Abstract
The role of MT (metallothionein) gene expression was investigated in rotenone-treated HeLa cells to induce a deficiency of NADH:ubiquinone oxidoreductase (complex I). Complex I deficiency leads to a diversity of cellular consequences, including production of ROS (reactive oxygen species) and apoptosis. HeLa cells were titrated with rotenone, resulting in dose-dependent decrease in complex I activity and elevated ROS production at activities lower than 33%. Expression of MT2A (MT isoform 2A), but not MT1A or MT1B RNA, was significantly inducible by rotenone (up to 7-fold), t-BHP (t-butyl hydroperoxide; 5-fold) and CdCl2 (50-fold), but not ZnCl2. Myxothiazol treatment did not elevate either ROS or MT2A levels, which supports a ROS-related mechanism for rotenone-induced MT2A expression. To evaluate the role of MT2A expression, MT2A and MT1B were overexpressed in HeLa cells and treated with rotenone. Compared with control and MT1B-overexpressing cells, ROS production was significantly lower and cell viability higher in MT2A-overexpressing HeLa cells when ROS production was enhanced by treatment with t-BHP. Mitochondrial membrane potential was noticeably less reduced in both MT-overexpressing cell lines. MT2A overexpression in rotenone-treated cells also significantly reduced or delayed apoptosis induction, as measured by caspase 3/7 activity and cytosolic nucleosome enrichment. We conclude that MT2A offers significant protection against the main death-causing consequences of rotenone-induced complex I deficiency in HeLa cells. Our results are in support of the protective role against oxidative stress ascribed to MTs and provide evidence that MT2A expression may be a beneficial downstream adaptive response in complex I-deficient cells.
Keywords: apoptosis, metallothionein, mitochondria, NADH:ubiquinone oxidoreductase, reactive oxygen species (ROS), rotenone
Abbreviations: Ct, cycle threshold; DCFDA, 2′,7′-dichlorofluorescin diacetate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MMLV, Moloney-murine-leukaemia virus; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MT, metallothionein; MT2A, MT isoform 2A; mtPTP, mitochondrial permeability transition pore; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; OXPHOS, oxidative phosphorylation; ROS, reactive oxygen species; t-BHP, t-butyl hydroperoxide; TMRM, tetramethylrhodamine methyl ester
INTRODUCTION
The production of ATP through the process of OXPHOS (oxidative phosphorylation) involves the successive transport of electrons through four mitochondrial enzyme complexes. The first of these complexes, NADH:ubiquinone oxidoreductase (complex I; EC 1.6.5.3), is assembled from 46 subunits of bigenomic origin [1]. Deficiencies of this complex are among the most common OXPHOS deficiencies [2] and may lead to a diversity of disease expression phenotypes [3]. The excessive formation of ROS (reactive oxygen species) as a contributing factor to the pathology of this deficiency has been well established, along with several other biochemical consequences, including loss of ATP production, loss of mitochondrial membrane potential, calcium regulation and apoptosis [4–8].
Recent studies using either inherited or rotenone-induced complex I-deficient cell lines have indicated that several nuclear and mitochondrial genes are differentially expressed in this disorder [9–11]. Among these, expression of MTs (metallothioneins) was induced in inherited complex I-deficient fibroblasts during carbon-source transition from glucose to galactose [9]. Although it was suggested that MT expression may impart a protective effect in complex I deficiency, the functionality of its expression in the context of complex I and possibly other deficiencies of the OXPHOS system remains to be established. MTs are small proteins (6–7 kDa) with a high cysteine content that can bind metals, particularly Zn and Cd, and scavenge ROS in a similar way to glutathione [12,13]. MT expression is regulated via cis-acting metal responsive elements and an antioxidant response element, both located in the proximal MT promoter, and is responsive to a wide range of effectors, including ROS [14,15]. In humans, MT1 and MT2 isoforms are thought to be ubiquitously expressed, with MT3 and MT4 only selectively expressed in neurons and squamous epithelial cells respectively [16–18]. MT2A (MT isoform 2A) appears to be the predominantly expressed MT isoform in human cell lines in vitro, including HeLa cells [19]. Although a clearly distinctive role for MT isoforms remains unclear, it is generally believed that MTs play an important role in metal ion homoeostasis and prevention of oxidative damage in cells [13,14,20].
