Abstract
Objective:
To determine the utility of radioguided parathyroidectomy for patients with hyperparathyroidism, we studied the properties of 180 resected, hyperfunctioning parathyroid glands.
Summary and Background Data:
Radioguided resection of hyperfunctioning parathyroid glands has been shown to be technically feasible in patients with parathyroid adenomas. Radioguided excision may obviate the need for intraoperative frozen section because excised parathyroid adenomas uniformly have radionuclide ex vivo counts >20% of background. The feasibility and applicability of radioguided techniques for patients with parathyroid hyperplasia are unclear.
Methods:
Between March 2001 and September 2002, 102 patients underwent neck exploration for primary (n = 77) and secondary/tertiary (n = 25) hyperparathyroidism. All patients received an injection of 10 mCi of Tc-99m sestamibi the day of surgery. Using a gamma probe, intraoperative scanning was performed, looking for in vivo radionuclide counts > background to localize abnormal parathyroid glands. After excision, radionuclide counts of each ex vivo parathyroid gland were determined and expressed as a percentage of background counts.
Results:
Although patients with single adenomas had higher mean background radionuclide counts, the average in vivo counts of all enlarged glands were higher than background. Notably, in vivo counts did not differ between adenomatous and hyperplastic glands, suggesting equal sensitivity for intraoperative gamma detection. Ectopically located glands were identified in 22 cases and all were accurately localized using the gamma probe. Postresection, mean ex vivo radionuclide counts were highest in the single parathyroid adenomas and lowest in hyperplastic glands. Importantly, in all hyperplastic glands, the ex vivo counts were >20%.
Conclusions:
In patients with hyperparathyroidism, radioguided surgery is a sensitive adjunct for the intraoperative localization of both adenomatous and hyperplastic glands. In this series, all 180 enlarged parathyroids were located with the gamma probe. We have also shown that the “>20% rule” for ex vivo counts not only applies to parathyroid adenomas but also to hyperplastic glands. Therefore, radioguided resection is equally effective and informative for both adenomatous and hyperplastic glands.
To determine the utility of radioguided parathyroidectomy for patients with primary, secondary, and tertiary hyperparathyroidism, we studied the properties of 180 resected, hyperfunctioning parathyroid glands. We found that radioguided resection is equally effective and informative for both adenomatous and hyperplastic parathyroid glands.
Surgery remains the only curative therapy for patients with hyperparathyroidism (HPTH). In experienced hands, parathyroidectomy is associated with high cure rates and minimal morbidity.1-7 Over the last decade, many novel techniques have been used to optimize outcomes of patients undergoing surgery for HPTH, including cervical block anesthesia,8 intraoperative intact parathyroid hormone (PTH) monitoring,5,9,10 and radioguided resection.11,12
Radioguided resection of parathyroid glands involves preoperative intravenous injection of technetium99m-sestamibi about 1-2 hours prior to surgery. Intraoperatively, a hand-held gamma probe is used to facilitate localization of hyperfunctioning parathyroid tissue. Radioguided parathyroidectomy has been shown to be technically feasible in patients with primary HPTH due to a parathyroid adenoma.11,13 Radioguided excision may obviate the need for intraoperative frozen section because excised parathyroid adenomas uniformly have radionuclide ex vivo counts >20% of background.12 However, because the reported experiences with radioguided parathyroidectomy have primarily focused on patients with parathyroid adenomas, the feasibility and applicability of radioguided techniques for patients with parathyroid hyperplasia are unclear. Therefore, to determine the utility of radioguided parathyroidectomy for all patients with HPTH, we studied the properties of 180 resected, hyperfunctioning parathyroids including 92 glands from patients with 4-gland hyperplasia.
