Abstract
NAADP (nicotinic acid–adenine dinucleotide phosphate) is fast emerging as a new intracellular Ca2+-mobilizing messenger. NAADP induces Ca2+ release by a mechanism that is distinct from IP3 (inositol 1,4,5-trisphosphate)- and cADPR (cADP-ribose)-induced Ca2+ release. In the present study, we demonstrated that micromolar concentrations of NAADP trigger Ca2+ release from rat hepatocyte microsomes. Cross-desensitization to IP3 and cADPR by NAADP did not occur in liver microsomes. We report that non-activating concentrations of NAADP can fully inactivate the NAADP-sensitive Ca2+-release mechanism in hepatocyte microsomes. The ability of thapsigargin to block the NAADP-sensitive Ca2+ release is not observed in sea-urchin eggs or in intact mammalian cells. In contrast with the Ca2+ release induced by IP3 and cADPR, the Ca2+ release induced by NAADP was completely independent of the free extravesicular Ca2+ concentration and pH (in the range 6.4–7.8). The NAADP-elicited Ca2+ release cannot be blocked by the inhibitors of the IP3 receptors and the ryanodine receptor. On the other hand, verapamil and diltiazem do inhibit the NAADP- (but not IP3- or cADPR-) induced Ca2+ release.
Keywords: calcium; cADP-ribose (cADPR); inositol 1,4,5-trisphosphate (IP3); nicotinic acid–adenine dinucleotide phosphate (NAADP); rat hepatocyte microsome; ryanodine receptor (RyR)
Abbreviations: cADPR, cADP-ribose; CICR, Ca2+-induced Ca2+ release; DTT, dithiothreitol; ER, endoplasmic reticulum; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; NAADP, nicotinic acid–adenine dinucleotide phosphate; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase; SR, sarcoplasmic reticulum
INTRODUCTION
Ca2+ is an essential and universal intracellular messenger, and, in most cells, intracellular stores play a prominent role in initiating Ca2+ responses [1]. NAADP (nicotinic acid–adenine dinucleotide phosphate) is the most recently established second messenger in intracellular Ca2+ signalling [2]. Together with IP3 (inositol-1,4,5-trisphosphate) [3] and cADPR (cADP-ribose) [4], NAADP plays a crucial role in the generation of intracellular calcium signals that are intimately involved in the regulation of a host of cellular processes in most of the cells [5]. The NAADP-induced Ca2+ release appears to be ubiquitous, as it has been described in various cell types ranging from sea-urchin eggs [6], in which it was first examined, to ascidian oocytes [7] (invertebrates), from higher plants [8] to some of the mammalian tissues, such as brain [9], heart [10], skeletal muscle [11], as well as pancreas acinar cells [12] and T-lymphocytes [13]. NAADP is an endogenous pyridine nucleotide synthesized from NADP+ via a base-exchange reaction catalysed by ADP-ribosyl cyclases or their homologue, CD38 [14], a reaction that is preferred at acidic pH. Synthesis of NAADP by a base-exchange reaction has been described in several mammalian tissues, including brain, heart, liver, spleen and kidney [15].
Compared with IP3 and cADPR, NAADP exhibits numerous unique properties. First, NAADP-mediated Ca2+ release is not sensitive to free cytosolic Ca2+ concentrations, thus it does not behave like a CICR (Ca2+-induced Ca2+ release) system [16]. Secondly, the effect of NAADP is not dependent on cytosolic pH, unlike that of IP3 and cADPR [17]. Furthermore, the response to a maximal NAADP concentration is eliminated by pre-treatment with a subthreshold concentration of NAADP [18]. Moreover, NAADP does not cross-desensitize with either IP3 or cADPR, showing that it induces Ca2+ release via a mechanism independent of IP3Rs (IP3 receptors) and RyRs (ryanodine receptors) [18]. This is also supported by the distinct pharmacological properties of the NAADP-sensitive mechanism. For example, NAADP-induced Ca2+ release is blocked by L-type Ca2+-channel modulators, such as nifedipine, verapamil and diltiazem, which have no effect on either of the other two Ca2+-release mechanisms [19]. In contrast, the NAADP-mediated Ca2+ release in certain mammalian systems (heavy sarcoplasmic reticulum [11] and nuclear envelope prepared from pancreatic acinar cells [20]) is sensitive to ryanodine and Ruthenium Red, thus it may function via the RyRs. Furthermore, NAADP failed to elicit Ca2+ release in Jurkat T-lymphocytes in which expression of RyR type 3 was knocked-down [21]. The NAADP-sensitive Ca2+ store, unlike cADPR and IP3 stores, is insensitive to thapsigargin and cyclopiazonic acid [22], potent and selective inhibitors of the SERCA (sarcoplasmic/endoplasmic reticulum Ca2+-ATPase). The identity of the Ca2+ stores targeted by NAADP has been examined extensively. The results of the membrane fractionation studies in sea-urchin eggs showed that the NAADP-sensitive stores did not co-purify with the ER (endoplasmic reticulum) or its counterpart in muscle cells, the SR (sarcoplasmic reticulum) [6], but co-migrated with lysosomal enzyme markers [23]. Taking into consideration these results, at least two separate Ca2+ stores exist in the cytoplasm: one is the ER/SR, gated by IP3 and cADPR, while the other ones, the acidic Ca2+ stores, such as lysosomes, endosomes and reserve granules, are sensitive to NAADP, a finding consistent with the acidic preference of the production of NAADP [23].
In the present study, we found that the NAADP-mediated Ca2+ release is indeed present in hepatocyte microsomes. This finding is in agreement with previous reports in which the presence of enzymatic synthesis of NAADP in liver extracts [15] and cellular concentrations of NAADP [24] in intact rat hepatocytes have been published. Cross-desensitization to IP3 and cADPR by NAADP did not occur in liver microsomes. We determined the unique self-desensitization pattern of the NAADP receptors, and our results show that the NAADP-mediated Ca2+ release was thapsigargin-dependent in hepatocyte microsomes. Finally, we characterized the extravesicular Ca2+- and pH-dependence, as well as the pharmacological properties, of the Ca2+ release elicited by NAADP in hepatocytes.
