with maintenance of potentially suppressive IL-10 production. These events may have negative consequences for host defense.
The percentages of circulating CD4+ and CD8+ T lymphocytes, the expression of CD4 and CD8 coreceptors on these cells, and the intracellular expression of IFNγ, IL-2, IL-4, and IL-10 by these T cell subsets after stimulation with bacterial superantigen were determined by flow cytometry at 1 and 7 days after major traumatic injury in 17 patients. Patients showed progressive diminution in the percentage of circulating T cells and intracellular pro-inflammatory cytokines during the first week after injury. Expression of intracellular IL-10 was maintained.
There is abundant evidence that serious injury, both thermal and traumatic, results in perturbations of both the innate and adaptive immune systems.1-3 Clinical observations and studies in appropriate animal models of injury have shown both activation and suppression of cells of the innate immune system following injury, with the majority of reports suggesting a protracted innate immune activation with production of pro-inflammatory mediators beginning shortly after injury.1-6 Most human and animal studies have demonstrated suppression of the adaptive immune system following major injury, reaching a nadir several days to more than a week following the initiating injury.6-13 The leading cause of death in seriously injured patients who survive initial resuscitation is the multiple organ dysfunction syndrome (MODS), usually associated with sepsis.1 Thus, injury-induced perturbations of the innate and adaptive immune systems have been the focus of a number of laboratories with the goal of defining mechanisms underlying the ineffective and/or inappropriate response of injured patients to pathogenic microorganisms.
A number of published reports have suggested that diminished capacity to produce the immunostimulatory cytokines interleukin-2 (IL-2) and interferon gamma (IFNγ) by T cells of the adaptive immune system is associated with diminished resistance to sepsis following serious injury.4-6,8,10-15 Diminished IL-2 and IFNγ production in some reports has been accompanied by increased production of the potentially immunosuppressive cytokines IL-4 and IL-10 by T cells, or possibly by cells of the innate immune system.5,11,15-20 However, in most published human and animal injury research, T cell cytokine production has been measured after in vitro exposure of populations of mononuclear leukocytes from the circulating blood or secondary lymphoid organs to polycloncal T cell activators, such as plant lectins, anti-T cell receptor antibodies, or phorbol esters plus calcium ionophores. There are at least 3 potential problems with such studies. (1) The results obtained are to a considerable degree dependent on the stimulus used, as Cavaillon and coworkers have pointed out,21,22 possibly related to the fact that the activating agents vary in their requirements for the participation of accessory cells from the innate immune system for optimal T cell activation. (2) The percentage of T cells in the responding mononuclear cell population has not been determined in the majority of reports; therefore, observed alterations in cytokine production might reflect changes in the percentage of T cells present rather than the capacity for cytokine production by the T cell population, to say nothing of the fact that the cell types producing the cytokines have not been clearly identified. (3) The stimulus used in most of the reported studies is clearly nonphysiologic, in that T cells ordinarily are activated in vivo by cognate antigen presented by cells of the innate immune system along with costimulatory signals and/or cytokines, again supplied by the antigen-presenting cells (APCs).23,24
In a few reported studies of burn and trauma patients, intracellular T cell cytokine expression has been assessed after maximal polyclonal activation with a phorbol ester and a calcium ionophore, which tests maximal cytokine production potential without the necessity for T cell receptor signaling. In a study of trauma patients, no overall alterations in T cell intracellular cytokine expression were noted; in those patients who had increased IL-4 expression, no relationship with outcome could be discerned.25 In a burn study, alterations in T cell cytokine expression were noted principally in the CD8+ T cell subset, where increased IL-4 expression was detected several days following burn injury.26
In the present study, we sought to clarify the effect of major traumatic injury on circulating T cell numbers, coreceptor expression, and cytokine production as indicated by intracellular cytokine staining of both CD4+ (helper) and CD8+ (cytotoxic) T cells after stimulating circulating mononuclear cells with a bacterial superantigen with the capacity to activate approximately 20% of T cells when presented by MHC II molecules displayed on APCs, and when the APCs also deliver appropriate costimulatory signals.27-30
MATERIALS AND METHODS
Patients
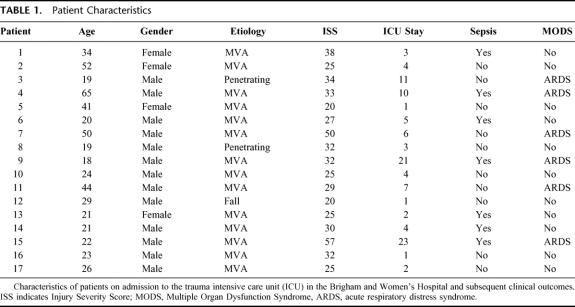
Twenty-milliliter venous blood samples were obtained from 17 consecutive adult trauma patients with ISS > 20 who were admitted to the Burn/Trauma Intensive Care Unit at the Brigham and Women’s Hospital and from whom informed consent could be obtained. Blood samples in all cases were drawn within the first 24 hours after injury and in 12 patients at 7 days after injury as well. On each occasion, blood was simultaneously drawn from an age- and sex-matched normal volunteer from a group of 12 who served as controls. The study was carried out in compliance with NIH guidelines and with the approval of the Human Research Committee of Partners HealthCare System, of which the Brigham and Women’s Hospital is a member. Patients were observed for signs of systemic inflammation (SIRS) using established criteria,5 sepsis [SIRS plus signs of invasive infection with culture of a pathogenic organism(s)], blood transfusions, organ dysfunction, and length of intensive care unit (ICU) stay. Patients with major head trauma were not included in the study. Control and patient blood samples were processed simultaneously. The demographics of the patient population along with initial ISS scores are listed in Table 1.
