Abstract
Introduction:
The incidence of hepatocellular carcinoma (HCC) in the United States has increased 75% in the last decade. Liver transplantation is gaining acceptance for the treatment of early HCC, even in patients with adequate liver function. The objective of this study was to determine the long-term outcome of patients with early HCC who would have been candidates for transplantation but were treated instead with partial hepatectomy.
Methods:
From August 1989 to November 2001, 611 patients with HCC were evaluated at our institution and entered into a prospective database. There were 180 (29%) patients who underwent partial hepatectomy, of whom 36 (20%) satisfied the currently accepted criteria for transplantation: 2 or 3 lesions each ≤ 3 cm in size or a solitary tumor ≤ 5 cm. Survival was determined by Kaplan-Meier analysis.
Results:
Median tumor size was 3.5 (range, 1.8-5) cm and the median number of lesions was 1 (range, 1-3). Patients had pathologically confirmed cirrhosis of the liver in 78% (28/36) of cases, and 86% had normal liver function (Child class A). Perioperative morbidity was 25%, the median length of hospital stay was 8 (range, 4-24) days, and there was 1 (2.8%) perioperative death. At a median follow-up of 35 months for survivors, the 1-, 3-, and 5-year overall survival was 85%, 74%, and 69%, respectively, with a median survival of 71 months. The 5-year disease-free survival was 48% with a median of 52 months.
Conclusions:
Partial hepatectomy in patients with early HCC who are otherwise eligible for transplantation can be performed with minimal morbidity and can achieve comparable 5-year survival to that reported for liver transplantation. Resection should be considered the standard therapy for patients with HCC who have adequate liver reserve.
The most effective therapy for patients with early hepatocellular carcinoma (HCC) and adequate liver reserve is unknown. We analyze the outcome of patients who would have been eligible for transplantation for HCC but were instead resected, and find that survival is comparable to transplantation in this patient population.
Hepatocellular carcinoma (HCC) is the fifth-leading cause of cancer in the world.1,2 In the United States, there will be 20,200 new cases of HCC this year, which is a 75% increase over the past decade and reflects the increased prevalence of chronic hepatitis infection.3 Three million people have chronic hepatitis C, and 1.2 million have chronic hepatitis B in the United States. These patients are estimated to develop HCC at a rate of 5% per year for chronic hepatitis C and 0.5% per year for hepatitis B.4,5 It is predicted that the number of deaths from HCC will rise dramatically over the next 10-15 years in this country.2,6,7
Partial hepatectomy has been the standard therapy for patients with HCC and good hepatic reserve when complete tumor resection is feasible. Five-year survival rates after resection have ranged from approximately 30 to 40%.8-14 Unfortunately, the majority of patients subsequently develop recurrent cancer in the liver remnant after resection.12,15 Adjuvant therapy has not been proven to be effective.16,17 In an attempt to achieve better results, surgeons have used liver transplantation for patients with HCC. The initial experience, in which transplantation was used liberally for patients with all stages of HCC, was discouraging, with 5-year survival rates ranging from 20-36%.18-22 It was noted, however, that patients with incidentally discovered HCC in the explanted liver and those with small tumors had similar survival comparable with patients undergoing liver transplantation for benign disease.23 Soon thereafter, the eligibility criteria for transplantation in patients with HCC were restricted. Mazzaferro et al24 published one of the first series to propose the currently accepted criteria for transplantation: a solitary tumor ≤ 5 cm, 2 or 3 tumors ≤ 3 cm in size, and the absence of vascular invasion. Four-year survival was reported at 75%, and 4 year recurrence free survival was 83%. Several other studies have confirmed these findings.20-23,25-28 Some authors have found the results to be so convincing that they are using liver transplantation as a first line treatment of patients with HCC and cirrhosis, regardless of the degree of liver dysfunction.29 However, the seemingly better results with transplantation versus partial hepatectomy may simply reflect the more stringent selection of patients with earlier stage HCC. In an attempt to determine whether partial hepatectomy or transplantation is a better treatment of early HCC, we examined the outcome of patients with early HCC treated with partial hepatectomy who would have been candidates for transplantation.
