Abstract
Objective:
To compare the detection rates for rectal cancer cells in blood and bone marrow in patients with or without preoperative chemoradiation.
Summary Background Data:
Previous reports have postulated a resistance of disseminated tumor cells to antiproliferative agents because of tumor cell dormancy.
Methods:
Blood samples from 142 patients (pre, intra-, and postoperative samples) and bone marrow samples from 127 patients undergoing resection of rectal adenocarcinoma were analyzed for tumor cells using a cytokeratin (CK) 20-reverse transcription polymerase chain reaction. The results were stratified according to preoperative therapy.
Results:
In patients without preoperative chemoradiation, tumor cell detection in blood and bone marrow correlated to tumor stage (Cochran Armitage trend test, P < 0.05). Tumor cells were detected in 34 of 103 (33%) bone marrow and 65 of 117 (55.6%) blood samples of patients without neoadjuvant treatment versus in 4 of 24 (16.7%) bone marrow and in 10 of 25 (40%) blood samples of patients with neoadjuvant treatment. The tumor cell detection rate was significantly lower in the group having undergone chemoradiation (binary logistic regression analysis, P < 0.05). The overall and disease-free survival were significantly worse in patients with tumor cell detection in the bone marrow after neoadjuvant therapy.
Conclusions:
Preoperative chemoradiation is associated with a decreased detection rate of rectal cancer cells in blood and bone marrow. These findings may explain the observed clinical benefit of patients with rectal cancer receiving chemoradiation. This is the first study suggesting that detection of disseminated rectal cancer cells may be useful for assessing the efficacy of neoadjuvant therapy.
Blood and bone marrow of 142 patients with or without preoperative chemoradiation for rectal cancer were tested for disseminated tumor cells. Preoperative chemoradiation was associated with a decreased detection rate of cancer cells. Patients without tumor cell detection in the bone marrow after neoadjuvant therapy had an excellent prognosis.
Surgical resection is the basis of therapy for patients with rectal cancer. Despite potentially curative resection, however, these patients are at high risk for systemic and local tumor recurrence caused by disseminated tumor cells not detected by current staging methods. One of the objectives of (neo-)adjuvant chemoradiation is the eradication of these tumor cells, thereby decreasing disease relapse and improving patient survival.1-3 In patients with nonresectable tumors (uT4), preoperative chemoradiation has the additional potential to downstage the tumor to allow a complete tumor resection.1-3
Because disseminated tumor cells rarely express proliferation-associated markers, it has been speculated that these cells may be resistant to antiproliferative agents.4,5 Currently, the efficacy of chemoradiation in the individual rectal cancer patient cannot be adequately assessed. The development of a surrogate marker to monitor the efficacy of (neo)-adjuvant treatment of rectal cancer would allow individualization of therapeutic regimes and thereby probably improve the management of these patients.
Polymerase chain reaction (PCR)-based protocols are sensitive and specific assays for detection of disseminated cancer cells, allowing the identification of approximately 1 neoplastic cell in 107 normal peripheral mononuclear blood cells.6,7 The detection of disseminated cancer cells by reverse-transcription PCR is based on detection of mRNA. Because blood and bone marrow contain sufficient RNAase to destroy extracellular RNA within a few seconds, the detection of mRNA in blood and bone marrow samples is generally accepted as an indicator for the presence of viable cells.8,9 Recently, we demonstrated the sensitivity and specificity of a cytokeratin (CK) 20-reverse transcription (RT)-PCR system in detecting disseminated colorectal cancer cells in blood and bone marrow.10-13 In this study, we compared the frequency of tumor cell detection in blood and bone marrow of patients with rectal cancer having either undergone preoperative chemoradiation or not to evaluate the efficacy of neoadjuvant therapy in eliminating disseminated cancer cells.
PATIENTS AND METHODS
Patients and Treatment
Informed consent was obtained from all patients. The study protocol was approved by the Ethics Committee of the University of Heidelberg. Patients (n = 154) with histologically confirmed primary rectal adenocarcinoma treated at the Departments of Surgery and Radiotherapy, University of Heidelberg were included (127 patients without, 27 patients with neoadjuvant therapy). Blood samples were available from 144 of the 154 patients (117 patients without, 27 patients with neoadjuvant therapy; in 2 patients receiving neoadjuvant therapy, only preoperative blood samples were obtained). Informed consent for bone marrow aspiration was obtained from 127 patients (103 patients without, 24 patients with neoadjuvant therapy). Patients with other malignant disease in their medical history were excluded. All patients were staged locally using endorectal ultrasonography, in case of suspected tumor infiltration into surrounding structures (uT4) a Hydro-CT scan of the pelvis was performed.