TABLE 1. Patient Characteristics
Reagents
Staphylococcal Enterotoxin B (SEB) and Brefeldin A were obtained from Sigma-Aldrich (St. Louis, MO). Culture medium for in vitro studies consisted of RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum, 1 mm glutamine, penicillin/streptomycin/fungizone, 10 mM HEPES buffer, 100 μM nonessential amino acids, and 2.5 × 10-5 M 2-mercaptoethanol, all purchased from Gibco Invitrogen Corporation (Grand Island, NY).
Blood Collection and Processing
Twenty-milliliter blood samples were collected in heparinized VACUTAINER CPT tubes (Becton Dickinson, Franklin Lakes, NJ). A control blood sample was drawn simultaneously. All CPT tubes were centrifuged for 30 min at 1500 × g within 1 hour of collection. The peripheral blood mononuclear cells (PBMC) interface was carefully removed by pipetting and was washed once in culture medium by centrifugation for 15 min at 300 × g. PBMC were resuspended in culture medium and counted with trypan blue for viability. PBMC were always >95% viable. Both patient and control PBMC were adjusted to a concentration of 5 × 106 cells/ml of culture medium.
T cell Phenotyping
Peripheral blood mononuclear cells from trauma patients and sex- and age-matched controls were incubated for 15 min at 4°C with human male AB+ serum (United States Biologic, Swampscott, MA) to prevent nonspecific binding with Fc receptors, then incubated for 20 min with fluorescein isothiocyonate (FITC)-labeled anti-CD4 or anti-CD8 antibody along with relevant isotype controls (Pharmingen, San Diego, CA), and fixed for 20 min with 100 μL of 2% paraformaldehyde in PBS (pH 7.4) at 4°C. One hundred thousand events were collected by flow cytometry, with gating based on forward versus side scatter using a FACScaliber instrument (Becton Dickinson, Mountain View, CA) and CD4+ and CD8+ T cell percentages and CD4 and CD8 mean fluorescence intensity were analyzed by the accompanying CELLQuestPro computer software program.
In Vitro Stimulation and Intracellular Cytokine Expression of T lymphocyte Subsets
Peripheral blood mononuclear cells in 200 μL volume were incubated in 96-well plates (Costar, Corning, NY) with 1 μg/ml of SEB or with culture medium alone at 37°C in 5% CO2 for 48 hours. For the final 6 hours of the incubation, brefeldin A (5 μg/ml) was added. Culture medium was then removed and, as described above, cells were incubated with human male AB+ serum (United States Biologic, Swampscott, MA) to prevent nonspecific binding with Fc receptors, labeled with FITC-conjugated anti-CD4 or anti-CD8 antibody (Pharmingen, San Diego, CA) for 20 min, and fixed for 20 min with 100 μL of 2% paraformaldehyde in PBS (pH 7.4) at 4°C. Following fixation, cells were washed once and then resuspended in 100 μL of permeabilization buffer (PBS, pH 7.4; 0.1% saponin; 1% BSA; 0.1% sodium azide) for 20 min at room temperature. Nonspecific antibody binding was blocked by pretreating the fixed and permeabilized cells with 25 μL of a 1 μg/ml solution of normal mouse and rat IgG (CALTAG Laboratories, Burlingame, CA) for 15 min. Cells were then stained with phycoerythrin (PE)-labeled antibodies specific for IL-2, IL-4, IL-10, or IFNγ and relevant isotype controls (Pharmingen, San Diego, CA) for 30 min. Intracellular cytokine expression was assessed via flow cytometry as noted above.
Statistics
Observations on patients at days 1 and 7 were compared by paired t test, as were observations made on patients and simultaneously processed controls. The χ2 test was used to evaluate differences in the incidence of complications between patient groups. The Prism 3.0 software (GraphPad, San Diego, CA) was used for all calculations. P < 0.05 was considered significant.
RESULTS
Circulating Mononuclear Cells
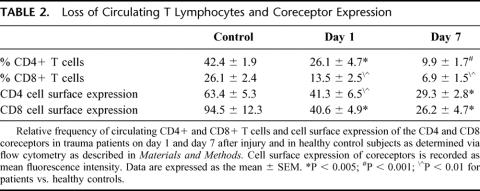
As shown in Table 2, the percentage of circulating CD4+ and CD8+ T cells in the PBMC population was significantly diminished in comparison with simultaneously studied controls on day 1 after injury. There was a further diminution in the percentages of both T cell populations on day 7. This was associated with a relative monocytosis and the appearance of immature myeloid cells within the PBMC population in patients, but not in controls. Similarly, there was diminished expression of the CD4 and CD8 coreceptors on the surface of circulating T cells on day 1 after injury in comparison with controls. Again, there was a further diminution of surface expression of these molecules by day 7.