MATERIALS AND METHODS
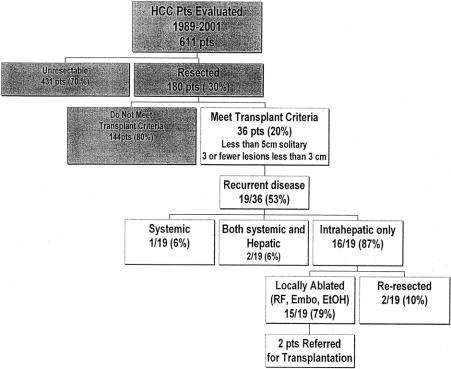
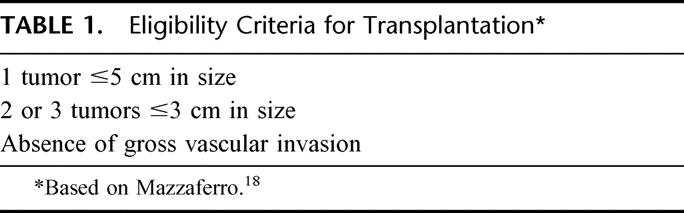
From August 1989 to November 2001, 611 patients with HCC were evaluated by members of the Hepatobiliary Service at Memorial Sloan-Kettering Cancer Center and entered into a prospective database (Fig. 1). There were 180 (29%) patients who underwent partial hepatectomy with complete gross resection of tumor. Of these, 36 (20%) patients met the currently accepted criteria for liver transplantation: a solitary tumor ≤5 cm, 2 or 3 tumors ≤3 cm in size, and the absence of vascular invasion. (Table 1). Patients over the age of 65 were included in the analysis because they are now undergoing transplantation with increasing frequency, especially since the advent of live donor organ procurement.
FIGURE 1. Summary of patients with HCC evaluated from 1989 to 2001.
TABLE 1. Eligibility Criteria for Transplantation
Serologic testing was performed preoperatively to determine hepatitis status as well as serum alpha fetoprotein level. Resected liver specimens were fixed in formalin, embedded in paraffin, and serially sectioned. Tumor number and size were confirmed on gross analysis, and microscopic analysis was performed to determine vascular invasion, microscopic tumor satellites, tumor differentiation, and status of the resection margin. Liver adjacent to tumor was examined for evidence of cirrhosis. Liver fibrosis was staged on a scale of 0-4, and advanced stage 3 and stage 4 were designated as cirrhosis.
The primary end point of the analysis was length of survival after partial hepatectomy. Survival estimates were determined using Kaplan-Meier analysis. All statistical analyses were performed using SPSS software (SPSS, Inc., Chicago, IL). A P value less than 0.05 was considered significant.
RESULTS
The clinical characteristics of patients with early HCC who were eligible for liver transplantation but treated with partial hepatectomy are listed in Table 2. The majority (82%) of patients were male with 10 (28%) patients age 65 or older. There were 28 (78%) patients with a pathologic diagnosis of cirrhosis of the liver. The majority (85%) of patients with hepatitis B or C were of Asian descent. The median tumor size was 3.5 (range, 1.8-5.0) cm and 83% of patients had a solitary lesion (Table 3). Only 2 (6%) patients had a positive microscopic resection margin and microscopic vascular invasion occurred in nearly one-third of patients
TABLE 2. Patient Characteristics (N = 36)
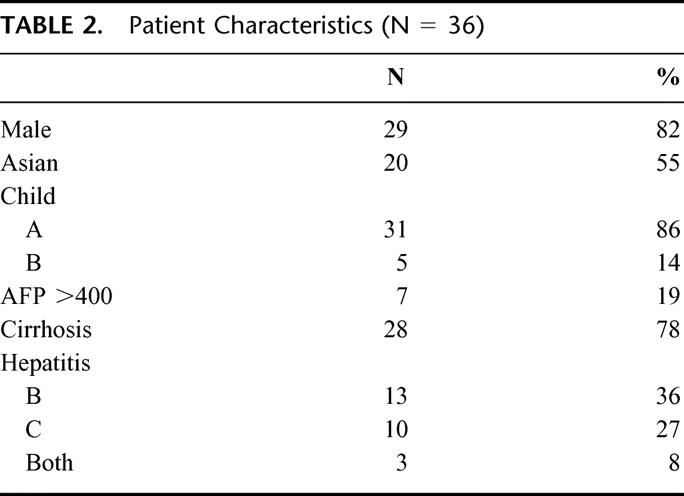
TABLE 3. Tumor Characteristics
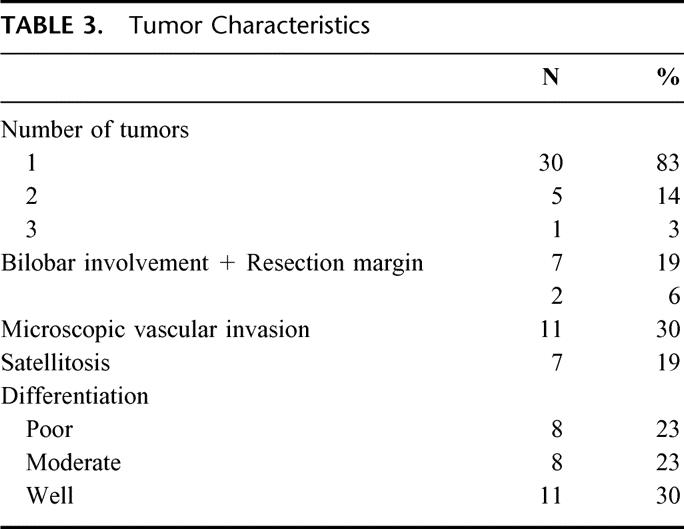
Most of the operations for these patients with small tumors were segmentectomies with 4 patients undergoing a wedge resection (Table 4). An anatomic resection was performed whenever possible. Only 28% of patients in the transplant eligible group required a hemihepatectomy or extended hepatectomy compared with nearly 70% of the patients who underwent hepatectomy and were not eligible for transplantation. The median hospital stay was 8 (range, 2-24) days for the transplant eligible group versus 13 days (range, 1-60) for the transplant ineligible resection group. Of the 36 patients who satisfied the criteria for transplantation and underwent partial hepatectomy, 1 (2.8%) patient died within a month after surgery from portal vein thrombosis and gastrointestinal hemorrhage. Perioperative morbidity occurred in 9 (25%) patients (Table 5). In contrast, in the transplant ineligible group (n = 144), perioperative morbidity and mortality were 45% and 5%, respectively.