METHODS
Between March 2001 and September 2002, 102 consecutive patients underwent neck exploration for primary (n = 77) and secondary/tertiary (n = 25) HPTH using radioguided techniques. All patients were injected with 10mCi of technetium99m-sestamibi on average 1-2 hours prior to surgery. In the operating room, background counts were obtained by placing an 11-mm collimated gamma probe (Neoprobe 2000, Ethicon Endo-Surgery Breast-Care, Cincinnati, OH) on the thyroid isthmus through the skin. After incision, intraoperative scanning was performed looking for radionuclide counts > background to localize abnormal parathyroid glands. The counts obtained by scanning on the identified enlarged parathyroid gland in situ were recorded as “in vivo” counts and expressed as a percentage of the background counts. After excision of the parathyroid, the tissue was placed on top of the gamma probe (directed away from the patient) to determine “ex vivo” counts. Ex vivo counts were expressed as a percentage of background counts. If the ex vivo parathyroid count was > 20% of background, frozen section analysis was not performed, based on the “20% rule” described by Norman.12
All data were recorded prospectively. Surgical cure was defined as a serum calcium level <10.5 mg/dL at 6 months after surgery. Recurrence was defined as a serum calcium level exceeding 10.5 mg/dL in consecutive samples at 6 months after surgery. Persistent disease was defined as a serum calcium level greater than 10.5 mg/dL within 6 months of surgery. The median follow-up was 17 months (range, 8-26 months). Data were recorded as mean ± sem. Statistical analysis was performed with SPSS software (SPSS Inc.) Statistical significance was defined as P < 0.05.
RESULTS
Patients and Outcomes
The mean age of the patients was 59 ± 1 years and 63% were female. The mean preoperative calcium and intact PTH levels were 11.3 ± 0.1 mg/dL and 231 ± 32 pg/mL, respectively. Twenty-five patients had had a previous neck exploration. After radioguided parathyroid surgery, 96% of all patients were cured of their HPTH. From these 102 patients, a total of 180 hyperfunctioning parathyroid glands were resected under radioguidance. Of these 180 enlarged glands, 59 were single adenomas, 29 were double/triple adenomas (asymmetric hyperplasia), and 92 were hyperplastic (enlargement of all parathyroid glands). Single adenomatous glands were generally larger than double/triple adenomas or hyperplastic glands (989 ± 203 vs. 650 ± 145 and 638 ± 66 mg, P = 0.013; Fig. 1).
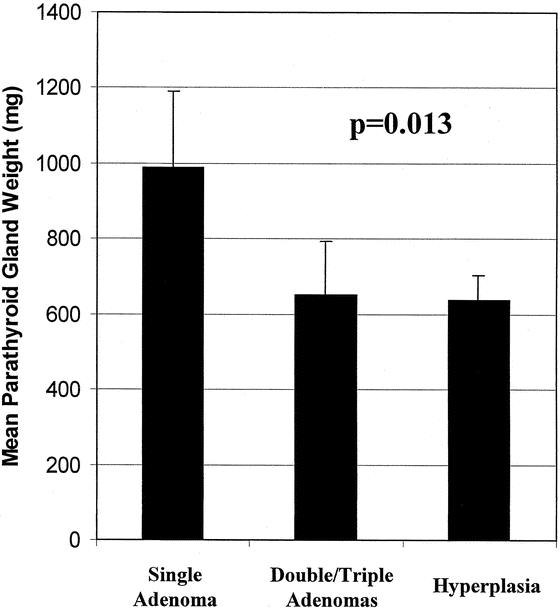
FIGURE 1. Mean weights of parathyroid glands resected by radioguidance. Single adenomas were on average larger than double/triple adenomas and hyperplastic glands.
Comparison of Patients Based on Etiology of HPTH
The patients were stratified based on the etiology of their HPTH (single adenoma, double/triple adenomas, and hyperplasia). Demographic and laboratory data among these 3 groups were then compared (Table 1). Patients in the hyperplasia group were on average younger (49 ± 2 vs. 63 ± 2 and 61 ± 1 year, P < 0.001). More patients in the single adenoma group were female (76% vs. 50% and 46%, P < 0.01). Patients in the hyperplasia group had slightly lower preoperative serum calcium levels (10.9 ± 0.2 vs. 11.4 ± 0.1 and 11.5 ± 0.2 mg/dL, P < 0.005) but higher preoperative intact PTH levels (475 ± 63 vs. 164 ± 24 and 210 ± 30 pg/mL, P < 0.001). As the number of resected glands increased, on average, the postoperative serum calcium levels decreased. However there was no difference in the postoperative mean intact PTH levels between the groups after surgery.