TABLE 2. Loss of Circulating T Lymphocytes and Coreceptor Expression
In the aggregate, these studies indicate that there is a significant reduction in the percentages of circulating CD4+ and CD8+ T cells by day 1 after serious traumatic injury, and that there is a further diminution in the percentages of these cells in the PBMC population by day 7. In the remaining CD4+ and CD8+ circulating T cell populations, there is also a significant decrease in the surface density of these coreceptors by day 1 with a further decrease by day 7.
T cell Cytokine Expression
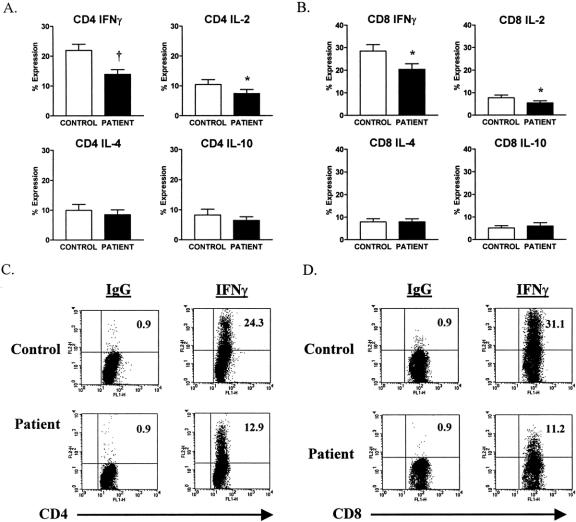
In Figure 1, the 17 patients are compared with controls on day 1 with respect to intracellular cytokine expression. It is apparent that the patients as a group showed diminished CD4+ T cell expression of IL-2 and IFNγ, but not IL-4 and IL-10 on day 1 when compared with controls. The same was true for CD8+ T cells. In Figure 1, representative fluorescence activated cell sorter (FACS) plots of intracellular expression of IFNγ by CD4+ and CD8+ T cells from a patient and a simultaneously studied normal control individual on day 1 after injury are shown. It is apparent that there is considerably less IFNγ expressed in the patient CD4+ and CD8+ T cells than in control cells. Background staining with isotype control antibody was consistently less than 1%, expressed as 0.9.
FIGURE 1. Decreased IFNγ and IL-2 expression in CD4+ and CD8+ T cells from trauma patients versus control subjects on day 1 after injury. (A and B) Decreased SEB-induced IFNγ and IL-2 but not IL-4 nor IL-10 intracellular expression in CD4+ and CD8+ T cells from patients, compared with control subjects. Data are expressed as the mean ± sem. *P < 0.05; †P < 0.001 for patients versus control subjects. (C and D) FACS plots of SEB-induced intracellular IFNγ expression and IgG isotype control in the gated T cell subsets. Results are representative of a trauma patient compared with a healthy control subject. The value shown in the upper right quadrant indicates the percentage of cytokine-positive cells.
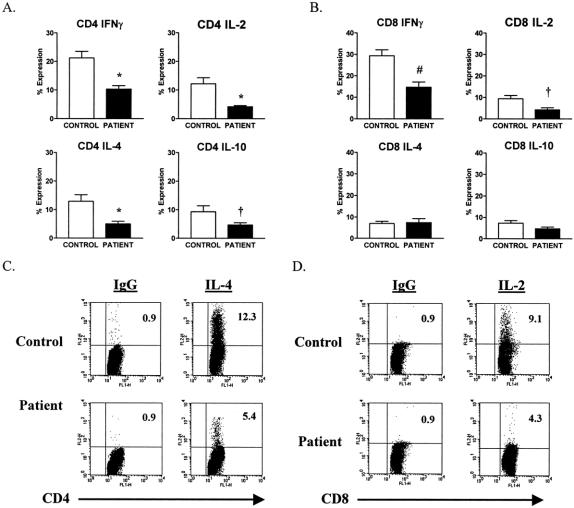
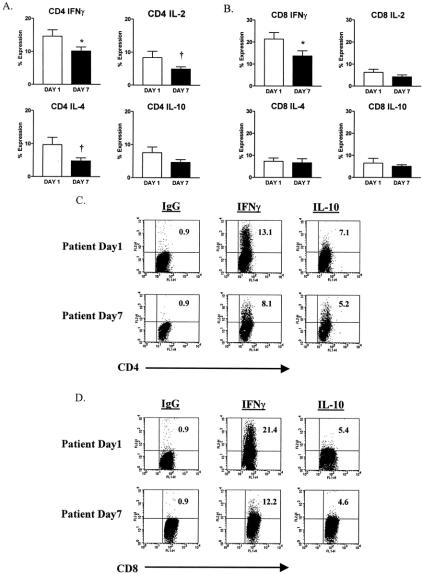
By day 7, CD4+ T cells showed significantly reduced expression of all 4 cytokines when compared with controls (Fig. 2), whereas CD8+ T cells showed diminished expression of IL-2 and IFNγ, but not IL-4 and IL-10 at the 7-day interval. As shown in Figure 2, by day 7 after injury, CD4+ T cells from a representative patient showed significantly diminished expression of IL-4 when compared with a simultaneously studied control, while CD8+ T cells from the same patient had reduced expression of IL-2.