MATERIALS AND METHODS
Preparation of microsomes
Liver microsomes were prepared as described previously by Fleschner and Kraus-Friedmann [25]. Briefly, Sprague–Dawley rat liver was homogenized in an ice-cold medium of 0.32 M sucrose, 20 mM Mops (pH 7.2) and 0.5 mM EGTA, also containing 1 mM DTT (dithiothreitol) and 0.2 mM PMSF as protease inhibitors, and was centrifuged at 2000 g for 15 min at 4 °C. The supernatant was centrifuged at 15000 g for 45 min, and the resulting supernatant was collected and centrifuged further at 100000 g for 90 min. Finally, the pellet was resuspended in a solution containing 0.32 M sucrose, 20 mM Mops (pH 7.2), 1 mM DTT and 0.2 mM PMSF. Protein concentration was set at ∼20 mg/ml which was measured using the Lowry assay [26] with BSA as a standard. The samples were frozen in liquid nitrogen and were stored at −80 °C until required.
Active loading of microsomes with Ca2+ and Ca2+-release assay
Ca2+ uptake and release were measured using 45Ca2+ to detect Ca2+ movements. The microsomes were diluted in a solution of 150 mM KCl, 20 mM Mops (pH 7.2), 0.5 mM MgCl2 and 10 μM Ca2+. In each experiment, 20–40 nCi of 45CaCl2 was used per assay point. The Ca2+ uptake was started by injecting 1 mM ATP into the solution at room temperature (22 °C). Ca2+ release was performed by adding 100 μM EGTA in the presence or absence of the Ca2+-releasing agent (10 μM IP3, 10 μM cADPR or 10 μM NAADP). The 45Ca2+ remaining in the vesicles was determined by filtration of 0.5 ml of microsome suspension through a Millipore HAWP nitrocellulose filter (0.45 μm pore size) under vacuum. The filters were washed with 5 ml of quench solution (150 mM KCl, 20 mM Mops, pH 7.2, 10 mM MgCl2 and 1 mM LaCl3) to lower the rate of non-specifically bound radioactivity. The radioactivity retained on the filter was measured by standard scintillation counting.
Passive loading of microsomes and Ca2+ release
Liver microsomes were passively loaded with 5 mM 45CaCl2 (20–40 nCi per assay point) by incubation for at least 5 h in an ice-cold medium containing 150 mM KCl, 20 mM Mops (pH 7.2), 45Ca2+ and 5 mM Ca2+. Passive loaded vesicles were diluted 10-fold into a Ca2+ releasing medium containing 150 mM KCl, 20 mM Mops (pH 7.2) and 500 μM of EGTA, to adjust the pCa to 6 at room temperature, and Ca2+-releasing agonists. The Ca2+ release was stopped by 5-fold dilution with the same quench solution described above, then the samples were filtrated through Millipore filters and washed with 5 ml of quench solution. The retained radioactivity was measured by standard scintillation counting.
RESULTS AND DISCUSSION
NAADP induces Ca2+ release from hepatocyte microsomes
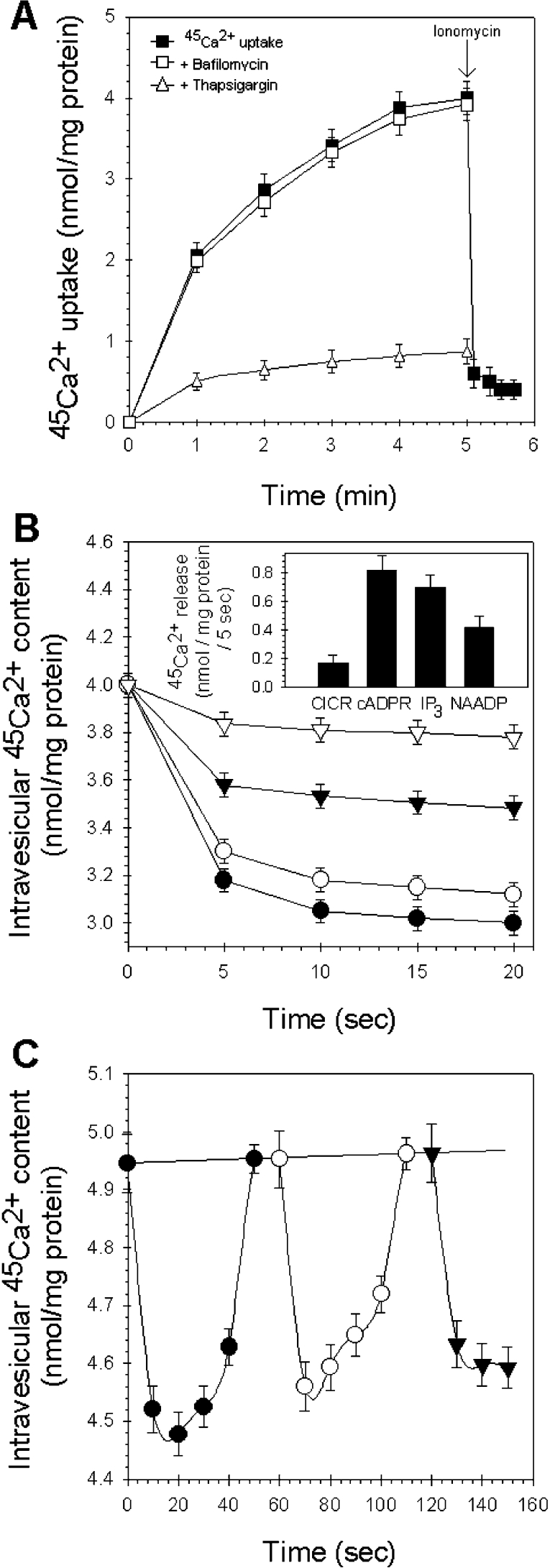
Hepatic microsomal vesicles rapidly sequestered 45Ca2+ in the presence of ATP (Figure 1A), with an uptake of 4.0±0.2 nmol/mg of protein (n=13). The maximum Ca2+ uptake was found within 5–10 min, which is later than that observed in experiments with intact or permeabilized cells, but consistent with previous reports [27]. Approx. 