Patients with suspected tumor infiltration into surrounding structures (uT4) and patients with very low tumors with the intention of sphincter preserving resection were subjected to neoadjuvant chemoradiation. Surgery was performed 5 to 7 weeks after termination of neoadjuvant treatment.
The tumor was resected according to the “no-touch isolation” technique with total mesorectal excision, either by abdominoperineal resection or by low anterior resection. All patients who received neoadjuvant treatment and the patients with uT3/4 or uN+ tumors were subjected to intraoperative radiotherapy (IORT).
Patients with stage II or III tumors, not being subjected to neoadjuvant treatment, received postoperative radiochemotherapy in addition to IORT. Tumor stage and grading were classified according to the 5th edition of the TNM classification of the UICC (International Union Against Cancer).14
Chemoradiation
Preoperative external beam radiotherapy was delivered with 23 MV photons. A 3-field technique (equally weighted posterior and lateral fields) was used with cephalad field borders after 3-dimensional treatment planning based on continuous computer tomographic slices. The mean neoadjuvant radiation dose was 41.1 Gy (29.3-50.4), which was determined to the reference point according to ICRU 50. Doses were delivered by conventional fractionation at 1.8 Gy per fraction, 5 fractions a week.
IORT was performed with an apparatus permanently installed in an operation theater of the surgical department. A magnetron-powered linear accelerator (Siemens Mevatron ME) provided electron beam energies from 6 to 18 MeV, corresponding to a depth of 24-54 mm down to the 90% isodose. The IORT dose was 10 to 15 Gy, depending on the applied neoadjuvant or planed adjuvant radiation dose.
In 17 patients, neoadjuvant chemotherapy was given as concomitant fractions on days 1-5 and 16-20 of the external beam radiotherapy (350 mg/m2 5-FU and 20 mg/m2 Leucovorin) and completed by additional 4 weekly cycles after the operation. Ten patients had weekly continuous chemotherapy (300 mg/m2 5-FU) during radiotherapy without postoperative chemotherapy.
Blood and Bone Marrow Samples
Three blood samples (10 mL) were obtained from each patient through a central venous catheter in the superior vena cava: the first after induction of anesthesia, the second after resection of the tumor, and the third 24 hours after the operation. Bone marrow samples (10 mL) were obtained after induction of general anesthesia by aspiration from both iliac crests (puncture site well outside irradiation field). The blood and bone marrow samples were diluted with 10 mL phosphate-buffered saline. After density centrifugation through Ficoll-Paque (Pharmacia; 30 minutes, 400 g) mononuclear cells were harvested from the interphase and washed twice in phosphate-buffered saline. The cell pellet was then shock frozen in liquid nitrogen and stored at −70°C.
RNA Extraction
RNA extraction from peripheral mononuclear blood cells, from bone marrow samples and from frozen tissue sections of tumors was conducted as previously described.10
RT-PCR
CK 20-RT-PCR was performed as previously described.10 PCR products were analyzed by electrophoresis on 2% agarose gels. PCR products were blotted onto nylon membranes (Hybond N+, Amersham Life Science, Buckinghamshire, UK) and hybridized with a chemoluminescence-labeled oligonucleotide probe as previously described.11 RNA quality and performance of reverse transcription of all analyzed samples was confirmed by RT-PCR amplification of glyceraldehyde phosphate dehydrogenase transcripts as previously described.10
Follow-Up and Statistical Analysis
Statistical computations were done using the software packages S-Plus (Insightful Corp.) and StatXact4 for Windows (Cytel Software Corp). A result was considered statistically significant when the P value was less than or equal to 5% (P ≤ 0.05). To provide quantitative information of the relevance of statistically significant results, 95% confidence intervals for odds ratios were computed. A binary logistic regression model was used to assess the effect of stage and neoadjuvant therapy on the detection rate of disseminated tumor cells.15 In patients undergoing neoadjuvant chemoradiation, the clinical stage was taken as basis for this analysis.16
The Cochran-Armitage trend test was used to determine the relationship between T, N category, stage, and the detection of disseminated tumor cells in patients not receiving neoadjuvant treatment.17 The time of follow up was calculated from the date of surgery. Survival was estimated according to the Kaplan-Meier method and compared using the log-rank test.18
RESULTS
Patient Characteristics
Included in this study were 154 patients with histologically confirmed primary rectal adenocarcinoma (99 male, 55 female; ages 27-96; mean, 61); 27 patients received neoadjuvant therapy, and 127 patients did not undergo neoadjuvant therapy. Indication for neoadjuvant therapy included suspected tumor infiltration into surrounding structures (clinically T4; n = 21) and patients with tumors in close proximity to the anal sphincter with the intention of sphincter preserving resection (n = 6). The stage distribution of patients without neoadjuvant chemoradiation is displayed in Table 1. The clinical stages of the patients undergoing neoadjuvant radiation were the following: Stage I: 0 patients, Stage II: 9 patients, Stage III: 11 patients, and Stage IV: 7 patients. The pathohistological (post-therapeutic) stages of these patients were the following: Stage I: 7 patients, Stage II: 4 patients, Stage III: 7 patients; and Stage IV: 7 patients. Two patients showed a complete histologic treatment response.