TABLE 4. Hepatic Resections in 36 Patients With Early Hepatocellular Carcinoma
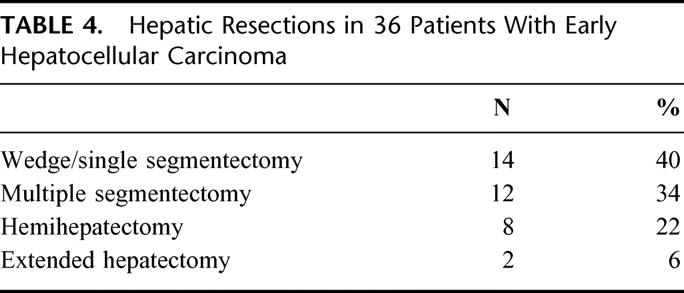
TABLE 5. Perioperative Outcome (N = 36)
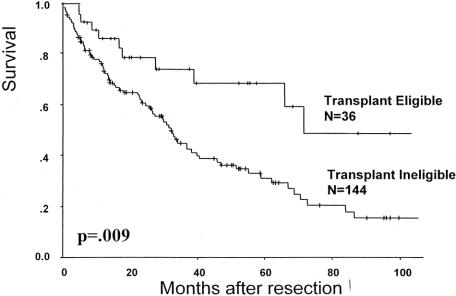
At a median follow-up of 35 months, the patients with early HCC who were treated with resection had a 1-, 3-, and 5-year overall survival of 85%, 74%, and 69%, respectively, and the median survival was 71 months (Fig. 2). In contrast, the 5-year actuarial survival for transplant ineligible resected patients (n = 180) was 31% with a median survival of 32 months (Fig. 3).
FIGURE 2. Overall survival after hepatectomy in patients with HCC.
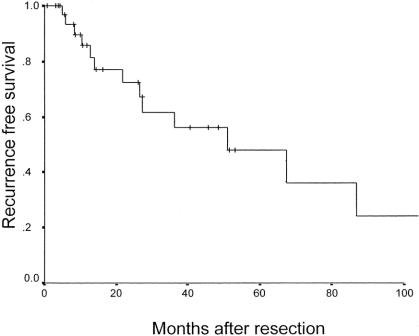
FIGURE 3. Recurrence after resection of early HCC (N = 36).
Twenty (55%) of the 36 patients with early HCC have developed tumor recurrence. The 5-year actuarial disease-free survival rate was 48% with a median recurrence free survival of 52 months (Fig. 3). As expected, patients who experienced tumor recurrence had a significantly worse survival (Fig. 4). Interestingly, in the 15 (42%) patients who remained free of recurrence, the 5-year overall survival was 93%, despite the presence of cirrhosis in 73% of these patients. The majority (85%) of recurrences were isolated to the liver (Fig. 1). For 80% of these patients, liver transplantation was still a possibility using the standard Milan criteria.24 All patients who recurred within the liver were treated with an ablative procedure consisting of hepatic artery embolization, radiofrequency ablation, cryoablation, or ethanol ablation. A second hepatectomy was performed in 2 patients. Recently, 2 patients have been referred for salvage transplantation; 1 received a liver transplant and the other is still on a transplant waiting list.