90% of the specifically retained microsomal Ca2+ was rapidly released by ionomycin (5 μM) (Figure 1A). This rate of decline of microsomal Ca2+ content defined the magnitude of the microsomal Ca2+ stores available for release. We found it important to identify the main Ca2+ transporter through which the microsomes are loaded. We determined the Ca2+ uptake of liver microsomes in the presence of 1 μM thapsigargin, a selective inhibitor of the SERCA, and 1 μM bafilomycin A1, an established blocker of the V-type ATPase [28]. The Ca2+ accumulation of microsomes was nearly abolished by thapsigargin, while bafilomycin did not affect substantially the Ca2+-uptake mechanisms of liver microsomes. In the light of these results, it is the SERCA that represents the main mechanism that is responsible for the active loading of liver microsomes. In the next step, we investigated whether NAADP could induce Ca2+ release from rat liver microsomes loaded actively with 45Ca2+ and compared it with IP3- and cADPR-induced Ca2+ release. In this assay, NAADP (10 μM), IP3 (10 μM) and cADPR (10 μM) induced a fast Ca2+ efflux, which differed significantly from control microsomes (CICR) (Figure 1B). The pattern of NAADP-mediated Ca2+ release appeared to be biphasic, with an initial rapid release followed by a sustained, but slower, phase of release. A similar pattern of Ca2+ release was observed when cADPR and IP3 were added (Figure 1B). After 5 s of Ca2+ release, the total amount of Ca2+ efflux elicited by CICR was 0.165±0.06 nmol/mg of protein (4.6% of ionomycin release; n=6–12). In the same set of experiments, NAADP released 0.42±0.08 nmol of Ca2+/mg of protein (11.8% of ionomycin release; n=15), while cADPR elicited 0.821±0.1 nmol of Ca2+/mg of protein (22.8% of ionomycin release; n=10) (Figure 1B, inset). Under the same conditions, IP3 released 0.7±0.09 nmol of Ca2+/mg of protein (19.6% of ionomycin release; n=8) (Figure 1B, inset). Thus NAADP is a potent, but somewhat less effective, Ca2+-releasing messenger than cADPR and IP3 in liver hepatocyte microsomes.
Figure 1. NAADP-induced 45Ca2+ release from active loaded hepatocyte microsomes.
(A) The time course of the Ca2+ uptake by liver microsomes was determined using 45Ca2+, as described in the Materials and methods section. Accumulation (■) of Ca2+ was started by addition of 1 mM ATP. The amount of mobilizable Ca2+ was determined by adding 5 μM ionomycin (arrow) to the medium. The effect on 45Ca2+ uptake of 1 μM thapsigargin (△) and 1 μM bafilomycin A1 (□) was also tested. Bafilomycin A1 was added to the microsomes 5 min before 45Ca2+ uptake was initiated. (B) Comparison of the Ca2+-mobilizing characteristics of IP3 (○), cADPR (●) and NAADP (▼) (10 μM each). CICR (▽) was determined by adjusting extravesicular free Ca2+ levels to pCa 6 using EGTA (100 μM). Results are means±S.E.M. for six to twelve determinations on at least four different experimental days. The inset shows the total amount of Ca2+ efflux triggered by IP3, cADPR and NAADP after 5 s of Ca2+ release. (C) Microsomes sequestered Ca2+ in the presence of an ATP-regenerating system (2 units/ml creatine-kinase and 4 mM phosphocreatine) and released calcium in response to subsequent addition of cADPR (●, 10 μM), IP3 (○, 10 μM) and NAADP (▼, 10 μM).
To determine further whether the NAADP-induced Ca2+-release mechanism in liver microsomes is distinct from the IP3- and cADPR-mediated Ca2+-release mechanism, we tested for possible agonist cross-desensitization. As shown in Figure 1(C), we tested subsequent Ca2+ release from actively loaded liver microsomes by cADPR, IP3 and NAADP (all applied at supramaximal concentrations, 10 μM) in the presence of an ATP-regenerating system. NAADP managed to elicit maximal Ca2+ efflux when applied after cADPR and IP3 had already been probed. Thus cross-desensitization to IP3 and cADPR by NAADP did not occur (Figure 1C). This result supports further the view that NAADP acts upon a Ca2+-release mechanism distinct from that of IP3 and cADPR from rat liver microsomes.