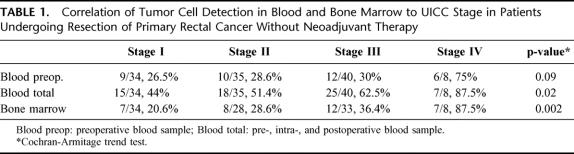
TABLE 1. Correlation of Tumor Cell Detection in Blood and Bone Marrow to UICC Stage in Patients Undergoing Resection of Primary Rectal Cancer Without Neoadjuvant Therapy
Tumor Cell Detection in Blood and Bone Marrow
Blood samples of 144 patients (117 patients without neoadjuvant therapy, 27 patients receiving neoadjuvant therapy) and bone marrow samples of 127 patients (103 patients without neoadjuvant therapy, 24 patients receiving neoadjuvant therapy) with rectal carcinoma were analyzed by CK 20-RT-PCR. The sensitivity of the CK 20-RT-PCR assay was determined in previous cell-spiking experiments. The assay allows the detection of 10 HT 29 cells in 10 mL of blood.10 The specificity of the CK 20-RT-PCR assay was determined in recent studies, 174 blood samples of 98 individuals and bone marrow samples of 30 patients without malignant disease consistently tested negative for CK 20 expression.10-13,19,20
In patients without neoadjuvant therapy, CK 20 transcripts were detected in at least 1 of the 3 blood samples taken (pre, intra-, and postoperative samples) in 55.6% of patients (65/117), in preoperative blood samples only in 31.6% of patients (37/117) and in bone marrow samples in 33% of patients (34/103). Tumor cell detection in blood (pre, intra- and postoperative samples) and bone marrow followed a statistically significant stage dependent trend (Cochran-Armitage trend test; Table 1).
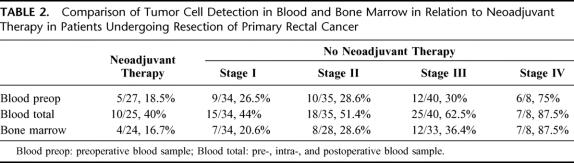
The results of tumor cell detection in blood and bone marrow of patients having received neoadjuvant chemoradiation in comparison to patients without this therapy are displayed in Table 2. Tumor cell detection rates in blood and bone marrow of patients having undergone neoadjuvant therapy were even lower than detection rates in stage I patients without neoadjuvant therapy.
TABLE 2. Comparison of Tumor Cell Detection in Blood and Bone Marrow in Relation to Neoadjuvant Therapy in Patients Undergoing Resection of Primary Rectal Cancer
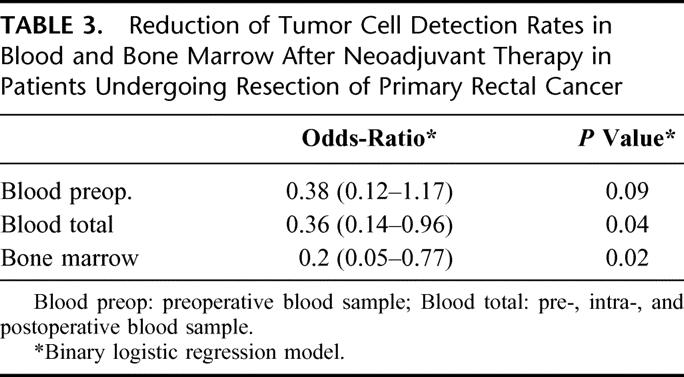
The effect of preoperative chemoradiation was then analyzed by a logistic regression model, which included tumor stage as additional risk factor. Neoadjuvant chemotherapy was associated with a significantly lower detection rate of tumor cells in bone marrow and in pre, intra- and postoperative blood samples in this analysis. This result was independent of tumor stage. The probability of detecting tumor cells in the bone marrow of patients having undergone neoadjuvant chemoradiation was 5 times lower than that of patients not having had neoadjuvant therapy, in blood samples this probability was still almost 3 times lower (Table 3).