TABLE 1. Comparison of Demographic and Laboratory Data
Radioguided Data
The median time from intravenous radionuclide injection to surgery was 90 minutes (range, 30 to 420 minutes). The radionuclide counts of the parathyroid glands in each group are shown in Table 2. The mean background counts on the thyroid isthmus was slightly higher in patients with single adenomas (241 ± 20 vs. 193 ± 14 and 198 ± 7, P = 0.027). Despite this small difference, the average in vivo counts of all enlarged glands were higher than background. Notably, the in vivo counts did not differ between adenomatous and hyperplastic glands, suggesting equal sensitivity for intrapoperative gamma detection. In fact, all hyperfunctioning parathyroid glands in the 3 groups had in vivo counts above background.
TABLE 2. Radioguided Excision Data
After excision of the 59 single adenomas, 58 (98%) had ex vivo counts greater than 20% of background (Table 2). Thus, intraoperative frozen section was not used in these cases. One patient with a 200mg single adenoma had ex vivo counts of 15% of background. However, although surgery was scheduled 1 hour after sestamibi injection, other operative emergencies led to a delay in this patient’s surgery. Therefore, this patient did not undergo surgery until 7 hours after sestamibi injection. Although the in vivo counts exceed background, the ex vivo counts were below 20% of background. Of the 29 double/triple adenomas, all 29 had ex vivo counts >20%. Importantly, all 92 hyperplastic glands also had ex vivo counts >20% of background.
The mean ex vivo radionuclide counts was highest in the single parathyroid adenomas and lowest in the hyperplastic glands (Table 2). Thus, ex vivo counts appeared to predict the etiology of the HPTH (single versus double/triple adenomas and hyperplasia). However, as shown in Figure 2, whereas the mean ex vivo counts differed between the groups, there was significant overlap in the distribution of ex vivo counts within the groups. Therefore, because of the large variation of counts seen in all groups, while an ex vivo count > 20% of background definitively confirmed resection of parathyroid tissue, the exact ex vivo count is not specific enough to predict the etiology of the HPTH.
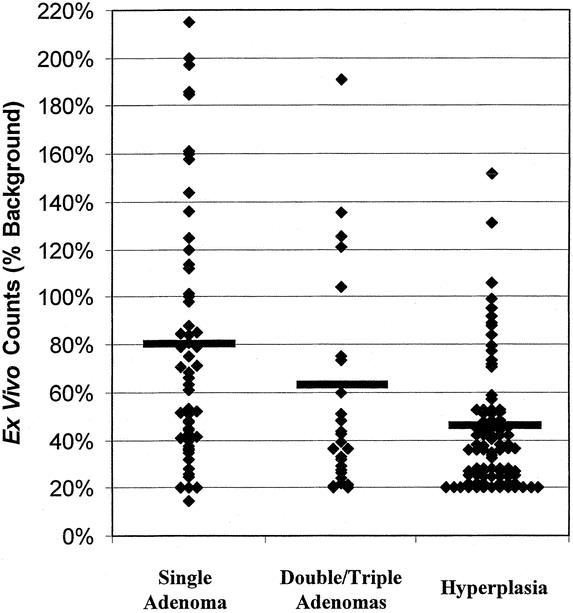
FIGURE 2. Ex vivo counts of radioguided resected parathyroid glands. Distribution of ex vivo gland counts group by etiology of HPTH. Gray line denotes mean ex vivo counts of the group.
To determine whether parathyroid gland weight correlated with ex vivo radionuclide counts, we performed a linear regression analysis. As shown in Figure 3, although very large glands (>2000 mg) tended to have high ex vivo counts, there was a large range of ex vivo counts in smaller glands. This translated into a very small linear trend (R2 = 0.25) that did not reach statistical significance. Therefore, gland weight did not directly correlate with ex vivo radionuclide counts.