FIGURE 2. Decreased TH1 and TH2 cytokine expression in CD4+ T cells but decreased TH1 cytokine expression only in CD8+ T cells from trauma patients compared with control subjects on day 7 after injury. (A) Decreased SEB-induced IFNγ, IL-2, IL-4, and IL-10 intracellular expression in CD4+ T cells from patients compared with control subjects. (B) Decreased SEB-induced IFNγ and IL-2 but not IL-4 nor IL-10 intracellular expression in CD8+ T cells from patients compared with control subjects. Data are expressed as the mean ± sem. †P < 0.05; *P < 0.01; and #P < 0.001 for patients versus control subjects. (C and D) FACS plots of SEB-induced IL-4 and IL-2 intracellular expression and IgG isotype control in the gated T cell subsets. Results are representative of a trauma patient compared with a healthy control subject. The value shown is the upper right quadrant indicates the percentage of cytokine-positive cells.
When comparison was made between the expression of intracellular cytokines by patients on day 1 versus day 7, it was apparent, as shown in Figure 3, that CD4+ T cells had significantly reduced expression of IFNγ on day 7, as compared with day 1. The same was true for CD8+ T cells. CD4+ T cells, but not CD8+ T cells, also showed significantly diminished expression of IL-2 and IL-4 at day 7 versus day 1. Neither T cell subpopulation showed a significant decrease in IL-10 expression on day 7, as compared with day 1. FACS plots of IFNγ and IL-10 expression by CD4+ and CD8+ T cells from a representative patient on days 1 and 7 are shown in Figure 3. Taken as a whole, these observations indicate that both CD4+ and CD8+ T cells show diminished expression of the immunostimulatory cytokine IFNγ by day 7 after injury, as compared with day 1. However, the same cells show no significant loss of the ability to express the suppressive cytokine IL-10 at the same time interval.
FIGURE 3. Decreased TH1 intracellular cytokine expression but not IL-10 expression in CD4+ and CD8+ T cells by trauma patients on day 7 compared with day 1 after injury. (A) Decreased SEB-induced IFNγ, IL-2, and IL-4 but not IL-10 intracellular expression in CD4+ T cells from patients on day 7 compared with day 1. (B) Decreased SEB-induced IFNγ and IL-2 but not IL-4 nor IL-10 intracellular expression in CD8+ T cells from patients on day 7 compared with day 1. Data are expressed as the mean ± sem. †P < 0.05; *P < 0.01 for patients on day 1 versus patients on day 7. (C and D) FACS plots of SEB-induced IFNγ and IL-10 intracellular expression and IgG isotype control in the gated T cell subsets and are representative of a trauma patient on day 1 compared with day 7. The value shown is the upper right quadrant indicates the percentage of cytokine-positive cells.
Correlation of Clinical Outcome With Circulating t cell Numbers and Cytokine Expression
Fifteen of the 17 patients met the criteria for SIRS early after injury. All patients received at least 1 blood transfusion. As noted in Table 1, 7 patients developed septic complications and 6 developed evidence of organ failure; in each instance, the acute respiratory distress syndrome (ARDS) was diagnosed by consensus conference criteria.31 There was no patient mortality. Eight patients had initial ISS scores of <30. Two patients in this group developed sepsis, and 1 developed ARDS. Nine patients had initial ISS scores of 30 or greater, and among this group 5 developed sepsis (3 with concomitant ARDS) and 2 developed ARDS only. The difference in the incidence of sepsis between these 2 patient groups was not significant by the χ2 test (P > 0.3). However, the incidence of ARDS was significantly higher in the patients with ISS > 30 (P < 0.04).
All patients who could not take adequate oral nourishment were placed on supplemental enteral nutrition to ensure an adequate calorie intake by way of the gastrointestinal tract. Similarly, all patients were carefully monitored for hyperglycemia, which was rigorously controlled by intravenous insulin infusion. It seems unlikely that either nutritional deficiency or hyperglycemia contributed to the suppressed proinflammatory cytkokine production noted in these patients. We did not measure catecholamine levels in this patient population, and it is possible that these mediators played a role in the results observed, particularly those recorded on day 1.
We could establish no correlation between absolute percentages of circulating CD4+ or CD8+ T cells, initial T cell cytokine expression, or the magnitude of the loss of IFNγ expression with the occurrence of either sepsis or ARDS in this patient population. On the other hand, all patients had diminished circulating T cell percentages versus controls, and nearly all patients demonstrated diminished IFNγ expression and stable IL-10 expression by day 7, as compared with the first 24 hours after injury. Only 2 patients failed to show appreciable (>30%) loss of IFNγ expression by day 7 in 1 or both T cell subsets, and only 1 showed a substantial (>30%) decrease in IL-10 expression by both T cell subsets on that day. The 1- and 7-day comparison data were admittedly somewhat skewed in this study, because patients who did not have a 7-day blood sample drawn were more likely to have had an uncomplicated hospital course. Indeed, only 1 of these 5 patients developed a septic complication, and none developed ARDS. Three of the 5 had initial ISS scores of <30. On the other hand, initial CD4+ and CD8+ T cell intracellular IFNγ expression in these 5 patients ran the gamut of that seen in the entire population, ranging from a low of 2.9% to a high of 30.1%.
Age also did not correlate with either outcome or IFNγ expression in this patient population. As noted in Table 1, 4 patients in the group were older than 40 years of age. Two of these patients developed sepsis, 1 with concomitant ARDS. The other 2 had a complication-free course. Initial IFNγ expression ranged from a low of 5.6. to a high of 31 in these older patients. IFNγ expression in both CD4+ and CD8+ T cells of the oldest patient in the group was within the error of the mean for the patient population as a whole on both day 1 and day 7.