TABLE 3. Reduction of Tumor Cell Detection Rates in Blood and Bone Marrow After Neoadjuvant Therapy in Patients Undergoing Resection of Primary Rectal Cancer
Follow-Up
The 20 patients who received neoadjuvant therapy and underwent R0 resection were included in the follow-up analysis. No patient died of unrelated disease and no patient was lost to follow-up. At the time of last follow-up 13 patients have no evidence of disease, 1 patient developed a local recurrence, 3 patients developed liver metastases, 2 patients developed pulmonary metastases, and 1 patient developed liver and pulmonary metastases. Four of the latter patients are alive with disease, and 3 patients have died of disease. The median follow up of these patients is 49 months (range, 15-72 months). The median overall and disease free survival has not yet been reached, the estimated 4-year overall survival is 79% and the disease-free survival is 60%.
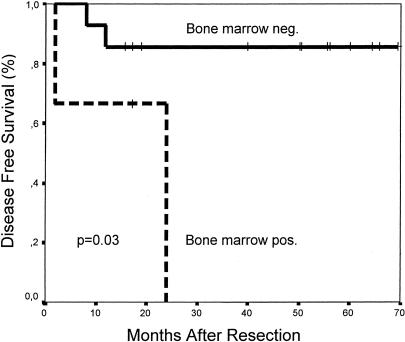
Bone marrow data were available for 17 of the patients. Tumor cells were detected in the bone marrow of 3 patients, 2 of which developed liver metastases (one patient has died of disease, the other is alive with disease). Of the 14 patients without detectable tumor cells in the bone marrow, only 2 patients have developed a recurrence. One patient developed lung metastases (alive with disease) and the other patient developed liver metastases (died of disease). The estimated 4-year disease free survival rate is 85% in patients without detectable tumor cells in the bone marrow (median survival has not yet been reached, median follow up is 50 months, range: 15-70 months) versus 0% in the bone marrow positive group (median survival 24 months; log-rank test, P = 0.03; Fig. 1). The estimated 4-year overall survival rates are 91% for the bone marrow negative group (median survival has not yet been reached) versus 0% for the bone marrow positive group (median survival: 42 months; log-rank test, P = 0.04).
FIGURE 1. Disease-free survival for R0 resected patients after neoadjuvant chemoradiation stratified according to detection of tumor cells in bone marrow.
Tumor cells were detected in pre, intra-, and postoperative blood samples in 7 of 20 patients having undergone neoadjuvant therapy. Three of the 7 patients with detectable tumor cells in the blood samples have in the meantime recurred (lung metastases n = 1, liver metastases n = 1, local recurrence n = 1). One of these patients has died of disease, the other 2 are alive with disease. Four of the 13 patients without detectable tumor cells in the blood have developed distant metastases. Two of these patients have died of disease, and the other 2 are alive with disease. The estimated 4-year disease free survival rates are 68% for the blood-negative group (median survival has not yet been reached, median follow-up is 50 months; range, 16-70 months) and 48% for the blood positive group (median survival: 48 months; P = ns), the estimated 4-year overall survival rates are 81% and 75% respectively (median survival has not yet been reached for both groups; P = ns).
DISCUSSION
This study investigated the association of neoadjuvant chemoradiation with hematogenous dissemination of rectal cancer. Monitoring the efficacy of neoadjuvant chemoradiation could potentially be of high value for patients with rectal carcinoma because therapeutic regimens could be more specifically tailored to the individual patient. This is of special importance because several studies have indicated that antiproliferative agents may not be effective against disseminated tumor cells as these cells rarely express proliferation-associated markers.4,5 In contrast to local downstaging, which can be assessed by imaging techniques, such as rectal endosonography or positron emission tomography,21,22 the response of disseminated tumor cells to systemic treatment cannot be adequately evaluated. Even though some authors have suggested that a significant tumor response to neoadjuvant chemoradiation is correlated to an improved survival, this has not been confirmed in other studies.23-25 Efficacy of antiproliferative drugs against disseminated tumor cells might be predictable by molecular characterization of the primary tumors, as rectal cancers without c-K-ras-mutations or with p53-mutations appear to be less sensitive to preoperative chemoradiation.26,27 The level of thymidylate synthase and polymorphisms of the repeated sequences in the enhancer of the thymidylate synthase gene promoter in tumor tissue may also allow prediction of the response to preoperative chemoradiation.28,29 However, prospective data from large patient cohorts regarding the value of these molecular markers are not yet available. In addition, cancers may be very heterogeneous; therefore assessment of the primary tumor may not accurately predict the behavior of disseminated cancer cells.30 Detection of disseminated rectal cancer cells in blood and bone marrow may prove to be a suitable surrogate marker for monitoring the efficacy of systemic therapy in rectal cancer patients. In breast cancer patients the validity of this concept has already been demonstrated.4,31 Comparable data has not yet been published for rectal cancer.