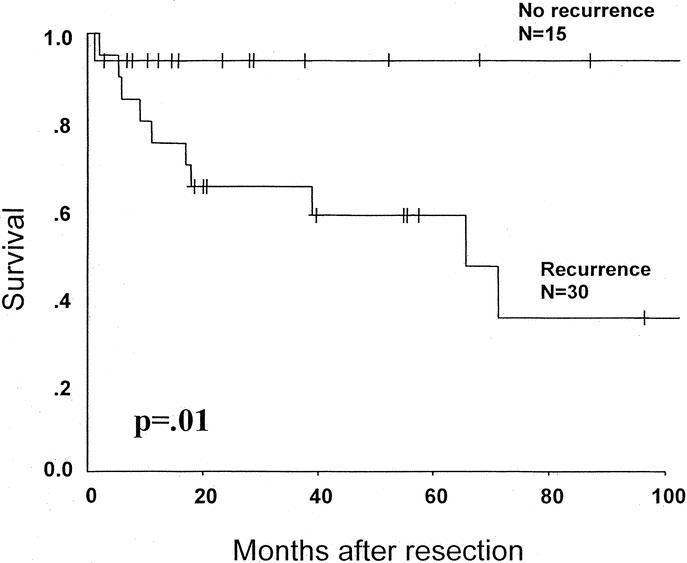
FIGURE 4. Overall survival of transplant-eligible patients based on the development of tumor recurrence. The 1 perioperative death is excluded.
DISCUSSION
There is little debate that the most appropriate treatment of patients with limited HCC who have moderate-to-severe cirrhosis is liver transplantation. By contrast, the management of patients with early HCC who have adequate liver reserve has engendered considerable controversy. This patient subset is the best group in which to determine whether partial hepatectomy or transplantation is better oncologic therapy. Ideally, a randomized prospective trial should be performed, but a variety of logistical issues make this unlikely to occur. Consequently, we can only extrapolate the relative efficacy of partial hepatectomy and transplantation from theoretical analyses30 and retrospective data.25,29 However, direct comparison between the published results of partial hepatectomy and transplantation for HCC is confounded by several methodologic problems. First, there is significant variability in patient selection for either resection or transplantation. For instance, in the Mazzaferro report, the median tumor size was only 1.5 cm whereas in the present series, it was 3.5 cm. Patients with fibrolamellar carcinoma, who may have a more favorable prognosis31 are often included in resection and transplantation data. Investigators do not always include perioperative deaths in the survival analysis. Many transplantation series contain patients with incidental tumors discovered during surgical exploration or pathologic examination of the liver explant.18 There is also a paucity of transplantation studies with long-term (>5 years) follow-up.
The potential advantages of liver transplantation for treating HCC may not be as relevant in patients with early HCC who have adequate liver function. Total hepatectomy may not be required because the patients may be less likely to have occult intrahepatic metastases and are not likely to develop progressive liver dysfunction, at least in the short-term. Several investigators claim that recurrence free survival may be improved in patients who undergo transplantation instead of partial hepatectomy. Indeed, recurrence-free survival after transplantation at 5 years has ranged from 53-60%,20,21,29,32 with others reporting 3- and 4-year recurrence free survival as high as 83%.23,24 By contrast, in this series, the actuarial recurrence free survival was 48% at 5 years after resection. However, recurrence free survival is misleading because it does not account for the late morbidity and mortality resulting from transplantation and immunosuppression. For example, in the Mazzaferro report,24 12 of the 14 nonoperative deaths were due to complications associated with transplantation and not from tumor recurrence.24 This was reflected in a disease free survival that was actually higher than the overall survival! We found that the patients who did not develop tumor recurrence in our series had over a 90% 5 year survival, indicating that there were not other major competing causes for death, even though the majority (73%) of these patients presented with cirrhosis at the time of their initial resection.
Perhaps the biggest disadvantage to liver transplantation is the limited supply of donor organs. Based on the Organ Procurement and Transplantation Network data as of April 15, 2003, there were 5326 total liver transplants performed in the United States in 2002, and 1065 (20%) were performed for HCC. Only 37 grafts were from live donors (Fig. 5). The sharp increase in the number of transplants for HCC in 2002 was a result of a change in the formula for organ allocation. Because of the disproportionately high percentage of patients with HCC receiving liver grafts and the overall limited supply of donor organs, the United Network for Organ Sharing (UNOS) has recently downgraded the status for transplantation in patients with HCC. There are 18,505 patients on the waiting list for transplantation and 40% of patients have been waiting for over 2 years. A major problem with much of the published transplantation data is that patients who die or become ineligible for transplantation while waiting for an organ are not generally considered in the analysis of outcome. Llovet addressed this issue in a nonrandomized trial. When patient “drop-out” from the waiting list was included in the survival analysis of patients selected for transplantation, 3-year survival decreased from 80 to 60%.25 Survival at 5 years was equivalent after resection or transplantation, even though many of the patients in the resection group exceeded the Milan eligibility criteria for transplantation.