DISCUSSION
The present studies support previous observations that the percentage of T cells in the circulating PBMC population is diminished after serious traumatic injury,1,10,25,34 and they also indicate that this proportionate loss of T cells is progressive over the first 7 days following injury and involves both CD4+ (helper) and CD8+ (cytotoxic) T cells. The explanation for this phenomenon is unclear at present. While there is ample evidence that sepsis is accompanied by extensive T lymphocyte apoptosis in human and animal models,32,33 the evidence that injury per se induces marked T cell apoptosis is lacking.33 There are reports of an increased percentage of circulating apoptotic mononuclear cells in injured patients,34,35 but these studies have not clearly established the cell types involved in this process. It therefore remains entirely possible that the diminished percentage of T cells found in the PBMC population of the trauma patients in the present study results from the dilutional effect caused by a relative monocytosis coupled with the appearance of immature myeloid forms in patient PBMC or migration of circulating T cells into the site of injury or into secondary lymphoid organs or a combination of these events.
The results of the present study generally support conclusions reached in prior reports from our own group and other investigators who have studied PBMC cytokine production in injured patients following in vitro stimulation with polyclonal T cell activators,4,8-10,13,14 namely that T cells following injury are less capable of producing the immunostimulatory cytokines IL-2 and IFNγ than simultaneously studied normal controls and that the loss of capacity to produce these cytokines is progressive for several days. The concordance between the present study of intracellular cytokine expression and prior studies of cytokine production is perhaps surprising, because most of the prior studies of T cell cytokine production in injured patients have not taken into account the relative percentage of T cells in the PBMC population studied. The present results further indicate that CD4+ T cells have a more profound diminution in stimulatory cytokine production after injury than CD8+ T cells, though there was a significant loss of IFNγ producing capacity in both T cell subsets by day 7.
The present studies do not completely support findings in some prior studies of cytokine release following polyclonal T cell stimulation of PBMCs from injured patients3,13,15,17, in that increased IL-4 and IL-10 expression at day 7 following injury was uncommon in the trauma patients reported here. On the other hand, IL-10 expression was maintained by both CD4+ and CD8+ T cells on day 7 in the present study, while IFNγ expression was significantly decreased, thus suggesting that the circulating T cells had assumed a more suppressive cytokine production profile.
The present studies made use of superantigen stimulation of patient and normal PBMCs as a potentially more physiologic activation signal for T cells than polyclonal activators, such as plant lectins, monoclonal anti-T cell receptor antibodies, or the use of a phorbol ester and a calcium ionophore. Though superantigens, like conventional antigens, require presentation by MHC II molecules on APCs, they can activate approximately 20% of a normal human T cell population, which express the appropriate Vβ chain of the T cell receptor.27-30 Thus, an immediate assessment of antigen-induced T cell cytokine production can be made without the necessity to wait for clonal expansion of the responding cell population, which would be required after activation with a conventional antigen. T cell responses to superantigens, as is the case with conventional antigens, ordinarily require appropriate costimulatory signals, as well as antigen presentation by APCs.22-30 Thus, it seemed that the use of superantigen stimulation would give an insight into the T cell cytokine response, which could be anticipated in vivo after exposure to antigenic products of invading microorganisms.
On the other hand, bacterial superantigens are known to bias T cells toward the production of proinflammatory type I cytokines,29,30 and it is of considerable interest that the circulating T cells in the injured patients in the present study had, in general, a progressive loss of type I cytokine expression (eg, IFNγ) and a maintenance of type II (eg, IL-10) cytokine expression over the first 7 days after injury.
The present results require comparison with 2 recent publications studying the expression of intracellular cytokines by circulating T cells in patients with serious traumatic injury, as reported by Wick et al,25 and in patients with major burn injury studied by Zedler et al.26 In both studies, T cell cytokine expression was measured after in vitro stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. In the study by Wick et al,25 only expression of IL-2 and IL-4 were measured and were used as markers for T helper 1 (TH1) and T helper 2 (TH2) cells in the circulating PBMC population. No measurements were made of IFNγ, considered by many investigators to be the signature cytokine of TH1 cells.23 In concert with the present results, Wick et al25 found that there was a highly significant decrease in the percentage of total circulating T (CD3+) cells. They also found a decrease in IL-2 expression, which did not quite reach statistical significance in the first week after injury. As was the case with CD8+ T cells in the present study, the percentage of IL-4 expressing T cells did not change during this time. In the study by Zedler et al,26 the burn patients showed an increase in CD8+ T cell expression of IL-4 on day 5 after injury, as compared with control individuals. The same cell population also expressed increased IFNγ. These authors did not show any decrease in the percentage of CD4+ cells after burn injury, though the percentage of CD8+ cells was significantly diminished. There are obvious differences in methodology between the present study and those of Wick et al25 and Zedler et al,26 in that IFNγ and IL-10 were not measured in the former study and IL-10 expression was not measured in the latter. The activation stimulus was also clearly different, in that PMA and ionomycin are generally considered a maximal stimulatory signal for T cells and bypass the necessity for T cell receptor engagement. In the present study, we have used antigen driven T cell activation, which we believe more closely mimics physiologic T cell activation in vivo. On the other hand, both the present study and the 2 reports discussed above indicate continued or increased IL-4 expression by some T cell populations during the course of the first week after injury. The somewhat different findings with regard to T cell numbers and CD8+ T cell cytokine expression in the burn patients studied by Zedler et al26 and the trauma patients studied here, might very well reflect an inherent difference in T cell responses to the 2 different forms of injury. However, because of methological differences between the 2 studies, this explanation remains speculative.