In this study, we determined the frequency of hematogenous tumor cell spread in patients with rectal cancer by CK 20-RT-PCR. Immunohistochemistry showed a similar rate of CK 20 expression in the resected rectal carcinomas of those patients having undergone neoadjuvant chemoradiation compared with published data of CK 20 expression in untreated colorectal carcinomas (data not shown).32 This suggests that neoadjuvant treatment does not lead to a down-regulation of CK 20-expression in tumor cells, which could have resulted in an increased rate of false negative findings in the used assay.
To exclude mere sampling errors as cause for the obviously different detection rates in the investigated groups the results were statistically analyzed. In patients not having had neoadjuvant therapy, we were able to demonstrate a significant correlation of the rate of tumor cell detection to tumor stage (Table 1). To eliminate the influence of tumor stage on the detection rates, the analysis of the potential effect of neoadjuvant therapy was performed by binary logistic regression. This analysis demonstrated, that preoperative chemoradiation of rectal cancer is significantly associated with a lower detection rate of disseminated cancer cells in blood (obtained pre, intra-, and postoperatively) and bone marrow and revealed a strong trend for the same association in preoperative blood samples (Table 3). As hematogenous tumor cell spread is a function of primary tumor stage, we used pretherapeutic stages, as assessed by rectal ultrasonography and CT scan, as basis for the analysis. Endorectal ultrasound is currently regarded as the most accurate modality for local staging of rectal cancer.21,33,34 We did not use the post-therapeutic stages for the above analysis since neoadjuvant therapy is known to result in downstaging in a considerable number of patients.2 The resulting lower tumor stages would have introduced a significant bias into the statistical analysis as tumor cell dissemination is predicted to be less in lower stages. The calculation of the effect of neoadjuvant therapy based on post-therapeutic stages would have probably underestimated the actual effect of this treatment on disseminated cells. In summary, our results seem to indicate an association of neoadjuvant chemoradiation to lower tumor cell detection rates in blood (pre, intra- and postoperative samples) and bone marrow.
Previous reports have postulated a resistance of disseminated tumor cells to antiproliferative agents because of tumor cell dormancy.4,5 The latter hypothesis, however, is in conflict with studies demonstrating improved survival of patients with rectal cancer having undergone chemoradiation which in turn suggests that this form of additional treatment may indeed be able to eliminate systemic tumor cell dissemination.1,3,35,36 Our results support this and may, at least partly, explain the observed clinical benefit of patients with rectal carcinoma having received chemoradiation. In our study, patients without detectable tumor cells in the bone marrow samples after neoadjuvant chemoradiation had a significant better overall and disease free survival compared with patients with detectable tumor cells. These data need to be confirmed by multivariate analysis of a larger patient cohort. The 4-year disease free survival rate of 85% and the 4-year overall survival rate of 91% in the group with negative bone marrow samples suggest a particularly good prognosis for this subgroup of patients. Further long-term follow-up is needed to clarify whether tumor cell detection in blood or tumor cell detection in bone marrow is more suitable to serve as a marker of efficacy for systemic therapy. In this study, the effect of neoadjuvant therapy was more evident on tumor cells detected in bone marrow samples and only tumor cell detection in bone marrow had any prognostic impact.
Based on our data, we hypothesize that neoadjuvant chemoradiation is associated with a lower frequency of disseminated tumor rectal cancer cells and that the detection of disseminated tumor cells by CK 20-RT-PCR may be able to serve as a marker to evaluate the efficacy of systemic therapy in patients with rectal cancer. The next step would be to perform serial examinations of patients with rectal cancer before and after chemoradiation to further substantiate our hypotheses. If the clinical validity of our data can indeed be confirmed, the treatment of rectal cancer may possibly be further individualized and guided by the detection of disseminated cancer cells.
Discussions
Dr. Harold J. Wanebo (Providence, Rhode Island): I want to congratulate Dr. Kienle and the Heidelberg Group on a very informative study illustrating a true in vivo tumor cell sensitivity assay and its potential in determining responsiveness to neoadjuvant therapy in rectal cancer patients. I have 3 questions:
Is there any relationship of the tumor cell detection to the CEA level, which has been shown to be at least a prognostic determinant in colorectal cancer in general?
Secondly, is there a correlation of the detected tumor cell level to the pattern of recurrence? I gather from your data that there is a higher pattern of distant failure, but is there a difference according to the pattern of the blood determined tumor cells versus the bone marrow determined tumor cells? For example, is there more likely a recurrence related to the presence of bone marrow detected cells?
Lastly, I think you alluded to it, but what would be your consideration in treating patients that do have persistence of tumor cells after neoadjuvant therapy, which would suggest that they are not responding. Is there some other option that might be done?
I want to thank the Association for the privilege of the floor and for a very informative contribution.