In light of the responsiveness of MTs to oxidative stress and the generally protective role associated with MTs against ROS, we hypothesized that MT expression would be responsive to a deficiency of complex I and, furthermore, that MTs may be involved in the pathology of such a deficiency. Although MT expression may be responsive to disruption of a number of mitochondrial functions that leads to oxidative stress, we focused on its responsiveness to an induced defect of the first component of the OXPHOS system, i.e. complex I, using rotenone. We investigated the expression of the predominant forms of MT, i.e. MT1 (isoforms A and B) and MT2A, in rotenone-treated HeLa cells against several control interventions, including metals, ROS-producing t-BHP (tert-butyl hydroperoxide) and the cytochrome c reductase inhibitor, myxothiazol. To evaluate the role of MT expression in complex I deficiency, we investigated the effect of overexpressed MT2A and MT1B on key parameters, including ROS production, ATP production, mitochondrial membrane potential and apoptosis in rotenone-treated HeLa cells.
EXPERIMENTAL
Materials
HeLa cells were purchased from the National Repository for Biological Materials of the National Cancer Association of South Africa. Tissue culturing reagents were obtained from Gibco, Invitrogen (Auckland, New Zealand). The pIRESneo2 expression vector was obtained from ClonTech, BD Biosciences (Mountain View, CA, U.S.A.), whilst all restriction endonuleases were purchased from Fermentas (Vilnius, Lithuania). MMLV (Moloney-murine-leukaemia virus) reverse transcriptase, random hexamer primers and the Apo-ONE Homogeneous Caspase 3/7 Assay kit were acquired from Promega (Madison, WI, U.S.A.). The QIAzol lysis reagent was purchased from Qiagen (Hilden, Germany). The X-tremeGENE Q2 transfection reagent and Cell Death Detection ELISAPlus were from Roche (Penzberg, Germany). The iQ SYBR Green Supermix was acquired from Bio-Rad (Hercules, CA, U.S.A.), and the probes DCFDA (2′,7′-dichlorofluorescein diacetate), TMRM (tetramethylrhodamine methyl ester) and Mitotracker Green were obtained from Molecular Probes (Eugene, OR, U.S.A.). All other reagents, including ATP assay reagents, were obtained from Sigma (St. Louis, MO, U.S.A.). MT cDNA clones with accession numbers as indicated in the text were obtained from The Resource Center of the German Human Genome Project (Berlin, Germany).
Cell culture and rotenone treatment
HeLa cells were cultured at 37 °C and 5% CO2 in a humidified incubator. The culturing medium, Dulbecco's modified Eagle's medium, was supplemented with 2 mM L-glutamine, 5% (v/v) fetal bovine serum, 50 units/ml penicillin and 50 μg/ml streptomycin. For rotenone and myxothiazol treatments, the medium of separate culture monolayers that were approx. 90% confluent was supplemented with inhibitors ranging from 0 to 2500 nM. Ethanol or DMSO content, which was used as solvents for inhibitors, was kept constant at 0.1%. Incubations were carried out for 24 h. When required, cells were collected by trypsinization unless otherwise stated and washed twice with PBS before analyses.
Enzyme assays
Rotenone-sensitive NADH:ubiquinone oxidoreductase (complex I) and antimycin A-sensitive cytochrome c reductase (complex III) activities were measured in enriched mitochondrial preparations isolated from 2×108 cells as described previously [21]. Enzyme activities were normalized to citrate synthase activity [22]. Protein content in these and other preparations was determined using the bicinchoninic acid method [23].