In the present study, the diminished expression of IFNγ and continued expression of the suppressive cytokine IL-10 by both CD4+ and CD8+ T cell populations a week after serious injury, could not be correlated with patient outcome, because all but 2 patients showed clearly (>30%) diminished IFNγ expression by day 7 and only 1 patient demonstrated diminished (30%) IL-10 production by both T cell subsets on that day. We were also unable to establish a significant correlation between the degree of alteration of intracellular cytokine expression and the degree of injury, the age of the patient, or the incidence of sepsis. This may represent a type II error caused by the modest size of the study population. On the other hand, in this population of seriously injured patients with uniformly depressed T cell function, the occurrence of sepsis may be a stochastic event determined more by the type and quantity of pathogenic microorganisms encountered by the individual patient than by subtle differences in the degree of cellular immune dysfunction. However, the occurrence of ARDS in this study population was significantly correlated with the severity of injury. Patients with an initial ISS > 30 had a higher incidence of this complication, suggesting that factors other than a diminished capacity for T cell cytokine production are important in the development of post injury ARDS.
We would conclude that following major traumatic injury there is a reduction in circulating CD4+ and CD8+ T lymphocytes and a diminished expression of CD4+ and CD8+ molecules on circulating T cells. Furthermore, both CD4+ and CD8+ T cells show decreased IFNγ expression by day 7 after injury when compared with day 1. IL-4 expression remains unchanged in CD8+ cells, though it is diminished in CD4+ cells. IL-10 expression remains unchanged in both T cell populations by day 7. The loss of immunostimulatory cytokine production and maintenance of inhibitory IL-10 production at day 7, along with loss of circulating T cell numbers, is likely to have negative consequences for host defense.
Discussions
Dr. Basil A. Pruitt, Jr. (San Antonio, Texas): These investigators have used staphylococcal enterotoxin B (SEB) as the antigenic stimulus to assess T-cell cytokine expression in what they consider to be a more physiologic fashion than with lectins or phorbol esters. That test system produced results that are somewhat unexpected since bacterial superantigens typically bias T-cells to produce pro-inflammatory Type I cytokines and they actually observed progressive loss of interferon gamma, which is the index product of TH-1 cells, and maintenance of IL-10, which is an index cytokine of TH-2 cells.
Since we know that in severely injured patients MHC Complex II molecules are decreased on monocytes and that superantigens, as you note, require presentation by MHC II molecules on APCs, could a decrease in MHC II molecules account for the changes you observed?
You have previously described a reciprocal relationship between IL-12 and IL-10 in injured patients. Do these studies represent further confirmation of that relationship or was IL-12 production maintained at irordinately high levels when assayed in the SEB system?
You have also previously shown that in IL-10 deficient mice TNF alpha levels are chronically elevated and as such can down-regulate T-cell activation and function. Does persistent elevation of TNF alpha levels explain the greater decrease of Type I cytokine production in CD4 cells at day 7 when IL-10 production was also decreased with respect to the controls?
Other factors that can influence leukocyte number and function include catecholamines and nutrition. Did the circulating level of catechols or the level of nutritional intake change in systematic fashion over the seven-day study period?
It is particularly troublesome that the observed changes didn’t correlate with clinical status and that the degree of alteration of intracellular cytokine expression couldn’t be related to sepsis or ARDS. You suggest that such may represent a Type II error but it may simply be the consequence of patient heterogeneity in terms of age, which ranged from 18 to 56 years, and ISS, which ranged from 20 to 57. If you plot cytokine production in response to SEB against age or ISS, does the alteration of T-cell function increase as those variables increase?
Lastly, you suggest that the use of SEB as the T-cell stimulant mimics the in vivo T-cell response to invading microorganisms. That raises the interesting possibility that the differences in cytokine response at day 1 and day 7 may represent an endotoxin-like tolerance to SEB induced by infection in these patients in the six-day interval between those measurements. To evaluate that possibility, can you tell us how many of the patients had a positive blood culture during the study period and whether the changes in cytokine response differed depending on the type of organism recovered; that is, gram negative or gram positive?
Dr. Thomas Murphy (Boston, Massachusetts): Thank you very much for those insightful comments and questions.
First, with regard to costimulatory molecules, particularly MHC Class II, yes, they are down-regulated after injury. Whether this contributed to the results we presented, I would think not. A critical loss of costimulatory molecules would be expected to prevent activation of both CD4 and CD8 T-cells, and clearly that is not what we are seeing in these patients.
Injured patients’ T-cells do show diminished Type I cytokine expression; however, their expression of the Type II cytokines, IL-4 and IL-10, is maintained. So these T-cells are not unresponsive to antigens.
Looking at IL-12 production, yes, we have previously demonstrated that IL-12 production by PBMC from injured patients is diminished at about 1 week after injury. This may well contribute to loss of Th-1 cytokine production. We did not measure TNF alpha in these experiments.