Dose-dependence of the NAADP-mediated Ca2+ release
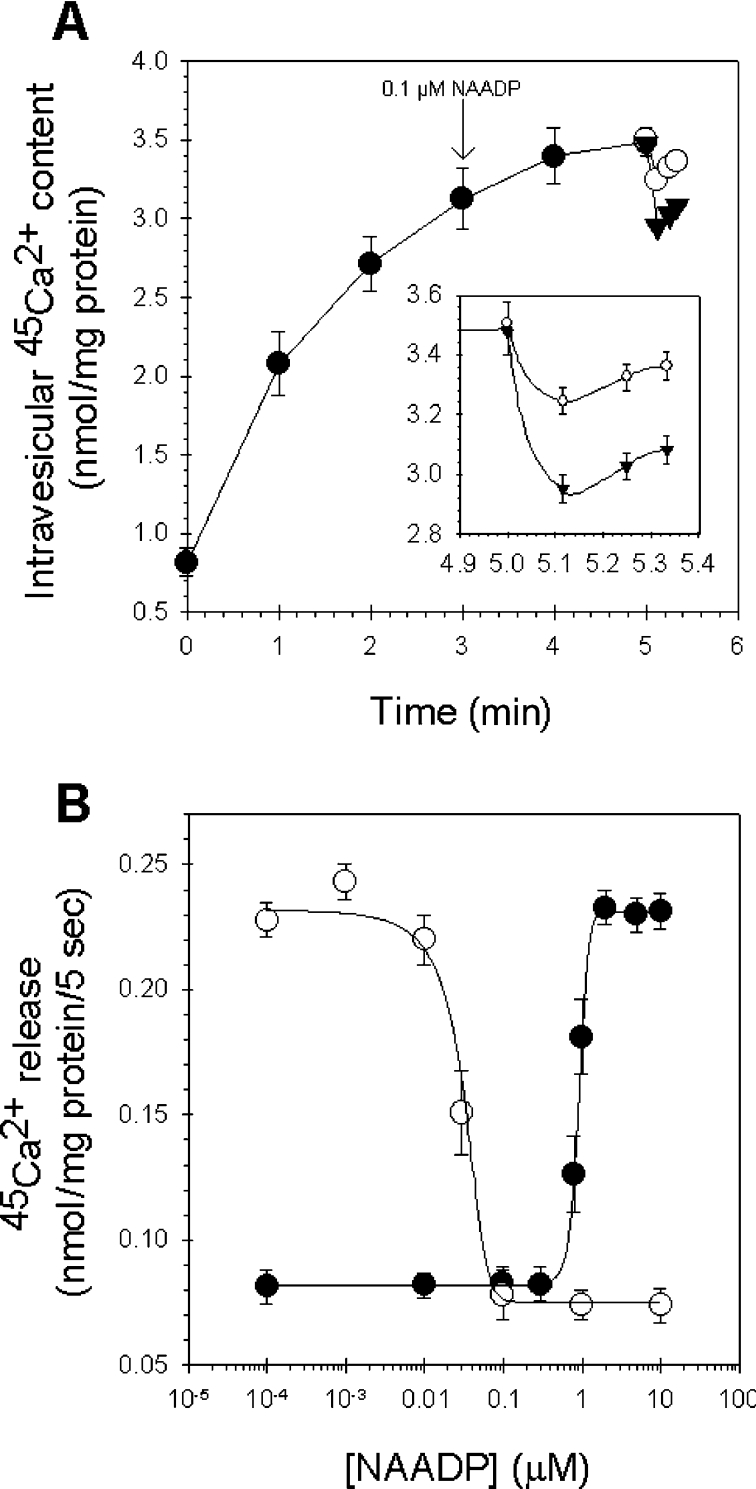
NAADP induced Ca2+ release in rat liver microsomes in a dose-dependent manner, with a half-maximal concentration (EC50) of 0.93±0.1 μM (Figure 2). Our results correspond with those of other authors who experimented with microsomes prepared from other mammalian tissues [9–11], whereas the EC50 for NAADP was reported to be one order of magnitude smaller in intact cells (approx. 100 nM) [12,13].
Figure 2. Dose-dependence of the NAADP-induced Ca2+ release in rat liver microsomes.
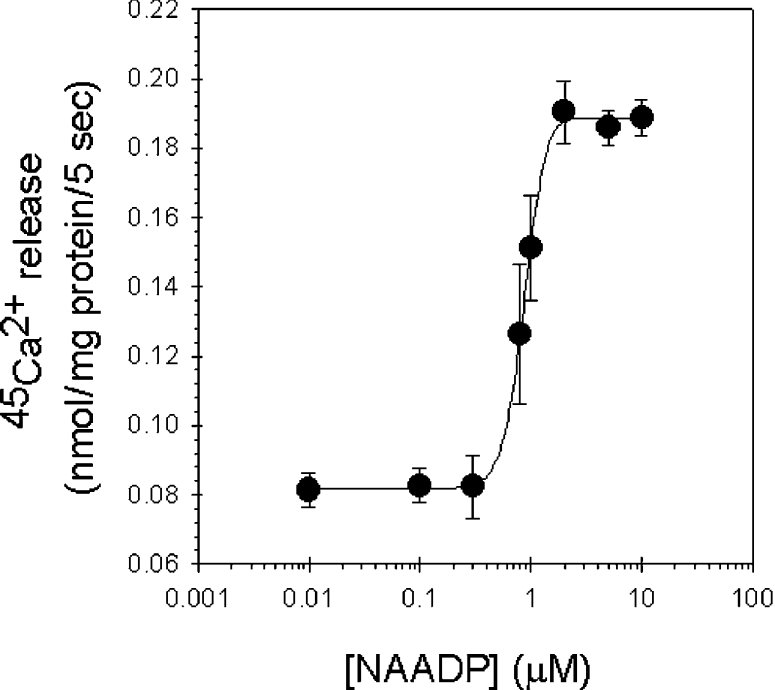
Microsomes were actively loaded with Ca2+ in the presence of 1 mM ATP and were assayed for Ca2+ release using different concentrations of NAADP in the range 0.01–10 μM. Results are means±S.E.M. for five independent experiments.
Unique homologous desensitization pattern of the NAADP receptors
We investigated the inactivation phenomenon of NAADP-induced Ca2+ release in liver microsomes. First, injection of subthreshold concentrations of NAADP (0.1 μM) into microsomes after 3 min during active loading did not result in substantial Ca2+ release by itself (Figure 3A). However, after 2 min of incubation, 10 μM NAADP released 0.14±0.04 nmol of Ca2+/mg of protein compared with 0.39±0.04 nmol of Ca2+/mg of protein released from non-pre-incubated microsomes.
Figure 3. Unique homologous desensitization pattern of the NAADP receptors.
(A) Homologous desensitization of NAADP receptors by subthreshold concentrations of NAADP. Actively loaded microsomes (●) were pre-treated with 0.1 μM NAADP for 2 min (starting 3 min after uptake was initiated, indicated by the arrow) and were then challenged to a supramaximal concentration of NAADP (10 μM, ○). NAADP-induced Ca2+ release from non-pre-treated microsomes (▼). The inset shows the Ca2+ efflux at 5 min of Ca2+ loading from microsomes incubated with non-activating concentrations of NAADP and non-pre-treated microsomes. (B) Dose–response curve of NAADP (●) and the residual Ca2+ release by a supramaximal concentration of NAADP (10 μM) after 2 min of pre-incubation with concentrations of NAADP between 0.1 nM and 10 μM (○).