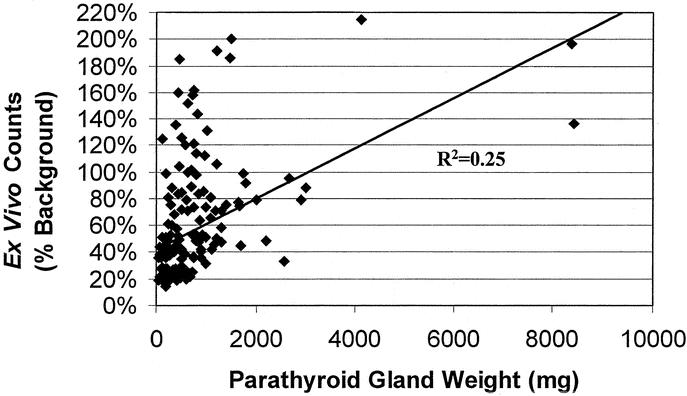
FIGURE 3. Parathyroid gland weights versus ex vivo counts. Line represents linear regression analysis.
Localization of Ectopic Glands
Ectopically located parathyroid glands were identified in 22 cases and all were accurately localized using the gamma probe. The most common ectopic location was the thymus (n = 9), followed by intrathyroidal (n = 4), mediastinal outside of the thymus (n = 4), retroesophageal (n = 4), and undescended (n = 1). Eighteen of these patients had primary HPTH, whereas 4 had secondary/tertiary HPTH.
DISCUSSION
The role of radioguided surgery in the management of patients with HPTH is not completely established. In this study, we describe our initial results with radioguided parathyroidectomy in the first 102 consecutive patients with primary, secondary, or tertiary HPTH who underwent this procedure at our institution. Using this technique, we successfully resected 180 enlarged, hyperfunctioning parathyroid glands. We found that radioguided surgery facilitated intraoperative localization, especially of ectopically located glands, allowed omission of intraoperative frozen section analysis, and was equally effective for adenomatous and hyperplastic parathyroids.
Radioguided parathyroidectomy has been shown to be very effective in patients with primary HPTH. In the largest published series to date (345 patients), Norman and colleagues11,12,14 have clearly shown that radioguided surgery is associated with high cure rates (99.5%) and minimal complications in patients with primary HPTH. Others have also shown that radioguided techniques may be of benefit. Flynn and associates found in 36 patients with primary HPTH that radioguided surgery decreased operative time and lower hospital costs.15 In 75 patients with primary HPTH, McGreal et al16 had a 97% success rate with radioguided localization. Goldstein reported that radioguided surgery in 20 patients with primary HPTH facilitated intraoperative localization, reduced operative time, lowered hospital charges, and decreased the use of intraoperative frozen section.17 Furthermore, we have recently reported that radioguided parathyroidectomy may reduce operative times in patients with tertiary HPTH undergoing bilateral neck exploration for hypercalcemia after kidney transplantation.18
Despite the many reports citing the potential benefits of radioguided surgery in patients with HPTH, several other investigators have not been as enthusiastic about this technology.19,20 Many cite the high success rate of parathyroidectomy with experienced surgeons and emphasize that radioguided techniques do not routinely provide any additional information.1,5,21 In a series of 60 patients with primary HPTH, Inabnet and colleagues13 found that although radioguided surgery was helpful in 40% of the time, it provided confusing or inaccurate information in 48% of cases. Jaskowick and associates22 found radioguided techniques only helpful 22% of the time in 57 patients with primary HPTH. In this relatively small series, the authors performed preoperative sestamibi scanning and ultrasound on all patients and routinely used frozen section analysis to confirm parathyroid tissue intraoperatively.22 However, despite their lack of enthusiasm for routine use of radioguided surgery for HPTH, both of these groups concede that these techniques may have potential benefit in select patients. Inabnet stated that “radioguided surgery may be beneficial in cases of persistent or recurrent HPTH and in the reoperative neck.”13 Jaskowiak added that gamma probe localization “proved crucial in several cases of reoperation and was helpful in other reoperations and in some cases with ectopic glands.22” Therefore, most authors would agree that radioguided surgery may play a role in the reoperative neck.11,21 Yet, to effectively use this potentially beneficial technology, it would seem logical that one would need some experience beforehand. In an invited critique following Inabnet et al’s report, Dr. Arthur Ross stated that “it is ironic that Inabnet et al suggest that we reserve the use of radioguidance for those patients who present the greatest challenge to even the most experienced parathyroid surgeons; yet their data suggest that they are unable to successfully use the technology in a simpler and less complicated patient population! … It seems that if we are to reserve this approach for our most challenging patients, we must be completely as comfortable and facile with its use in “routine” patients where success of the procedure may not be greatly dependent on the satisfactory application of the technology.13”