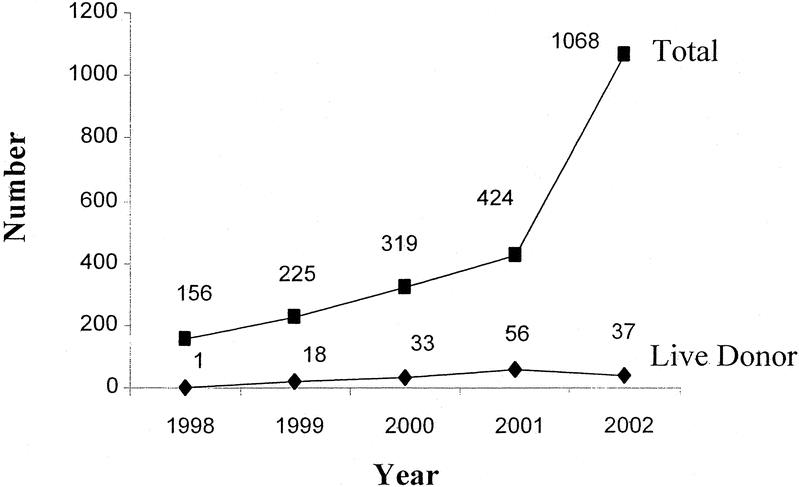
FIGURE 5. Current use of liver transplantation for HCC in the United States. There were 5326 liver transplantations in 2002, 1068 (20%) of which were performed for HCC. Based on OPTN data, April 15, 2003.
In attempt to circumvent the shortage of available cadaveric liver grafts, transplant surgeons introduced live donor transplantation. However, the indications for live donor transplantation are unclear, and its ethical justification is uncertain.33 Complication rates in the healthy donors have been reported at 14%, including a 6% biliary complication rate and 1 mortality.33 Conversely, biliary complications in the transplant recipient of a graft from a live donor range from 22 to 40%.33-35 There are only very preliminary outcome data regarding live donor transplantation in patients with HCC. Nevertheless, because of the perceived relative benefit of transplantation when compared with resection and the potentially unlimited supply of live donor organs, some surgeons are already advocating liver transplantation in patients who do not meet UNOS criteria.36,37 In a recent report of 27 patients with HCC who received a graft from a live donor and then were followed for a median of 8 months, there were 3 (11%) perioperative deaths and 2 other deaths (1 from early tumor recurrence).36
We found that patients with early HCC who underwent partial hepatectomy had a 5-year survival comparable to the reported results of liver transplantation. We have previously found tumor size to be a predictor of outcome after resection.10 In our series, liver resection could be performed safely (2.8% perioperative mortality) with minimal associated morbidity. Thus, the results in the present study argue against transplantation as a first-line therapy for patients with early HCC who do not have significant liver dysfunction. In 1985, Blumgart et al38 first proposed that resection was more appropriate than transplantation for large hepatocellular cancers. Our current findings extend these earlier results to patients with small tumors and preserved liver function. Thus, expanding the Milan criteria for transplantation as proposed by some investigators to include larger and more numerous tumors seems inappropriate.29,36,37 Indeed, in half of our patients, transplantation may not have provided any advantage while exposing them to the potential morbidity associated with a liver graft and immunosuppression. Transplantation would have been unnecessary in the 48% of patients expected to be recurrence free at 5 years by actuarial analysis. Furthermore, transplantation may have been pointless in the 8% of patients who developed extrahepatic disease (which presumably reflects unfavorable tumor biology) at the time of their initial recurrence. Moreover, 80% of those who developed recurrent disease were still eligible for salvage transplantation. In light of recent theoretical analyses,39,40 we have considered transplantation for selected patients who develop tumor recurrence following partial hepatectomy. Indeed, 1 of our patients did undergo transplantation and another patient is still on the transplant waiting list. Although salvage transplantation is a means to ration available organs, it is an unproven strategy.39 Patients with recurrent HCC are also eligible for a number of other treatments. Repeat hepatectomy has proven effective41-43 and was performed in 2 patients in this series.
In summary, although patients with early HCC who have adequate liver function represent a small subset of patients with HCC, they have comparable survival when treated with partial hepatectomy as opposed to liver transplantation. Partial hepatectomy remains the gold standard for these patients. The current paradigm of transplanting patients who have early HCC and preserved liver function who are also eligible for resection remains dubious.
Discussions
Dr. Russell W. Strong (Queensland, Australia): This is a timely presentation which challenges the ever-increasing premise that liver transplantation should be the first and only option for HCC when the patient’s disease is within the restrictive criteria as outlined by Mazzaferro.
The unprecedented growth in liver transplantation for HCC in the United States in 2002 to 20% of all of the transplants was due to a change in the formula for organ allocation but is also a manifestation of the burgeoning problem already upon us and its magnitude is likely to become even greater. It could, in due course, overwhelm liver transplantation to the further disadvantage of patients with nonmalignant disease. The downgrading of the status for liver transplantation in patients with HCC will undoubtedly swing the pendulum back towards the nonmalignant recipients, whereupon the HCC patients are disadvantaged and are likely to have disease progression during a more prolonged waiting period and may therefore be rendered too advanced and nontransplantable.