MT RNA expression analysis
Rotenone and myxothiazol incubations were performed as described in the previous paragraph. In addition, as controls for MT expression, treatments with CdCl2 (12.5 μM) and ZnCl2 (250 μM) were performed. To increase ROS levels in cells, t-BHP at concentrations of 0.5, 0.8 and 1.0 mM was included and incubated for 3 h before cells were harvested. Total RNA was isolated from 2×106 cells using QIAzol reagent according to the manufacturer's instructions, and the RNA integrity was verified by agarose-gel electrophoresis and ethidium bromide staining. RNA (3 μg) was reverse-transcribed with 200 units of MMLV reverse transcriptase in a volume of 40 μl using 0.5 μg of random hexamer primers. In addition to MT transcripts, several other (housekeeping gene) transcripts were evaluated for suitability as normalization controls. The primers for real-time PCR (in 5′–3′ notation) for the various genes (with GenBank® accession numbers in parentheses) are as follows: GAPDH (glyceraldehyde-3-phosphate dehydrogenase; NM_002046), β-actin (NM_001101), β2-microglobulin, (NM_004048) and RNA polymerase II (X63564) were used as reported previously [24]; 18 S rRNA (X03205) forward primer GTGCATGGCCGTTCTTAGTT and reverse primer CGGACATCTAAGGGCATCAC; MT1A (NM_005946) forward primer TCCTGCAAATGCAAAGAGTG and reverse primer TTCCAAGTTTGTGCAGGTCA; MT1B (NM_005947) forward primer GAACTCCAGGCTTGTCTTGG and reverse primer GATGAGCCTTTGCAGACACA; MT2A (NM_005953) forward primer TCCTGCAAATGCAAAGAGTG and reverse primer CAGGTTTGTGGAAGTCGCGT. Real-time PCR was performed using an iCycler iQ (Bio-Rad) in a final volume of 20 μl using SYBR Green for detection. The PCR reaction consisted of 10 μl of iQ SYBR Green Supermix, 500 nM of forward and reverse primers and 75 ng of cDNA (3 ng for 18 S rRNA primers). The method included an initial denaturation step (3 min at 95 °C) followed by 35 cycles of denaturation at 95 °C for 20 s, primer annealing at 60 °C for 10 s, extension at 72 °C for 20 s and an additional step at 82 °C (84 °C for 18 S rRNA primers) with a single fluorescence measurement. A final extension at 72 °C for 5 min followed by a melting curve analysis (55–95 °C with a heating rate of 0.5 °C per 5 s and fluorescent measurement every 5 s) concluded the run. All samples were amplified in triplicate and the mean value was used for further calculations. Every assay included a no-template control, five serial dilution points (in steps of 5-fold) of a cDNA mixture, and each of the test cDNAs. Mean results [Ct (cycle threshold) values] from the iCycler iQ Real-time Detection system (iCycler iQ Real-time Detection System Software, version 3.0; Bio-Rad) were analysed by Statistica Version 7 software (StatSoft, Tulsa, OK, U.S.A.) and the BestKeeper software tool [24]. PCR efficiency for each primer set was calculated by serial dilutions method using the REST software tool [25]. The relative expression quantities for each sample were calculated by the comparative Ct method, and gene expression stability was analysed using the GeNorm software tool [26].
MT analysis
For MT protein analysis, the same interventions were performed as described for RNA analysis. After collection, cells were sonicated on ice for three bursts of 3 s. MT content was determined in the 13000 g supernatants of homogenates by a highly sensitive RIA as previously described [27]. This antibody fully cross-reacts with MT1 and MT2 isoforms, but not with MT3, and has been validated for human MT2A (generously provided by Dr Milan Vasak, Institute of Biochemistry, University of Zurich, Zurich, Switzerland).
MT-overexpressing HeLa cell lines
The cDNA encoding human MT1B (GenBank® accession no. NM_005947) was amplified using 5′-CCTAGGAACTCCAGGCTAGC-3′ as a forward primer and 5′-AAAGAATGTAGCA-AACCGGTC-3′ as a reverse primer. Human MT2A (GenBank® accession no. NM_005953) cDNA was amplified with 5′-GCGAACCCGCGTGCAACCGGTCCC-3′ as forward primer and 5′-CAGGTTTGTGGAAGTCGCGT-3′ as reverse primer. After confirming the sequences, the MT1B and MT2A cDNAs were cloned into the EcoRV/PinAI and EcoRV/EcoRI sites of the pIRESneo2 mammalian expression vector respectively. Expression with this vector is driven by the CMV (cytomegalovirus) major immediate early promoter. After verifying the sequence of the constructs, HeLa cells were transfected with the MT expression constructs as well as the base vector, pIRESneo2, using X-tremeGENE Q2 transfection reagent according to the manufacturer's instructions. Cell lines successfully transfected with pIRESneo2-MT1B, -MT2A and pIRESneo2 constructs were denoted as MT1B-, MT2A-overexpressing cells and control cells respectively. Selection of transfected cells was performed with 1 mg/ml Geneticin in addition to the standard medium supplements. After a 3-week selection period, the standard culturing medium contained 200 μg/ml Geneticin throughout all subsequent incubations and analyses. The presence of the MT cDNAs was confirmed by PCR. Briefly, pIRESneo2-specific primers, 5′-TAATACGACTCACTATAGG-3′ (forward) and 5′-GCCCTAGATGCATGCTCG-3′ (reverse), were used to amplify the cDNAs using isolated DNA from clones. DNA was isolated by phenol/chloroform extraction and ethanol precipitation based on the procedure originally described by Maniatis et al. [28]. The presence of the correct-length amplicons was used to confirm the presence of the cDNAs, and MT RNA and protein expression were evaluated as described in the previous two sections.