In our hands, radioguided techniques successfully localized all enlarged, hyperfunctioning parathyroid glands. All 180 resected parathyroid glands had in vivo counts higher than background. We believe that radioguidance directed the surgeon to minimize the extent of dissection in most of our cases. Furthermore, in 22 cases, it facilitated localization of ectopic glands. In 9 cases (6 primary and 3 secondary/tertiary HPTH) an enlarged parathyroid was found in the thymus. Four retroesophageal adenomas were localized with the gamma probe. One patient with persistent primary HPTH had an undescended parathyroid adenoma that was not detected by preoperative sestamibi scans but intraoperatively found with the aid of the gamma probe. Ectopic parathyroid glands were present in the thyroid gland in 4 patients. In each of these cases, the use of the gamma probe led to a partial thyroidectomy and resection of the hyperfunctioning parathyroid gland. Lastly, 4 patients had mediastinal parathyroid adenomas; 2 had undergone previous bilateral neck explorations after an initial negative sestamibi scan and were subsequently localized with another imaging modality while the other 2 presented initially with a positive sestamibi scan showing a mediastinal lesion. All 4 patients had successful radioguided surgery; 1 through a sternotomy and the other 3 by radioguided video-assisted thoracoscopic surgery.23 We found the gamma probe very useful in distinguishing mediastinal fat and lymph nodes from parathyroid tissue during video-assisted thoracoscopic surgery.23 Although we routinely perform preoperative sestamibi scanning on all patients with primary HPTH, we believe that radioguided surgery facilitates localization in the majority of cases.
Many surgeons have reported that radioguided surgery allows omission of intraoperative frozen section to confirm parathyroid tissue.2,11,12,15-18,23 This is based upon Norman’s observation that almost all parathyroid adenomas have ex vivo counts >20% of background.12 In our series, 179 of 180 resected parathyroid glands had ex vivo counts >20%. Therefore, we did not use frozen section analysis in these cases. In 1 patient with a 200 mg parathyroid adenoma, the ex vivo counts were only 15%, prompting us to perform a frozen section to confirm parathyroid tissue. The low counts in this patient were most likely a result of the delay of 7 hours between his sestamibi injection and surgery. Although we believe that radioguided surgery allows omission of frozen section, there are surgeons who do not rely upon the probe and also do not require intraoperative frozen section.
Although most studies, including ours, universally report that single adenomas almost always have ex vivo counts >20%, our study is the first to show that hyperplastic parathyroid glands also have ex vivo counts >20%. Of the 29 double/triple adenomas and the 92 hyperplastic glands resected in our patients, 100% had ex vivo counts exceeding 20% of background. Our results differ from other smaller series. In analyzing 9 hyperplastic glands, McGreal and colleagues found the mean ex vivo count to be 10.5% of background.16 Murphy and Norman12 reported a mean ex vivo count of 58 hyperplastic glands in patients with primary HPTH of 7.5%. We believe these differences could be due to several reasons. In our protocol, we set the background counts based upon the thyroid isthmus, similar to the technique used by Inabnet.13 Norman and colleague use “the operative basin.12” In addition, although previous series have focused on patients with primary HPTH exclusively, our series includes those with secondary and tertiary HPTH.
It is interesting to note that in our hands, the ex vivo counts appear to predict pathology. On average, single parathyroid adenomas had higher ex vivo counts than double/triple adenomas, which were higher than hyperplastic glands. However, as shown in Figure 2, there is significant overlap in the range of counts seen with the different pathologies. Therefore, we feel that while ex vivo counts definitely allow identification of parathyroid tissue, they are not specific enough to define pathology. Therefore, we routinely use the intraoperative PTH assay to confirm curative resection intraoperatively. We strongly feel that radioguided techniques complement the intraoperative PTH assay but cannot replace it.
In conclusion, in patients with HPTH, radioguided surgery is a sensitive adjunct for the intraoperative localization of both adenomatous and hyperplastic glands. In this series, all 180 enlarged parathyroids were located with the gamma probe. We have also shown that the “>20% rule” for ex vivo counts not only applies to parathyroid adenomas but also to hyperplastic glands. Therefore, radioguided resection is equally effective and informative for both adenomatous and hyperplastic glands.