Most would accept that an early HCC with advanced cirrhosis is best treated by liver transplantation. But can we reduce the burden on liver transplant programs by resection of limited HCC when the patient has adequate liver reserve? In an intention-to-treat analysis, the Barcelona Group found that in patients with Child’s A cirrhosis and portal hypertension who were selected to undergo resection, achieved better results than those patients assigned to transplantation which was hampered by patients becoming nontransplantable through disease progression during a prolonged waiting period and the Memorial Group have given us a reasonable argument towards this end. However, I believe there are a few points that I would like the authors to clarify.
In general, transplantation for HCC in the noncirrhotic is a contraindication because of the ability to resect major volumes of parenchyma, and if because of size and distribution this is impossible, the patient is unlikely to benefit from liver transplantation.
Twenty-two percent of your patients were noncirrhotic and therefore would be unlikely to be considered for transplantation. Were these patients the predominant recipients of the 28% of major hepatectomies and did they show less recurrent disease and therefore skew the results?
Was there any relationship between the 30% who demonstrated microvascular invasion on histology and recurrence? If this was the case, do you consider that this high risk of recurrence would warrant early liver transplantation as advocated by the Barcelona Group rather than waiting for recurrence and then referring for liver transplantation if still within the restricted criteria?
I thank you for the opportunity to comment.
Dr. Leslie H. Blumgart (New York, New York): Thank you very much, Dr. Strong.
I’d like to address the first point regarding the 20% or so of patients without cirrhosis. There really was no difference at all in outcome. For instance, the major resections were performed in patients with cirrhosis. We were unable to show any difference at all in the usual clinical and pathologic features as predictors of outcome in this study. So I really don’t think that it has had any effect on the results.
The question of the drop-outs and the equivalent survival shown by the Barcelona Group between transplantation and resection is interesting and you raise the question of possible differences in outcomes for patients with vascular invasion. In this regard, it should be noted that the whole concept of performing transplantation for early HCC is based on the Mazzaferro paper from Milan. It is interesting that in our current study we had a median size of tumor of 3.5 cm. The Mazzaferro paper had a median tumor size of 1.5 cm for transplanted patients. There was also no vascular invasion seen in any of the patients in that study. Indeed they were a good risk group of patients and would have been predicted to do well. In addition, there was only a two-year follow-up. So I am not convinced that the results from Milan are such an excellent guide for the selection of patients with good liver function and small tumors as has been claimed.
Dr. John Wong (Hong Kong, China): We are very interested in the results just published because they have helped to confirm our experience of 135 such patients, published last year, with respect to whether patients should undergo primary resection or transplantation.
The group that seemed likely to benefit from primary transplantation, as mentioned by Dr. Strong, are those who underwent resection and then developed intrahepatic recurrence. Nevertheless, most of these patients are still eligible for transplantation even after developing recurrence.
I have 3 questions for Dr. Blumgart.
Did your group analyze those patients with intrahepatic recurrence to determine the risk factors that identify who might be better selected for primary transplantation?
The second question is, were any of the 611 patients who had the same criteria of transplant eligibility but were not resected? For example, did they have tumors that were very close to the cava or were there patients with 2 separate small tumors on different lobes of the liver?
Finally, have you considered using radiofrequency ablation for these small tumors rather than a major resection as the primary treatment? Thank you.
Dr. Leslie H. Blumgart (New York, New York): There are 3 points there, Dr. Wong.
Firstly, in relation to risk factors. This was a small group. We did look at predictors in this group of patients but the numbers are really too small for firm conclusions. However, as far as we could judge they were still transplant eligible.
The question you asked about the nonresected group is very interesting and important. Yes, of course, there were some patients with small tumors in inappropriate anatomic locations or with multiple tumors who were treated either with 1 of the ablative methods or were referred for transplantation. Indeed we have increasingly referred patients for transplantation in recent years. So the answer is yes, we do consider some selected patients (I think that is what is inherent in your question) for primary transplantation. This applies especially to young patients with hepatitis C and patients with badly placed small tumors, which would need a major resection of functional liver tissue. However, some of our reluctance in referring patients for transplantation has been what we perceive as a tendency to use live related donors in these patients - an approach with which we have some apprehension.
As regards RFA - yes, of course, we have used radiofrequency ablation. It is interesting that indeed it is being used as a bridging procedure in some transplant units. However, if you look at the results of the Barcelona Group, who studied RFA-treated patients in the liver explants, it was shown that very few patients actually have had full control of tumors. RFA is popular but unproven. In addition, it requires placement of a large probe often into a small tumor and there is some risk of dissemination. I was speaking to Jordi Bruix from the Barcelona Group the other day and he is somewhat reluctant to accept RFA for that reason. Indeed the results of alcohol injection combined with embolization are just as good. So yes, we have used RFA, but I am not sure the evidence for efficacy is there.
Dr. William C. Chapman (St. Louis, Missouri): I too would like to compliment the authors on their excellent results with a difficult group of patients. And while I think no 1 would dispute the excellent short-term and medium-term results reported today, significant questions remain regarding the best long-term treatment approach for patients with cirrhosis and early-stage HCC because of the risk of intrahepatic recurrences from small lesions that cannot be detected in the remnant liver or from second primaries.