Catecholamines - this is why we excluded the patients with major head trauma. Patients with head trauma, as we all know, have increased intracranial pressure and have an ongoing catecholamine surge. There have been published papers showing that increased levels of catecholamine will drive a Th-2 type response. It is certainly possible that catecholamines played a role in the results observed, particularly early after injury. We did not measure catecholamines in these patients.
We have looked at age and sex as variables and in this modest-sized cohort of patients we could see no obvious effects on cytokine responses or the development of subsequent septic events.
Did our findings in some patients represent a form of gram negative endotoxin tolerance? There were 7 patients who had proven sepsis. There were 4 pneumonias, 2 patients with abdominal sepsis, and 1 wound sepsis. This broke down to 4 cases of gram positive sepsis and 3 cases of gram negative sepsis. Most septic events occurred at or beyond 7 days. In any case, the type of organism did not appear to influence the T-cell cytokine profile.
Dr. Anthony A. Meyer (Chapel Hill, North Carolina): I want to thank you for the opportunity to hear your presentation on the very challenging work of trying to do these studies on patients rather than experimental animals. In our institution, where we have been studying this in an animal model, our findings in CD8 cells noted there was really not a diminution. But again, there are many differences in a very controlled system compared with yours.
I just want to ask, were there any concomitant issues of management such as nutritional assessment, maintenance of nutrition and/or blood transfusions that occurred to try to sort out some of the differences that you see in your patient population compared with what we see in a very tightly controlled animal system looking at injury?
Dr. Thomas Murphy (Boston, Massachusetts): The mean number of blood transfusions was less 3 units in these patients, though all patients had at least 1 transfusion. Nutritional status was carefully monitored in these patients who received enteral feeding if p.o. intake was in any way inadequate. The mean age of these patients was 24 years. They were mostly healthy young males, a typical trauma population.
Dr. Charles E. Lucas (Detroit, Michigan): I rise not to confuse the faithful membership at this late hour about alphabet soup, but to ask a philosophical question.
Most patients with ISS 34 who have had an average of 3 blood transfusions by day 7 are getting ready for discharge and organ function has been restored to normal unless you have some long bone fractures or you have supervening sepsis.
Most of your patients and most of everyone’s patients in that category of injury are about ready for discharge, have you have considered that your findings may be totally unrelated to the immune response? Are these measurements the overall gold standards for measuring the immune response?
Dr. Thomas Murphy (Boston, Massachusetts): Most patients, you are right, are ready for discharge at a relatively early time point. Five patients did not have the 7-day measurements because of very early discharge. There is a subset of patients that are prone to sepsis after injury. Approximately 40% of patients develop this complication.
We cannot find any correlation in this study between T-cell cytokine expression and the development of septic events or organ failure. It is possible that patients sepsis is simply a random event after serious injury due to the dose and type of organisms encountered in the setting of generalized immune depression. We don’t know. But I think it is still important to do this research on patients, because there is a significant proportion of seriously injured patients that will develop septic complications and organ failure.
Footnotes
Reprints: John A. Mannick, MD, Brigham and Women’s Hospital, 75 Francis St., Boston, MA 02115.
Supported in part by USPHS grant GM35633-17, the David Brook Fund for Surgical Research, and the Julian and Eunice Cohen fund for Surgical Research.
REFERENCES
- 1.Deitch EA. Multiple organ failure, pathophysiology and potential future therapy. Ann Surg. 1992;216:117-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore FA, Sauaia A, Moore EE, et al. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501-512. [DOI] [PubMed] [Google Scholar]
- 3.Mannick JA, Rodrick ML, Lederer JA. The immunologic response to injury. J Am Coll Surg. 2001;3:237-244. [DOI] [PubMed] [Google Scholar]
- 4.Livingston DH, Appel SH, Wellhausen Sr, et al. Depressed interferon gamma production and monocyte HLA-DR expression after severe injury. Arch Surg. 1988;123:1309-1312. [DOI] [PubMed] [Google Scholar]
- 5.Bone RC. Towards and epidemiology and natural history of SIRS (Systemic Inflammatory Response Syndrome). JAMA. 1992;268:3452-3455. [PubMed] [Google Scholar]
- 6.Schwacha MG, Schneider CP, Chaudry IH. Differential expression and tissue compartmentalization of the inflammatory response following thermal injury. Cytokine. 2002;17:266-274. [DOI] [PubMed] [Google Scholar]