In Figure 3(B) we compared the dose–response curve of the NAADP-induced Ca2+ release with the curve for residual Ca2+ release by supramaximal NAADP (10 μM) after 2 min of pre-incubation of microsomes with different concentrations of NAADP (between 0.1 nM and 10 μM). In this manner, NAADP may function as its own specific antagonist with an IC50 of 30 nM. The two curves form a U-shape as NAADP desensitizes its receptors with an IC50 that is one order of magnitude lower than the EC50. Thus we found evidence that, similarly to invertebrates [18], full desensitization of the NAADP receptors by subthreshold NAADP concentrations is possible without any need for previous substantial Ca2+ release. This phenomenon is in contrast with the self-desensitization mechanism for IP3 and cADPR (cross-desensitization) [18].
The effect of thapsigargin and bafilomycin A1 on the NAADP-evoked Ca2+ release in rat liver microsomes
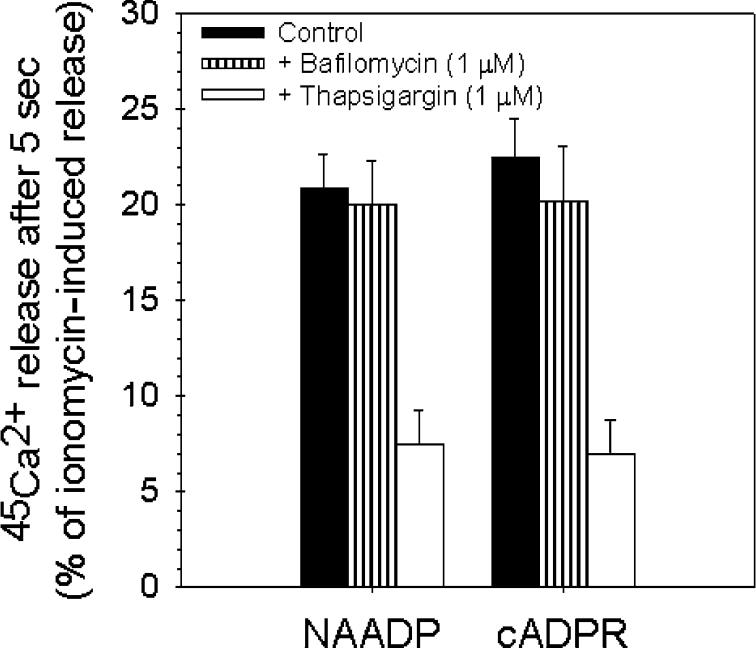
The NAADP-sensitive Ca2+ stores are insensitive to thapsigargin in sea-urchin eggs [22], as well as in several intact mammalian cell types (e.g. arterial smooth muscle [29] and pancreatic acinar cells [30]), and can be localized to the lysosomal compartment [23,28] (acidic thapsigargin-insensitive pool). Therefore it seemed important to test whether the NAADP-mediated Ca2+ release from rat liver microsomes is dependent on acidic pools. One way of interfering with organellar acidification is to pre-treat with bafilomycin A1, which is a blocker of the vacuolar-type H+-ATPase [28]. When actively loaded microsomes were incubated for at least 5 min with bafilomycin A1 (1 μM), we found that both NAADP (10 μM) and cADPR (10 μM) elicited an entirely normal Ca2+-release response (Figure 4) (n=4). No substantial change was observed in the response of the Ca2+ release elicited by cADPR and NAADP to bafilomycin A1 when longer incubation times (10 and 20 min) were applied (M. Mándi and J. Bak, unpublished work). These results indicate that the Ca2+ released from liver microsomes induced by NAADP in unlikely to come from acidic compartments.
Figure 4. Effects of thapsigargin and bafilomycin A1 on the cADPR- and NAADP-elicited Ca2+ release in rat liver microsomes.
The actively loaded vesicles were pre-incubated with thapsigargin (1 μM) for at least 2 min and with bafilomycin A1 (1 μM) for at least 5 min before Ca2+ release was induced with supramaximal concentrations of cADPR and NAADP (both 10 μM). Closed bars represent the Ca2+ release from non-pre-treated microsomes, while open bars show the Ca2+ efflux from microsomes treated with thapsigargin (1 μM) and hatched bars represent the effect of bafilomycin A1 (1 μM).
Furthermore, microsomes were treated with maximal concentration of thapsigargin (1 μM), a potent and selective inhibitor of the SERCA, for at least 2 min when Ca2+ uptake reached the plateau. The amount of Ca2+ efflux elicited by 10 μM NAADP in liver microsomes pre-treated with thapsigargin was reduced to 7.48±1.75% of the ionomycin release while NAADP released 20.86±1.8% of ionomycin released in non-pre-treated microsomes (Figure 4). The effect of cADPR was similarly affected by pre-treatment with thapsigargin (6.94±1.85% of ionomycin release in pre-incubated microsomes compared with 22.51±2% of ionomycin release in the absence of thapsigargin). Our results show that the NAADP-mediated Ca2+ release was thapsigargin-dependent as was that of cADPR. The ability of thapsigargin to block the NAADP-sensitive Ca2+ release is in contrast with the results published for sea-urchin eggs [22] or intact mammalian cells [29,30]. The Ca2+ release from microsomes can be described as a one-pool model [31]. The microsomal Ca2+ store is in fact a mixture of Ca2+ stores deriving from both lysosomes and the ER, moreover it is filled mainly by SERCA (see Figure 1A) and contains IP3Rs, RyRs and NAADP receptors. This type of fusion of the different intracellular Ca2+ stores is an artifact of the preparation process itself.