In the submitted abstract, you reported that the presence of hepatitis B or C was a significant predictor of poor survival following hepatectomy. But this factor was not discussed in the paper. I wonder if you would comment on this aspect.
Since the most common indication for liver transplantation in the U. S. today is hepatitis C-associated liver disease and since, as you point out, the risk of developing HCC is estimated to be increased by upwards of 100-fold in this subgroup, should resection be considered as a first modality under consideration in this subgroup?
At the risk of significantly oversimplifying this issue, partial hepatectomy for HCC in the setting of cirrhosis and active hepatitis has been compared with performing segmental colectomy for colon cancer in the setting of chronic ulcerative colitis with plans for follow-up surveillance of the remaining colon. Clearly, the significant organ shortage that we currently face significantly complicates this issue.
I would like to thank the authors for asking me to discuss this paper and the Association for the privilege of the floor.
Dr. Leslie H. Blumgart (New York, New York): Thank you, Dr. Chapman. Your point regarding hepatitis C is important. As I have just said in response to 1 of the previous questions, we would consider transplanting patients with hepatitis C, particularly with multiple lesions or poorly situated tumors.
As to your second question, what you are really referring to, I think, is recurrence-free survival. Improved recurrence-free survival has been much touted and heavily based on the original Milan series. However, there are but few transplant series that have reached five-year survival figures. There are only a handful of papers I could find with five-year follow-up data. And in those papers, recurrence-free survival is around 50% at 5 years. This is very similar to our data with a five-year survival at 48%.
The reported 81% survival in the Milan paper is very difficult to accept. It should be recognized, as I have already stated, that these tumors were small and without vascular invasion and in addition there was previous ablation in half of the cases. The follow-up was only 2 years and no account was taken of patients who drop out of the transplant waiting list due to progressive disease.
I am not pretending we have the final answer to this question. It is a wide-open debate and something which will be ongoing. What I am pleased about is that we have stimulated discussion and I thank you for your comments.
Dr. Henri Bismuth (Villejuif, France): I congratulate you, Dr. Blumgart, for with 68% five-year survival you have excellent results. But five-year disease-free survival is 48%. In our series of transplanted patients with HCC, on 116 patients with the same selection, we have 73% five-year overall survival and 73% disease-free survival. This identical overall and disease-free survival means that the disease is controlled, which is not apparently the case in your resected patients.
I do not think that today liver resection in this group of patients may be considered as the standard therapy. But you said that you have a super selected group of patients with no recurrence after resection. So my question is how you may select preoperatively this subgroup of patients who will not have recurrence after resection?
Dr. Leslie H. Blumgart (New York, New York): I suppose with the same difficulty as you select them for transplantation. But you are giving a paper later today on the same topic, Henri. Unlike the United Nations, I don’t think the French should be allowed 2 tries to affect the debate. (Laughter)
Footnotes
Reprints: Ronald P. DeMatteo, MD, Hepatobiliary Service, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021. E-mail: dematter@mskcc.org.
REFERENCES
- 1.Akriviadis EA, Llovet JM, Efremidis SC, et al. Hepatocellular carcinoma. Br J Surg. 1998;85:1319-1331. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36:S30-S34. [DOI] [PubMed] [Google Scholar]
- 3.El S, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [DOI] [PubMed] [Google Scholar]
- 4.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942-1956. [DOI] [PubMed] [Google Scholar]
- 5.Di B, Simpson LH, Lotze MT, et al. Development of hepatocellular carcinoma among patients with chronic liver disease due to hepatitis C viral infection. J Clin Gastroenterol. 1994;19:222-226. [DOI] [PubMed] [Google Scholar]
- 6.Alter MJ, Kruszon M, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562. [DOI] [PubMed] [Google Scholar]
- 7.Williams I. Epidemiology of hepatitis C in the United States. Am J Med. 1999;107:2S-9S. [DOI] [PubMed] [Google Scholar]
- 8.The Liver Cancer Study Group of Japan. Predictive factors for long term prognosis after partial hepatectomy for patients with hepatocellular carcinoma in Japan. Cancer. 1994;74:2772-2780. [DOI] [PubMed] [Google Scholar]
- 9.Bismuth H, Houssin D, Ornowski J, et al. Liver resections in cirrhotic patients: a Western experience. World J Surg. 1986;10:311-317. [DOI] [PubMed] [Google Scholar]