- 7.Munster AM, Eurenius K, Katz RM, et al. Cell-mediated immunity after thermal injury. Ann Surg. 1973;117:139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood JJ, Rodrick ML, O’Mahony JB, et al. Inadequate interleukin 2 production: a fundamental immunological deficiency in patients with major burns. Ann Surg. 1984;200:311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrick ML, Wood J, O’Mahony JB, et al. Mechanisms of immunosuppression associated with severe nonthermal traumatic injuries in man: production of interleukin 1 and 2. J Clin Immunol. 1986;6:310-318. [DOI] [PubMed] [Google Scholar]
- 10.Faist E, Kupper TS, Baker CC, et al. Depression of cellular immunity after major injury. Its association with posttraumatic complications and its reversal with immunomodulation. Arch Surg. 1986;121:1000-1005. [DOI] [PubMed] [Google Scholar]
- 11.Mack VE, McCarter MC, Naama HA, et al. Dominance of T-helper 2-type cytokines after severe injury. Arch Surg. 1996;131:1303-1308. [DOI] [PubMed] [Google Scholar]
- 12.Kox WJ, Bone RC, Krausch D, et al. Interferon gamma-1b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: proof of principle. Arch Intern Med. 1997;157:389-393. [PubMed] [Google Scholar]
- 13.Mokart D, Capo C, Blache JL, et al. Early postoperative compensatory anti-inflammatory response syndrome is associated with septic complications after major surgical trauma in patients with cancer. Brit J Surg. 2002;89:1450-1456. [DOI] [PubMed] [Google Scholar]
- 14.De AK, Kodys KM, Pellegrini J, et al. Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin Immunol. 2000;96:52-66. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan ST, Lederer JA, Horgan AF, et al. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222:482-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiPiro JT, Howdieshell TR, Goddard JK, et al. Association of interleukin-4 plasma levels with traumatic injury and clinical course. Arch Surg. 1995;130:1159-1163. [DOI] [PubMed] [Google Scholar]
- 17.Lyons A, Kelly JL, Rodrick ML, et al. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann Surg. 1997;226:450-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherry RM, Cue J, Goddard JK, et al. Interleukin-10 is associated with the development of sepsis in trauma patients. J Trauma. 1996;40:613-616. [DOI] [PubMed] [Google Scholar]
- 19.Ayala A, Lehman DL, Herdon CD, et al. Mechanism of enhanced susceptibility to sepsis following hemorrhage. Arch Surg. 1994;129:1172-1178. [DOI] [PubMed] [Google Scholar]
- 20.Toliver-Kinsky TE, Varma TK, Lin CY, et al. Interferon-γ production is suppressed in thermally injured mice: decreased production of regulatory cytokines and corresponding receptors. Shock. 2002;18:322-330. [DOI] [PubMed] [Google Scholar]
- 21.Muret J, Marie C, Fitting C, et al. Ex vivo T-lymphocyte derived cytokine production in SIRS patients is influenced by experimental procedures. Shock. 2000;13:169-174. [DOI] [PubMed] [Google Scholar]
- 22.Cavaillon JM, Adib-Conquy M, Cloez-Tayarani I, et al. Immunodepression in sepsis and SIRS assessed by ex vivo cytokine production is not a generalized phenomenon: a review. J Endo Res. 2001;7:85-93. [PubMed] [Google Scholar]
- 23.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111-147. [DOI] [PubMed] [Google Scholar]
- 24.Kelly JL, O’Suilleabhain CB, Soberg CC, et al. Severe injury triggers antigen-specific T-helper cell dysfunction. Shock. 1999;12:39-45. [DOI] [PubMed] [Google Scholar]
- 25.Wick M, Kollig E, Muhr G, et al. The potential pattern of circulating lymphocytes TH1/TH2 is not altered after multiple injuries. Arch Surg. 2000;135:1309-1314. [DOI] [PubMed] [Google Scholar]
- 26.Zedler S, Faist E, Ostermeier B, et al. Postburn constitutional changes in T-cell reactivity occur in CD8+ rather than in CD4+ cells. J Trauma. 1997;42:872-881. [DOI] [PubMed] [Google Scholar]
- 27.Kell MR, Kavanagh EG, Goebel A, et al. Injury primes the immune system for an enhanced and lethal T-cell response against bacterial superantigen. Shock. 1999;12:139-144. [DOI] [PubMed] [Google Scholar]
- 28.Muraille E, Pajak B, Urbain J, et al. Role and regulation of IL-12 in the in vivo response to staphylococcal enterotoxin B. Int Immunol. 1999;11:1403-1410. [DOI] [PubMed] [Google Scholar]
- 29.Arad G, Levy R, Kaempfer R. Superantigen concomitantly induces TH1 cytokine genes and the ability to shut off their expression on re-exposure to superantigen. Immunol Lett. 2002;82:75-78. [DOI] [PubMed] [Google Scholar]
- 30.Luxembourg A, Grey H. Strong induction of tyrosine phosphorylation, intracellular calcium, nuclear transcription factors and interferon γ, but weak induction of IL-2 in naïve T cells stimulated by bacterial superantigen. Cell Immunol. 2002;219:28-37. [DOI] [PubMed] [Google Scholar]
- 31.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818-824. [DOI] [PubMed] [Google Scholar]
- 32.Hotchkiss RS, Swanson PE, Knudson MC, et al. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148-4156. [PubMed] [Google Scholar]
- 33.Hotchkiss RS, Tinsley KW, Swanson PE, et al. Sepsis-induced apoptosis cause progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952-6963. [DOI] [PubMed] [Google Scholar]
- 34.Teodorczyk-Injeyan JA, Cembrzynska-Nowak M, Lalani S, et al. Immune deficiency following thermal trauma is associated with apoptotic cell death. J Clin Immunol. 1995;15:318-328. [DOI] [PubMed] [Google Scholar]
- 35.Pellegrini JD, De AK, Kodys K, et al. Relationships between T lymphocyte apoptosis and anergy following trauma. J Surg Res 2000;88:200-206. [DOI] [PubMed] [Google Scholar]