ROS production
Cell lines were seeded in microtitre plates at 2×104 cells per well and treated with rotenone, myxothiazol and metals as described in the ‘Cell culture and rotenone treatment’ section. Incubations with t-BHP (0.5–1.0 mM) were used to induce elevated ROS production. The fluorescent probe, DCFDA (10 μM), was used to measure ROS production essentially as described previously [29]. Fluorescence (excitation at 485 nm and emission at 530 nm) was measured and the mean for eight samples was used in data analysis and expressed relative to protein content.
Cell viability assay
Cells were seeded into microtitre plates at a density of 2×104 cells. Rotenone and t-BHP incubations were performed as described in the ‘Cell culture and rotenone treatment’ section. As a positive control for loss of cell viability, a 30 min incubation with 6% (v/v) acetic acid was included. Cell viability [MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) assay] was determined by measuring formazan formation as described previously [30]. The mean for three replicates was calculated and expressed relative to protein content.
Membrane potential assessment
The potentiometric fluorescent dye, TMRM, was used in confocal microscopy analyses to estimate visually mitochondrial membrane potential. Cell lines were seeded on sterile glass coverslips in 6-well plates (Nunc) at densities of 2×105 cells per well in 2 ml of culture medium. After overnight incubations to allow attachment, rotenone was added and incubated for 24 h. TMRM (0.5 μM) and Mitotracker Green (0.5 μM) were added and incubated for 30 min at 37 °C, after which the coverslips were washed with three changes of media. The coverslips with adherent cells were placed in an applicable flow cell bath in the presence of 2 ml of medium. Confocal images were monitored using a Nikon (PCM2000) inverted confocal microscope. Ar and He/Ne Spectra-Physics lasers, with excitation at 475 and 505 nm and emission at between 505 and 568 nm (green) as well as long pass >565 nm (red), were employed. In order to minimize photobleaching of the sample and free radical formation in the cells, the smallest available pinhole was used (0.5 μm), together with a neutral density filter of 10%. Magnification was obtained with a Nikon ×60/1.40 Apo Planar oil objective, and bars in the micrographs indicate size. Laser power and capturing settings were kept constant in comparative experiments, to enable quantitative analysis. A scan speed of 3 μs/scan was typically used and capturing was averaged to obtain representative micrographs.
ATP and apoptosis analyses
To determine the ATP content in rotenone-treated cells, a luminescence-based assay reagent was used as instructed by the manufacturer and expressed relative to protein content. Cells were seeded in microtitre plates at 2.0×104 cells per well, allowed to adhere overnight and incubated with rotenone for 8 h before ATP measurements. Caspase 3/7 activity in treated cells was measured fluorimetrically using a commercial kit as instructed by the manufacturer. Briefly, cells were seeded in microtitre plates at densities of 2.0×104 cells per well, allowed to adhere overnight and incubated with rotenone as described in the ‘Cell culture and rotenone treatment’ section. A 2 h incubation with 1 μg/ml staurosporine was included as positive control for apoptosis. Assays were carried out kinetically and expressed relative to protein content. As an indicator for DNA degradation during apoptosis, cytosolic nucleosome enrichment in cells was determined using a commercial ELISA as instructed by the manufacturer.
Statistical analyses
All results were analysed with Statistica (version 7) software. Statistical comparisons of MT expression in HeLa cells were made using ANOVA with post hoc comparison (Tukey test). For statistical analysis of values obtained from different MT-overexpressing and control cell lines, two-way ANOVA was performed. For these analyses and interpretation of results, the interactions of the concentration (of either rotenone or t-BHP) and MT expression were evaluated as indicated by a significant F-value (test statistic). Statistical significance was considered when P<0.05.