Ca2+- and pH-dependence of the NAADP-induced Ca2+ release
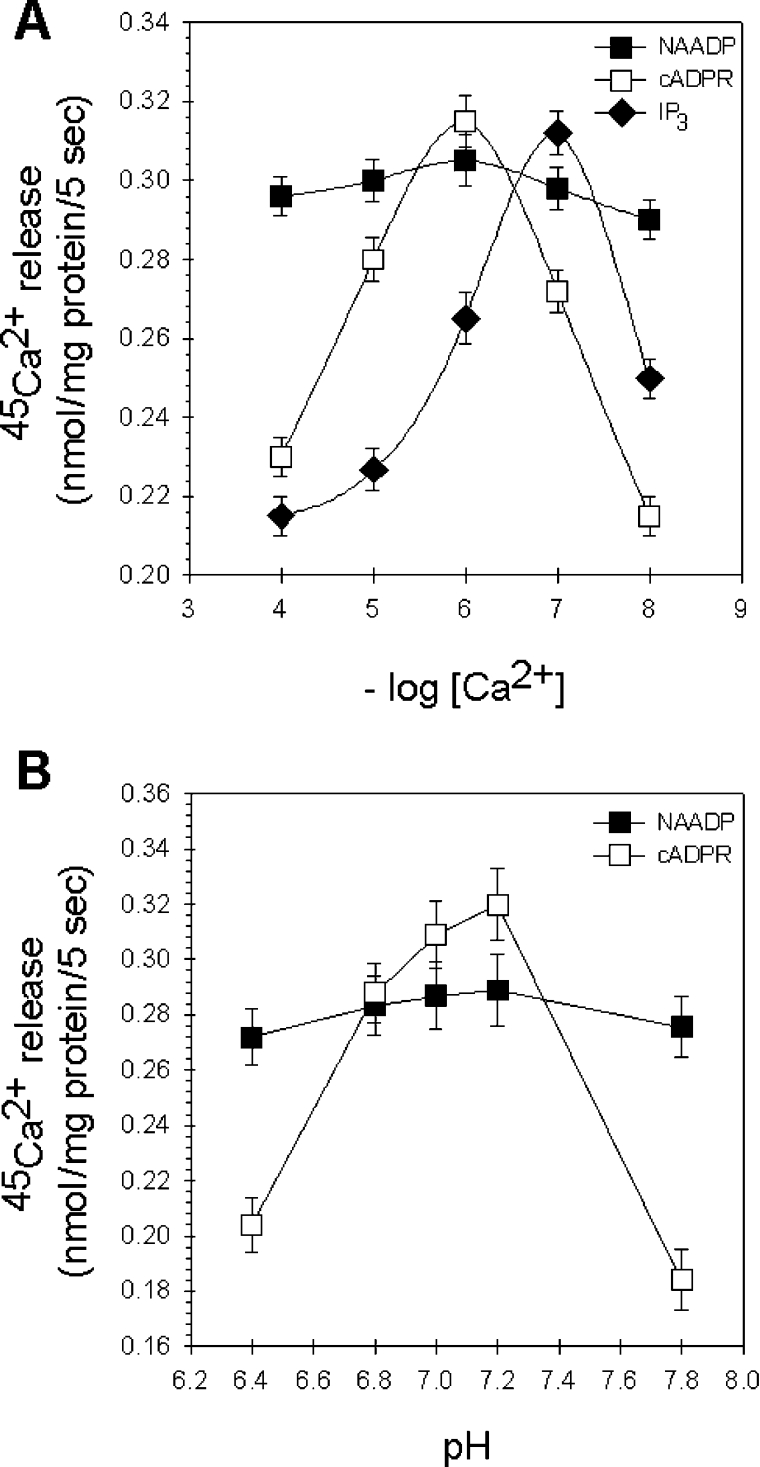
In the next set of experiments, we investigated the effect of free extravesicular Ca2+ concentration upon the Ca2+-release induced by NAADP, IP3 and cADPR (Figure 5A). Passive loading of microsomes has the advantage over ATP-driven loading that the concentration of free extravesicular Ca2+ can be set more accurately with Ca2+-complexing agents, such as EGTA. The activation of both IP3Rs and RyRs often shows similar bell-shaped dependence on the concentration in the vicinity of the cytoplasmic face of the release [32,33]. Similarly, in Figure 5(A), we show that the pCa response curves of the IP3 and cADPR appeared to be bell-shaped, with an optimal pCa at 7 and 6 respectively. However, the NAADP-induced Ca2+ release we found to be fairly independent of the extravesicular Ca2+ concentration. This finding is one of the unique characteristics that NAADP displays in all cell types.
Figure 5. Ca2+- and pH-dependence of the NAADP-induced Ca2+ release.
(A) Extravesicular free Ca2+ concentration-dependence of the IP3-, cADPR- and NAADP-mediated system in passively loaded liver microsomes. Extravesicular pCa (4–8) was set by EGTA (200–750 μM), NAADP (■), IP3 (◆) and cADPR (□) were applied at supramaximal concentrations (10 μM). (B) Differential effect of pH on the cADPR- and NAADP-sensitive Ca2+-releasing system. The pH of the Ca2+-release medium was changed from 6.4 to 7.8, and the amount of 45Ca2+ released by 10 μM cADPR (□) and 10 μM NAADP (■) was determined.
It was described previously that the NAADP-induced calcium release in sea-urchin egg homogenates [17] and rat mesangial cell microsomes [34] was not affected by the pH changes of the incubation medium. In contrast, cADPR-induced Ca2+ release was inhibited by alkalinization of the medium [17]. We found that the NAADP-induced Ca2+ release in hepatocyte microsomes was not affected by changing the pH of the incubation buffer from 6.4 to 7.8 (Figure 5B). We propose that protonation and deprotonation of relevant amino acids with pKa values in the range of physiological pH has no effect upon the gating property of the putative channel that is activated by NAADP and, by extension, upon the binding of NAADP to its receptor [35]. However, the response to cADPR was mostly dependent on pH, showing an optimal pH of 7.2. The peak Ca2+ efflux evoked by cADPR was at least 50% higher than at pH values one unit lower or higher. Alkalinization of the medium may alter the binding of cADPR to its receptor or may affect activation of RyRs by pharmacological agonists [17]. NAADP- and cADPR-triggered Ca2+ release from liver microsomes was differentially affected by pH, providing further evidence that these agonists signal through functionally distinct pathways.