Dr. Christian Herfarth (Heidelberg, Germany): Thank you very much for your questions, Dr. Wanebo. I can’t tell you anything about the correlation of CEA levels to tumor cell detection because we did not specifically analyze that. The main objectives of our study were to investigate how tumor cell detection was related to tumor stage and what influence neoadjuvant therapy had on the tumor cell detection rate. After having shown that the tumor cell detection rate in blood and bone marrow indeed increases with more advanced tumor stages, the next step would be to perform a larger prospective trial where the tumor cell detection rate using CK-20-RT-PCR is compared with CTA levels and other established risk factors.
Concerning your question on correlation of tumor cell detection and pattern of recurrence we cannot answer this because the follow-up has not yet been completed for the whole patient cohort. In the group of patients having undergone neoadjuvant therapy only 7 patients developed a recurrence and we failed to find any specific pattern here. However, this probably has little significance due to the small sample size.
That brings us to your third question, what are our future plans, especially in regard to the treatment of those patients with disseminated tumor cells. Well, we are now planning to perform a prospective trial looking at disseminated tumor cells in bone marrow before and after neoadjuvant chemoradiation in the same patients. We initially couldn’t do that because the local ethics committee would not give us permission to aspirate bone marrow without general anesthesia before the surgery within this study. They argued that there was too little evidence that chemoradiation indeed had any influence on the detection rate of disseminated cells and that this had any pathologic meaning in the cause of the disease. Now, after having shown that neoadjuvant therapy is associated with a lower tumor cell detection rate we are optimistic that we will get ethics committee approval to look for disseminated tumor cells in the bone marrow before chemoradiation. Our long-term goal is to use disseminated tumor cells as a surrogate marker for the effectiveness of systemic therapy. Patients with persistence of tumor cells after systemic therapy might benefit from a change of therapeutic regimen.
Dr. Olga Jonasson (Chicago, Illinois): Approximately 50 years ago Dr. Warren Cole, a former president of this Society, had his laboratory study cancer cells in circulating blood. Some of these papers were presented at these meetings. The techniques used then were highly labor intensive.
Many of us were studying peripheral smears with - with Papanicolau’s technique. And certainly the cancer cells were there. But what surprised us then and in subsequent reports, using other more elegant staining technologies or identification techniques, such as yours, have continued to show these circulating cancer cells in the blood and in the bone marrow. Not all of these patients with these cells, though, go on to die of their disease, and I am certain there are many factors which bear upon the cell’s ability to establish distant disease. I wonder if you might speculate on those mechanisms.
Dr. Christian Herfarth (Heidelberg, Germany): Thank you very much for this very interesting and essential question. It is well known that disseminated tumor cells can regularly be found in patients with tumors. I would like to recall the investigations of Dr. Fidler from Houston, who analyzed isolated tumor cells in earlier experiments and found tumor cells in the blood already in early tumor stages. The main point is that not every tumor cell forms a metastasis, only about 1 of 10,000 or even more tumor cells develops into an overt metastasis.
The risk of formation of metastases is probably dependent on the number and the biologic behavior of the circulating tumor cells. As the PRC assay we used was not quantitative we cannot say anything about the number of detected tumor cells, however, a higher number of disseminated tumor cells probably increases the risk of a recurrence when considering the above estimates for metastases formation. Further studies are needed to better characterize these cells.
Dr. W. Douglas Wong (New York, New York): I congratulate Dr. Kienl and Dr. Weitz and Dr. Herfarth and their colleagues from the University of Heidelberg for a very thought-provoking and intriguing study in investigating the association of neoadjuvant chemoradiation with hematogenous dissemination of rectal cancer. I have a couple questions.
A comment first. Since this was not a randomized trial and there was considerable heterogeneity of the patients, it is not entirely clear to me whether the lower CK 20 positivity rate seen in the neoadjuvant group was truly due to the receipt of preoperative chemoradiation or to some other factor not represented in the statistical modeling. In the study, your CK positivity was very, very strong and length to stage IV disease. Since it may be more beneficial to monitor CK 20 levels and potentially cure those staged patients, I wonder if you eliminated the stage IV patients from your analysis and limited it just to the stage I to III patients, does the use of neoadjuvant chemoradiation still correlate with reduced CK 20 levels?
My second comment is that the correlation between the CK 20 positivity and relapse was not entirely tight, and since 20 to 40% of stage I patients are CK 20 positive yet we would expect only about a 10% relapse rate in stage I patients, my question is, do you have a sense of the predictive value for relapse in early-stage patients who did not receive any adjuvant therapy?
Then my final question pertains to the methodology. In this study you reported the RT-PCR is either positive or negative. That may be somewhat subjective, depending on what the band looks like and who is interpreting it. Have you considered or have you used quantitative PCR analysis? That may provide more objective data. When you reported your data, the PCR is reported either as positive or negative. I just wondered if you had used quantitative PCR analysis to give a more objective result. Thank you.