Discussions
Dr. Robert Udelsman (New Haven, Connecticut): I would like to congratulate Dr. Chen, Dr. Mack, and Dr. Starling for their continued contributions in the field of endocrine surgery. I also appreciation Dr. Chen’s courtesy in allowing me to review the transcript well in advance of today’s meeting. It was particularly pleasing for me to note an overall cure rate in this series of 96%, which is a testament to the skill of these surgeons. I have 4 specific questions:
You conclude that radio-guided excision obviates the need for intraoperative frozen section. Yet Norman Thompson and other groups, including our own, have abandoned routine frozen section during parathyroid surgery as this practice does not contribute significantly to intraoperative decision making, especially in the area of intraoperative parathyroid and hormone assay. I would be interested in your comments about frozen sections.
Two, you refer in your manuscript to triple adenomas. I find this term confusing. I suspect that what you describe as triple adenomas are in fact cases of asymmetric multigland hyperplasia where 1 of the 3 glands is of relatively normal size. This semantic distinction is important because multigland hyperplastic patients have increased recurrence rates and there are ramifications for family screening and screening for familial syndrome. I would appreciate your comments on that.
Three, did you obtain preoperative Sestamibi scans in some or perhaps all of your patients? And if so, how much if any additional information was obtained from the additional Sestamibi required to employ the intraoperative gamma probe?
Finally, in the area of ever-increasing cost scrutiny 1 must weigh the relative contributions of complementary perioperative adjuncts. Is it appropriate for every patient to undergo 1 preoperative Sestamibi scan in addition to two, another intraoperative Sestamibi scan, as well as complementary use of the intraoperative parathyroid hormone assay?
Finally, Dr. Chen, I would like to congratulate you for your success and contributions, and I thank the American Surgical Association for the privilege of the floor.
Dr. Herbert Chen (Madison, Wisconsin): Thank you, Dr. Udelsman. First of all, I would like to thank you because I certainly could not have performed a lot of these surgeries without the training I received from you during my residency and fellowship at Johns Hopkins. I will always be indebted to you for that. I will take your questions in the order you asked them.
You first asked about frozen section and the need to do it. And I do agree with you, especially as you get more and more experienced the need for frozen section decreases, and especially since most institutions are moving towards using intraoperative PTH that probably makes frozen section obsolete. The nice thing about having the gamma probe is that any doubt in your mind is taken out because you instantaneously know that that the resected tissue is parathyroid tissue.
Your second question is regarding triple adenomas. And I do agree that is a bit of a nomenclature, that perhaps triple adenomas could represent asymmetric hyperplasia. And in truth, only 2 of our patients in this series had what we term triple adenomas, because we saw 3 distinctly enlarged glands and 1 very small normal gland.
Your third question had to do with preoperative Sestamibi. For patients with primary hyperparathyroidism, which are 77 in this group, we would get a Sestamibi scan to determine if they are a candidate for the minimally invasive procedure. Those with secondary hyperparathyroidism do not get a Sestamibi scan.
That leads into your fourth question about costs and scans. If we get a positive Sestamibi scan and they are a candidate for our minimally invasive procedure we will target that gland and they will get a preoperative Sestamibi injection but not a second scan. In none of these patients was a second scan obtained at the time of surgery, the nuclear medicine people simply inject them the morning of surgery and then sent over to the OR.
So that is the main cost that we have with this type of procedure. We already use the gamma probe for sentinel node procedures in breast and melanoma. About half of these patients in this series weren’t even charged for that injection because our nuclear medicine people couldn’t figure out a way to charge for an injection. Since then, they have started to dictate reports detailing the injections. But I think the amount they can bill for that is the very little. So our patients really do not have increased costs.
Dr. Orlo H. Clark (San Francisco, California): Congratulations, Dr. Chen, on this fine paper. Endocrine surgery, and specifically parathyroid surgery, is very successful, as Dr. Chen mentioned. Despite the excellent success rate, we need to make parathyroid operations 100% successful and even less invasive. I have several questions for Dr. Chen.