Pharmacological properties of the NAADP-elicited Ca2+ efflux
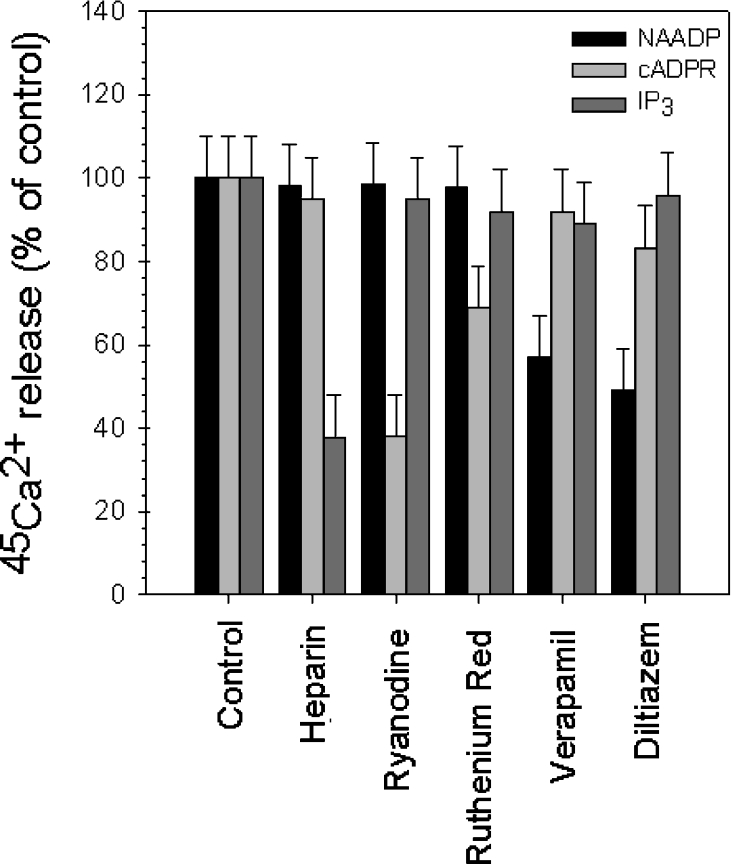
We examined the pharmacological properties of the NAADP-mediated Ca2+ release to gather more evidence that it is distinct from those mediated by IP3 and cADPR. Heparin (100 μg/ml), a well-established inhibitor of the IP3Rs [19], inhibited the Ca2+ release elicited by IP3 by 62.15±7% and did not alter the effect of cADPR and NAADP (Figure 6). The RyR antagonists, ryanodine (5 μM) and Ruthenium Red (5 μM), blocked the cADPR-induced Ca2+ efflux by 62±6% and 31.19±4% respectively, leaving that of IP3 and NAADP unaltered. The L-type Ca2+ receptor blockers, verapamil (100 μM) and diltiazem (100 μM) [18], abolished specifically, but only partially (up to 43±4% and 50.82±6% of inhibition respectively) the Ca2+-releasing effect of NAADP in rat liver microsomes. On the other hand, they had minimal effect on the Ca2+ release by IP3 and cADPR (less than 15%) (Figure 6). To sum up, neither heparin, nor ryanodine and Ruthenium Red were able to block substantially the NAADP-induced Ca2+ release, while verapamil and diltiazem were effective inhibitors of NAADP receptors (Figure 6). Our results suggest that the NAADP-mediated Ca2+ release is indeed a distinct pathway in rat liver microsomes.
Figure 6. Pharmacological properties of the intracellular Ca2+ channels mediated by IP3, cADPR and NAADP.
The 45Ca2+ release by supramaximal concentrations of IP3, cADPR and NAADP (all 10 μM) was challenged in the presence of heparin (100 μg/ml), ryanodine (5 μM), Ruthenium Red (5 μM), verapamil (100 μM) and diltiazem (100 μM).
Acknowledgments
We thank Dr Gábor Blaskó and Dr Miklós Péter Kalapos for fruitful discussions.
References
- 1.Berridge M. J. Cell signalling: a tale of two messengers. Nature (London) 1993;361:315–325. doi: 10.1038/365388a0. [DOI] [PubMed] [Google Scholar]
- 2.Lee H. C. Calcium signalling: NAADP ascends as a new messenger. Curr. Biol. 2003;13:R186–R188. doi: 10.1016/s0960-9822(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 3.Patel S., Joseph S. K., Thomas A. P. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999;25:247–264. doi: 10.1054/ceca.1999.0021. [DOI] [PubMed] [Google Scholar]
- 4.Lee H. C. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 5.Berridge M. J., Lipp P., Bootman M. D. The versatility and universatility of calcium signalling. Nature Rev. Mol. Cell Biol. 2001;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 6.Lee H. C., Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- 7.Albrieux M., Lee H. C., Villez M. Calcium signalling by cyclic ADP-ribose, NAADP and inositol triphosphate are involved in distinct functions in Ascidian oocytes. J. Biol. Chem. 1998;273:14566–14574. doi: 10.1074/jbc.273.23.14566. [DOI] [PubMed] [Google Scholar]
- 8.Navazio L., Bewell M. A., Siddequa A., Dickinson G. D., Galione A., Sanders D. Ca2+ release from endoplasmatic reticulum of higher plants elicited by NADP metabolite nicotinic acid adenine dinucleotid phosphate. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8693–8698. doi: 10.1073/pnas.140217897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bak J., White P., Timár G., Missaen L., Genazzani A. A., Galione A. Nicotinic acid-adenine dinucleotid phosphate triggers Ca2+ release from brain microsomes. Curr. Biol. 1999;9:751–754. doi: 10.1016/s0960-9822(99)80335-2. [DOI] [PubMed] [Google Scholar]
- 10.Bak J., Billington R. A., Timár G., Dutton A. C., Genazzani A. A. NAADP receptors are present and functional in the heart. Curr. Biol. 2001;11:1–20. doi: 10.1016/s0960-9822(01)00269-x. [DOI] [PubMed] [Google Scholar]
- 11.Hohenegger M., Suko J., Gscheidlinger R., Drobny H., Zidar A. Nicotinic acid-adenine dinucleotide phosphate activates the skeletal muscle ryanodine receptor. Biochem. J. 2002;367:423–431. doi: 10.1042/BJ20020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masgrau R., Churchill G. C., Morgan A. J., Ashcroft S. J. H., Galione A. NAADP: a new second messenger for glucose-induced Ca2+ responses in clonal pancreatic β cells. Curr. Biol. 2002;13:247–251. doi: 10.1016/s0960-9822(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 13.Berg I., Potter B. V. L., Mayr G. W., Guse A. H. Nicotinic acid adenine dinucleotid phosphate (NAADP) is an essential regulator of T-lymphocyte Ca2+ signalling. J. Cell. Biol. 2000;150:581–588. doi: 10.1083/jcb.150.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aarhus R., Graeff R. M., Dickey D. M., Walseth T. F., Lee H. C. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J. Biol. Chem. 1995;270:30327–30333. doi: 10.1074/jbc.270.51.30327. [DOI] [PubMed] [Google Scholar]