- 10.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagao T, Inoue S, Goto S, et al. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987;205:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagasue N, Kohno H, Chang YC, et al. Liver resection for hepatocellular carcinoma. Results of 229 consecutive patients during 11 years. Ann Surg. 1993;217:375-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vauthey JN, Klimstra D, Franceschi D, et al. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg. 1995;169:28-34. [DOI] [PubMed] [Google Scholar]
- 15.Shirabe K, Takenaka K, Taketomi A, et al. Postoperative hepatitis status as a significant risk factor for recurrence in cirrhotic patients with small hepatocellular carcinoma. Cancer. 1996;77:1050-1055. [PubMed] [Google Scholar]
- 16.Lai EC, Lo CM, Fan ST, et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg. 1998;133:183-188. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M, Arii S, Sugahara K, et al. Adjuvant oral chemotherapy to prevent recurrence after curative resection for hepatocellular carcinoma. Br J Surg. 1996;83:336-340. [DOI] [PubMed] [Google Scholar]
- 18.Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;110:726-734. [PubMed] [Google Scholar]
- 19.Soreide O, Czerniak A, Blumgart LH. Large hepatocellular cancers: hepatic resection or liver transplantation? Br Med J (Clin Res Ed). 1985;291:853-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemming AW, Cattral MS, Reed AI, et al. Liver transplantation for hepatocellular carcinoma. Ann Surg. 2001;233:652-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwatsuki S, Starzl TE, Sheahan DG, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pichlmayr R, Weimann A, Steinhoff G, et al. Liver transplantation for hepatocellular carcinoma: clinical results and future aspects. Cancer Chemother Pharmacol. 1992;31(Suppl):S157-S161. [DOI] [PubMed] [Google Scholar]
- 23.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [DOI] [PubMed] [Google Scholar]
- 25.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [DOI] [PubMed] [Google Scholar]
- 26.Olthoff KM, Millis JM, Rosove MH, et al. Is liver transplantation justified for the treatment of hepatic malignancies? Arch Surg. 1990;125:1261-1266. [DOI] [PubMed] [Google Scholar]
- 27.Olthoff KM, Rosove MH, Shackleton CR, et al. Adjuvant chemotherapy improves survival after liver transplantation for hepatocellular carcinoma. Ann Surg. 1995;221:734-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone MJ, Klintmalm GB, Polter D, et al. Neoadjuvant chemotherapy and liver transplantation for hepatocellular carcinoma: a pilot study in 20 patients. Gastroenterology. 1993;104:196-202. [DOI] [PubMed] [Google Scholar]
- 29.Figueras J, Jaurrieta E, Valls C, et al. Resection or transplantation for hepatocellular carcinoma in cirrhotic patients: outcomes based on indicated treatment strategy. J Am Coll Surg. 2000;190:580-587. [DOI] [PubMed] [Google Scholar]
- 30.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [DOI] [PubMed] [Google Scholar]
- 31.Van T, Carr B, Iwatsuki S, et al. The 11-year Pittsburgh experience with liver transplantation for hepatocellular carcinoma: 1981-1991. J Surg Oncol Suppl. 1993;3:78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto J, Iwatsuki S, Kosuge T, et al. Should hepatomas be treated with hepatic resection or transplantation? Cancer. 1999;86:1151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown RS, Russo MW, Lai M, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818-825. [DOI] [PubMed] [Google Scholar]
- 34.Kawachi S, Shimazu M, Wakabayashi G, et al. Biliary complications in adult living donor liver transplantation with duct-to-duct hepaticocholedochostomy or Roux-en-Y hepaticojejunostomy biliary reconstruction. Surgery. 2002;132:48-56. [DOI] [PubMed] [Google Scholar]
- 35.Testa G, Malago M, Valentin G, et al. Biliary anastomosis in living related liver transplantation using the right liver lobe: techniques and complications. Liver Transpl. 2000;6:710-714. [DOI] [PubMed] [Google Scholar]
- 36.Gondolesi G, Munoz L, Matsumoto C, et al. Hepatocellular carcinoma: a prime indication for living donor liver transplantation. J Gastrointest Surg. 6:102-107. [DOI] [PubMed]
- 37.Kaihara S, Kiuchi T, Ueda M, et al. Living-donor liver transplantation for hepatocellular carcinoma. Transplantation. 2003;75:S37-S40. [DOI] [PubMed] [Google Scholar]
- 38.Blumgart LH, Soreide O, Czerniak A. Large hepatocellular cancers: hepatic resection or liver transplantation. Br Med J (Clin Res Ed). 1985;291:1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majno PE, Sarasin FP, Mentha G, et al. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:899-906. [DOI] [PubMed] [Google Scholar]
- 40.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235:373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu RH, Lee PH, Yu SC, et al. Surgical resection for recurrent hepatocellular carcinoma: prognosis and analysis of risk factors. Surgery. 1996;120:23-29. [DOI] [PubMed] [Google Scholar]
- 42.Shimada M, Matsumata T, Taketomi A, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 1994;115:703-706. [PubMed] [Google Scholar]
- 43.Wu MC, Chen H, Yan YQ. Rehepatectomy of primary liver cancer. Semin Surg Oncol. 9:323-326. [DOI] [PubMed] [Google Scholar]