In a patient who had abnormal parathyroid glands identified preoperatively, how often did the results vary in the operating room when you used the probe? Did it help you in any patient, such as in a patient whose sestamibi scan was negative preoperatively, but positive in the operating room? I am surprised you did not use this technique in patients with secondary hyperparathyroidism since they have multiple abnormal parathyroid glands, and sestamibi scanning preoperatively is not very accurate.
In the patients with multiple abnormal parathyroid glands and primary hyperparathyroidism, was there a difference in counts in normal versus abnormal parathyroid glands? Having now completed your investigation, do you believe that you and your senior collaborators will be using radio-guided parathyroidectomy a year from now?
Dr. Herbert Chen (Madison, Wisconsin): Thank you, Dr. Clark, for your questions.
First of all, regarding preoperative Sestamibi scan, I think how you read the Sestamibi really depends - because if you are trying to get as many patients to your minimally invasive approach you may overread a couple of Sestamibis to try to include those patients for a minimally invasive procedure.
At our institution approximately 10% of all patients who we think there is a single adenoma based on the preoperative Sestamibi, at the time of surgery, a second adenoma will be found. Truthfully, a lot of those are determined by the PTH assay. If you check intraoperative PTH, the level will not fall, and then you will further your exploration looking for that second adenoma.
With regard to the multigland patients, we do see equal uptake of the tracer in patients with hyperplastic disease, as shown in the 92 glands we presented here. Their in vivo counts were no different from those with single glands and their ex vivo counts, although on average were lower, all exceeded 20%. So if you do get a scan on a patient with hyperplastic disease, it doesn’t light up on the film. For some reason having that probe right on the parathyroid gland can give you a little more sensitivity than an x-ray machine that is a couple of feet away from the patient’s neck.
Finally, you asked, will we use this technique a year from now? Certainly I feel it gives you some additional information. I can’t predict in which patients the probe will be helpful. And because I can’t predict it, I think we will continue to use it for all patients. We have a small study that we have just recently presented showing that the gamma probe saves time even when you are going to a bilateral exploration. So I think in a year we will be using it still.
Dr. Quan-Yang Duh (San Francisco, California): Dr. Chen, I really enjoyed your paper. I have a very short question for you. The reason to use many of these localization studies is to help you do minimally invasive parathyroidectomies. How many of the patients had a focused operation or unilateral exploration and how many needed 4 gland exploration?
Dr. Herbert Chen (Madison, Wisconsin): Thanks, Dr. Duh. A minimally invasive procedure, as I think all of you would agree, is for patients with primary hyperparathyroidism in which you suspect they have a single adenoma.
Seventy-seven patients in this study had primary hyperparathyroidism. About 80% of them had a single gland that was localized by Sestamibi scanning in which we started with the minimally invasive approach. In 90% of these patients, we were able to complete that minimally invasive procedure. But because of either a lack of a 50% drop in intraoperative PTH levels or persistent counts that we detected with the gamma probe we converted 10% of them to an open operation and found in some patients 1 additional gland, in some patients more than 1 additional enlarged gland. About 50% of the time we find it is a double or triple adenoma, 50% we find it is a hyperplasia.
Footnotes
Reprints: Herbert Chen, MD, University of Wisconsin Medical School Department of Surgery, H4/750 CSC 600 Highland Avenue, Madison, WI. E-mail: chen@surgery.wisc.edu.