Dr. Christian Herfarth (Heidelberg, Germany): Thank you very much for these questions. I want to answer the last question first. Quantitative or semi-quantitative CK 20 PRC is probably not more accurate than the used RT-PCR for detecting disseminated cells in the study setting because CK-20 expression is known to be very heterogeneous in different tumor cells of the same patient. Therefore quantitative PCR not necessarily reflects the true number of tumor cells but merely the expression rate of the investigated marker gene. The used RT-PCR method is purely qualitative with a very high sensitivity, 1 tumor cell can be detected among 10 to 7 mononuclear cells.
To answer your other question, probably due to this very high sensitivity it is possible to find disseminated tumor cells also in a rather large number of patients with stage I disease. This again stresses the point, that detection of disseminated tumor cells in blood or bone marrow does not mean that the patient has a 100% chance of developing a recurrence. Therefore, not all these patients will indeed develop metastases, but they may have an increased risk for recurrence. This needs to be confirmed by the further oncological follow-up.
In this trial we could show that neoadjuvant chemoradiation reduces the tumor cell detection rate independent of the tumor stage, as the performed multivariate analysis was designed to correct for stage. Therefore also the number of patients with stage IV included in the study could not bias the result of the analysis.
Dr. Jose G. Guillem (New York, New York): It is well shown that following a long course of preoperative RT chemo that perhaps 12 to 15% of rectal cancers will demonstrate a complete pathologic response. In our institution’s experience it appears that this subset goes on to have few if any failures at 5 years, suggesting that local response may also be a surrogate marker of overall outcome and efficacy of systemic therapy. My question therefore is, although your sample size is small, did you see any relationship between local response and level of CK 20?
Dr. Christian Herfarth (Heidelberg, Germany): We had 2 patients with local recurrence who had a complete remission after neoadjuvant chemoradiation. Those patients did not have any disseminated tumor cells. Obviously a larger patient cohort needs to be examined to further clarify the role of disseminated tumor cells. I, however, want to point out, that the potential prognostic impact of local response to neoadjuvant treatment is currently debated in the literature. Detection of disseminated tumor cells might prove to be a valid diagnostic tool in this context.
Footnotes
Reprints: J. Weitz, MD, PhD, Department of Surgery, University of Heidelberg, INF 110, d-69120 Heidelberg, Germany. E-mail: juergen_weitz@med.uni-heidelberg.de.
P. K. and M. K. contributed equally to this study.
REFERENCES
- 1.Påhlmann L. Neoadjuvant and adjuvant radio- and radio-chemotherapy of rectal carcinomas. Int J Colorectal Dis. 2000;15:1-8. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler JD, Warren BF, Jones AC, et al. Preoperative radiotherapy for rectal cancer: implications for surgeons, pathologists and radiologists. Br J Surg. 1999;86:1108-1120. [DOI] [PubMed] [Google Scholar]
- 3.Ailouni M. The role of radiation therapy in the adjuvant treatment of rectal cancer. Curr Opin Gastroenterol. 2001;17:86-90. [DOI] [PubMed] [Google Scholar]
- 4.Braun S, Kentenich C, Janni W, et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. 2000;18:80-86. [DOI] [PubMed] [Google Scholar]
- 5.Pantel K, Schlimok G, Braun S, et al. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst. 1993;85:1419-1424. [DOI] [PubMed] [Google Scholar]
- 6.Pantel K, von Knebel Doeberitz M. Detection and clinical relevance of micrometastatic cancer cells. Curr Opin Oncol. 2000;12:95-101. [DOI] [PubMed] [Google Scholar]
- 7.Johnson P, Burchill S, Selby P. The molecular detection of circulating tumor cells. Br J Cancer. 1995;72:268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macfarlane DE, Dahle CE. Isolating RNA from whole blood - the dawn of RNA-based diagnosis. Nature. 1993;362:186-188. [DOI] [PubMed] [Google Scholar]
- 9.Ghossein R, Bhattacharya S, Rosai J. Molecular detection of micrometastases and circulating tumor cells in solid tumors. Clin Cancer Res. 1999;5:1950-1960. [PubMed] [Google Scholar]
- 10.Weitz J, Kienle P, Lacroix J, et al. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin Cancer Res. 1998;4:343-348. [PubMed] [Google Scholar]
- 11.Weitz J, Kienle P, Magener A, et al. Detection of disseminated colorectal cancer cells in lymph nodes, blood and bone marrow. Clin Cancer Res. 1999;5:1830-1836. [PubMed] [Google Scholar]