- 15.Chini E. N., Dousa T. P. Enzymatic synthesis and degradation of nicotinate adenine dinucleotide phosphate (NAADP), a Ca2+-releasing agonist, in rat tissues. Biochem. Biophys. Res. Commun. 1995;209:167–174. doi: 10.1006/bbrc.1995.1485. [DOI] [PubMed] [Google Scholar]
- 16.Chini E. N., Dousa T. P. Nicotinic acid adenine dinucleotid phosphate-induced Ca2+ release does not behave as a Ca2+-induced Ca2+-releasing system. Biochem. J. 1996;316:708–711. doi: 10.1042/bj3160709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chini E. N., Liang M., Dousa T. P. Differential effect of pH upon cyclic ADP-ribose and nicotinic acid adenine dinucleotid phosphate-induced Ca2+ release systems. Biochem. J. 1998;335:499–504. doi: 10.1042/bj3350499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genazzani A. A., Empson R. M., Galione A. Unique inactivation properties of NAADP-sensitive Ca2+ release. J. Biol. Chem. 1996;271:11599–11602. doi: 10.1074/jbc.271.20.11599. [DOI] [PubMed] [Google Scholar]
- 19.Genazzani A. A., Mezna M., Dickey D. M., Michelangeli F., Walseth T. F., Galione A. Pharmacological properties of the Ca2+-release mechanism sensitive to NAADP in the sea urchin egg. Br. J. Pharmacol. 1997;121:1489–1495. doi: 10.1038/sj.bjp.0701295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerasimenko J. V., Maruyama Y., Yano K., Dolman N. J., Tepikin A. V., Petersen O. H., Gerasimenko O. V. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J. Cell Biol. 2003;163:271–282. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langhorst M. F., Schwarzmann N., Guse A. H. Ca2+ release via ryanodine receptors and Ca2+ entry: major mechanisms in NAADP-mediated Ca2+ signaling in T-lymphocytes. Cell. Signalling. 2004;16:1283–1289. doi: 10.1016/j.cellsig.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Genazzani A., Galione A. A. Nicotinic acid adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochem. J. 1996;315:721–725. doi: 10.1042/bj3150721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 24.Churamani D., Carrey E. A., Dickinson G. D., Patel S. Determination of cellular nicotinic acid-adenine phosphate (NAADP) levels. Biochem. J. 2004;380:449–454. doi: 10.1042/BJ20031754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleschner C. R., Kraus-Friedmann N. The effect of Mg2+ on hepatic microsomal Ca2+ and Sr2+ transport. Eur. J. Biochem. 1986;154:313–320. doi: 10.1111/j.1432-1033.1986.tb09399.x. [DOI] [PubMed] [Google Scholar]
- 26.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Lilly L. B., Gollan J. L. Ryanodine-induced calcium release from hepatic microsomes and permeabilized hepatocytes. Am. J. Physiol. 1995;268:G1017–G1024. doi: 10.1152/ajpgi.1995.268.6.G1017. [DOI] [PubMed] [Google Scholar]
- 28.Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinnear N. P., Boittin F. X., Thomas J. M., Galione A., Evans A. M. Lysosome-sarcoplasmatic reticulum junctions. J. Biol. Chem. 2004;279:54319–54326. doi: 10.1074/jbc.M406132200. [DOI] [PubMed] [Google Scholar]
- 30.Yamasaki M., Masgarau R., Morgan A. J., Churchill G. C., Patel S., Ashcroft S. J. H., Galione A. Organelle selection determines agonist-specific Ca2+-signals in pancreatic acinar and β cells. J. Biol. Chem. 2003;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 31.Cancela J. M., Charpentier G., Peterson O. H. Co-ordination of Ca2+ signalling in mammalian cells by the new Ca2+-releasing messenger NAADP. Eur. J. Physiol. 2003;446:322–327. doi: 10.1007/s00424-003-1035-x. [DOI] [PubMed] [Google Scholar]
- 32.Hagar R. E., Burgstahler A. D., Nathanson M. H., Erlich B. E. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature (London) 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mészáros L. G., Bak J., Chu A. Cyclic ADP-ribose as an endogenous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature (London) 1993;364:76–79. doi: 10.1038/364076a0. [DOI] [PubMed] [Google Scholar]
- 34.Yusufi A., Cheng J., Thomson M., Chini E. N., Gande J. NAADP elicits specific microsomal Ca2+ release from mammalian cells. Biochem. J. 2001;353:531–536. [Google Scholar]
- 35.Billington R. A., Genazzani A. A. Characterization of NAADP+ binding in sea urchin eggs. Biochem. Biophys. Res. Commun. 2000;276:112–116. doi: 10.1006/bbrc.2000.3444. [DOI] [PubMed] [Google Scholar]