REFERENCES
- 1.Udelsman R. Six hundred fifty-six consecutive explorations for primary hyperparathyroidism. Ann Surg. 2002;235:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H. Surgery for primary hyperparathyroidism: what is the best approach? Ann Surg. 2002;236:552-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, Parkerson S, Udelsman R. Parathyroidectomy in the elderly: do the benefits outweigh the risks? World J Surg. 1998;22:531-535. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Zeiger MA, Gordon TA, et al. Parathyroidectomy in Maryland: effects of an endocrine center. Surgery. 1996;120:948-952. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Sokoll LJ, Udelsman R. Outpatient minimally invasive parathyroidectomy: a combination of sestamibi-SPECT localization, cervical block anesthesia, and intraoperative parathyroid hormone assay. Surgery. 1999;126:1016-1021. [DOI] [PubMed] [Google Scholar]
- 6.Nichol PF, Starling JR, Mack E, et al. Long-term follow-up of patients with tertiary hyperparathyroidism treated by resection of a single or double adenoma. Ann Surg. 2002;235:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Girotto JA, Harmon JW, Ratner LE, et al. Parathyroidectomy promotes wound healing and prolongs survival in patients with calciphylaxis from secondary hyperparathyroidism. Surgery. 2001;130:645-650. [DOI] [PubMed] [Google Scholar]
- 8.Ditkoff BA, Chabot J, Feind C, et al. Parathyroid surgery using monitored anesthesia care as an alternative to general anesthesia. Am J Surg. 1996;172:698-700. [DOI] [PubMed] [Google Scholar]
- 9.Irvin GL III, Sfakianakis G, Yeung L, et al. Ambulatory parathyroidectomy for primary hyperparathyroidism. Arch Surg. 1996;131:1074-1078. [DOI] [PubMed] [Google Scholar]
- 10.Irvin GL III, Molinari AS, Figueroa C, et al. Improved success rate in reoperative parathyroidectomy with intraoperative PTH assay. Ann Surg. 1999;229:874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman J, Denham D. Minimally invasive radioguided parathyroidectomy in the reoperative neck. Surgery. 1998;124:1088-1092. [DOI] [PubMed] [Google Scholar]
- 12.Murphy C, Norman J. The 20% rule: a simple, instantaneous radioactivity measurement defines cure and allows elimination of frozen sections and hormone assays during parathyroidectomy. Surgery. 1999;126:1023-1028. [DOI] [PubMed] [Google Scholar]
- 13.Inabnet WB III, Kim CK, Haber RS, et al. Radioguidance is not necessary during parathyroidectomy. Arch Surg. 2002;137:967-970. [DOI] [PubMed] [Google Scholar]
- 14.Norman JG, Jaffray CE, Chheda H. The false-positive parathyroid sestamibi: a real or perceived problem and a case for radioguided parathyroidectomy. Ann Surg. 2000;231:31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn MB, Bumpous JM, Schill K, et al. Minimally invasive radioguided parathyroidectomy. J Am Coll Surg. 2000;191:24-31. [DOI] [PubMed] [Google Scholar]
- 16.McGreal G, Winter DC, Sookhai S, et al. Minimally invasive, radioguided surgery for primary hyperparathyroidism. Ann Surg Oncol. 2001;8:856-860. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein RE, Blevins L, Delbeke D, et al. Effect of minimally invasive radioguided parathyroidectomy on efficacy, length of stay, and costs in the management of primary hyperparathyroidism. Ann Surg. 2000;231:732-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichol PF, Mack E, Bianco J, et al. Radioguided parathyroidectomy in patients with secondary and tertiary hyperparathyroidism. Surgery., in press. [DOI] [PubMed]
- 19.Udelsman R. Is unilateral neck exploration for parathyroid adenoma appropriate? Adv Surg. 2000;34:319-329. [PubMed] [Google Scholar]
- 20.Saaristo RA, Salmi JJ, Koobi T, et al. Intraoperative localization of parathyroid glands with gamma counter probe in primary hyperparathyroidism: a prospective study. J Am Coll Surg. 2002;195:19-22. [DOI] [PubMed] [Google Scholar]
- 21.Udelsman R. Surgery in primary hyperparathyroidism: the patient without previous neck surgery. J Bone Miner Res. 2002;17(Suppl 2):N126-N132. [PubMed] [Google Scholar]
- 22.Jaskowiak NT, Sugg SL, Helke J, et al. Pitfalls of intraoperative quick parathyroid hormone monitoring and gamma probe localization in surgery for primary hyperparathyroidism. Arch Surg. 2002;137:659-668. [DOI] [PubMed] [Google Scholar]
- 23.O’Herrin JK, Weigel T, Wilson M, et al. Radioguided parathyroidectomy via VATS combined with intraoperative parathyroid hormone testing: the surgical approach of choice for patients with mediastinal parathyroid adenomas? J Bone Miner Res. 2002;17:1368-1371. [DOI] [PubMed] [Google Scholar]