- 12.Weitz J, Koch M, Kienle P, et al. Detection of hematogenic tumor cell dissemination in patients undergoing resection of liver metastases of colorectal cancer. Ann Surg. 2000;232:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch M, Weitz J, Kienle P, et al. Comparative analysis of tumor cell dissemination in mesenteric, central and peripheral venous blood in patients with colorectal cancer. Arch Surg. 2001;136:85-89. [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH, Wittekind C. UICC: TNM-Classification of Malignant Tumors. London: John Wiley &cjs0038; Sons, Inc., Publications; 1997. [Google Scholar]
- 15.Harrell FE. Regression Modeling Strategies. New York: Springer; 2001. [Google Scholar]
- 16.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 1989. [Google Scholar]
- 17.Lehmann EL. Nonparametrics: Statistical Methods Based on Ranks. San Francisco: Holden-Day, Inc.; 1975. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457-481. [Google Scholar]
- 19.Weber T, Lacroix J, Weitz J, et al. Expression of cytokeratin 20 in thyroid carcinomas and peripheral blood detected by reverse transcription polymerase chain reaction. Br J Cancer. 2000;82:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Güdemann C, Weitz J, Kienle P, et al. Detection of hematogenous micrometastasis in patients with transitional cell carcinoma. J Urol. 2000;164:532-536. [PubMed] [Google Scholar]
- 21.Glaser F, Kuntz C, Schlag P, et al. Endorectal ultrasound for control of preoperative radiotherapy of rectal cancer. Ann Surg. 1993;217:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guillem JG, Puig-La Calle J, Akhurst T, et al. Prospective assessment of primary rectal cancer response to preoperative radiation and chemotherapy using 18-fluorodeoxyglucose positron emission tomography. Dis Colon Rectum. 2000;43:18-24. [DOI] [PubMed] [Google Scholar]
- 23.Luna-Perez P, Trejo-Valdivia B, Labastida S, et al. Prognostic factors in patients with locally advanced rectal adenocarcinoma treated with preoperative radiotherapy and surgery. World J Surg. 1999;23:1069-1074. [DOI] [PubMed] [Google Scholar]
- 24.Janjan NA, Crane C, Feig BW, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol. 2001;24:107-112. [DOI] [PubMed] [Google Scholar]
- 25.Onatis MW, Noone RB, Fields R, et al. Complete response to neoadjuvant chemoradiation for rectal cancer does not influence survival. Ann Surg Oncol. 2001;8:801-806. [DOI] [PubMed] [Google Scholar]
- 26.Luna-Perez P, Segura J, Alvarado I, et al. Specific c-K-ras gene mutations as a tumor-response marker in locally advanced rectal cancer treated with preoperative chemoradiotherapy. Ann Surg Oncol. 2000;7:727-731. [DOI] [PubMed] [Google Scholar]
- 27.Luna-Perez P, Arriola EL, Cuadra Y, et al. P53 protein overexpression and response to induction chemoradiation therapy in patients with locally advanced rectal adenocarcinoma. Ann Surg Oncol. 1998;5:203-208. [DOI] [PubMed] [Google Scholar]
- 28.Villafranca E, Okruzhov Y, Dominguez MA, et al. Polymorphisms of the repeated sequences in the enhancer region of the thymidylate synthase gene promoter may predict downstaging after preoperative chemoradiation in rectal cancer. J Clin Oncol. 2001;19:1779-1786. [DOI] [PubMed] [Google Scholar]
- 29.Okonkwo A, Musunuri S, Talamonti M, et al. Molecular markers and prediction of response to chemoradiation in rectal cancer. Oncol Rep. 2001;8:497-500. [PubMed] [Google Scholar]
- 30.Allegra C. Thymidylate synthase levels: prognostic, predictive or both? J Clin Oncol. 2002;20:1711-1713. [DOI] [PubMed] [Google Scholar]
- 31.Braun S, Hepp F, Kentenich C, et al. Monoclonal antibody therapy with edrecolomab in breast cancer patients: monitoring of elimination of disseminated cytokeratin-positive cells in bone marrow. Clin Cancer Res. 1999;5:3999-4004. [PubMed] [Google Scholar]
- 32.Moll R, Löwe A, Laufer J, et al. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140:427-447. [PMC free article] [PubMed] [Google Scholar]
- 33.Hawes RH. New staging techniques. Endoscopic ultrasound. Cancer. 1993;71:4187-4192. [DOI] [PubMed] [Google Scholar]
- 34.Heriot AG, Grundy A, Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86:17-28. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhry V, Nittala M, Prasad ML. Preoperative chemoradiation and coloanal J pouch reconstruction for low rectal cancer. Am Surg. 2000;66:387-393. [PubMed] [Google Scholar]
- 36.Grann A, Feng C, Wong D, et al. Preoperative combined modality therapy for clinically resectable UT3 rectal adenocarcinoma. Int J Radiation Oncology Biol Phys. 2001;49:987-995. [DOI] [PubMed] [Google Scholar]