RESULTS
MT expression and ROS production in rotenone-treated cells
The treatment of HeLa cells with varying concentrations of rotenone (0–2500 nM) resulted in a dose-dependent decrease in residual complex I activities as measured in enriched mitochondrial preparations (Table 1). Values were similar as reported previously for fibroblasts [31] and resulted in a useful range of complex I activities, ranging between 0 and 100%, to compare the responsiveness of the parameters that were investigated in the present study. Similarly, complex III could be inhibited with myxothiazol treatment over a range of 0–1000 nM that resulted in a dose-dependent decrease of activity. Treatments with rotenone and myxothiazol were limited to 24 h to limit the contribution of media composition changes on cellular function.
Table 1. Complex I and III activities in rotenone- and myxothiazol-treated HeLa cells.
Rotenone-sensitive complex I and antimycin A-sensitive complex III activities were determined in mitochondrial-enriched preparations that were prepared from rotenone- or myxothiazol-treated (24 h) HeLa cells. Values are means±S.D. (n=4, *P<0.05) with percentage activities relative to untreated cells given in parentheses. UCS, units of citrate synthase.
| Complex I (nmol·min−1·UCS−1) | Complex III (nmol·min−1·UCS−1) | |
|---|---|---|
| Rotenone (nM) | ||
| 0 | 108.7±13.4 (100%) | 61.7±2.5 (100%) |
| 10 | 55.9±6.3* (51%) | 60.6±1.5 (98%) |
| 100 | 36.1±3.5* (33%) | 64.6±7.7 (105%) |
| 1000 | 16.8±4.5* (15%) | 62.8±5.9 (102%) |
| 2500 | 0.0±3.8* (0%) | 59.8±4.0 (97%) |
| Myxothiazol (nM) | ||
| 10 | 111.6±3.8 (103%) | 42.9±4.2* (70%) |
| 100 | 106.2±4.5 (98%) | 27.9±4.0* (45%) |
| 500 | 105.1±2.1 (97%) | 6.8±1.5* (11%) |
| 1000 | 106.7±7.2 (98%) | 0.0±0* (0%) |
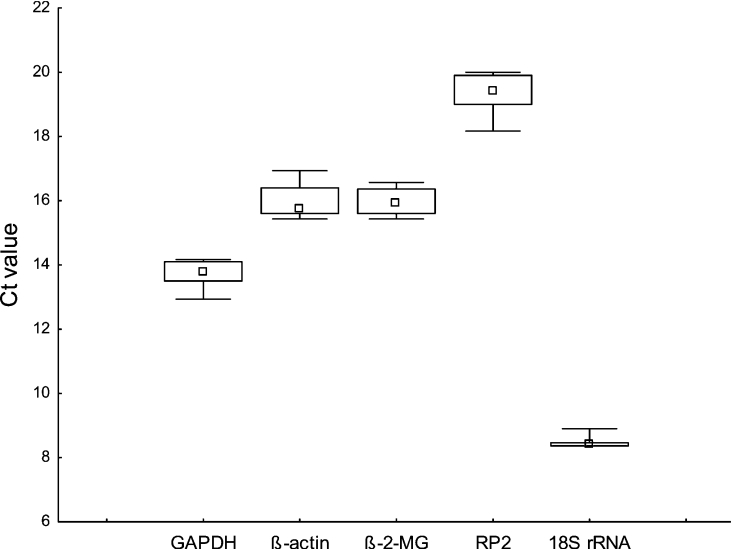
Real-time PCR was performed on total RNA samples obtained from HeLa cells that were treated with rotenone. We included known inducers of MT expression, CdCl2 and ZnCl2 as well as the ROS inducer, t-BHP, as controls for MT expression. In addition, myxothiazol was included to evaluate the mechanistic possibilities of MT expression. For RNA expression studies, Northern blotting gave poor, unspecific results, which were probably due to the similarity that exists between the different MT isoforms. Real-time PCR was used, which allowed isoform RNA expression analysis. The stability of several commonly used ‘housekeeping genes’ involved in diverse biological activities, including glycolysis, cytoskeleton structure and kinetics, immune response, gene expression and protein biosynthesis, were investigated under the interventions mentioned. To ensure comparability between the analyses of all five housekeeping genes as well as MT isoforms, we determined the reaction efficiency of each individual assay by measuring serial dilutions of 75 ng of cDNA in triplicate [25]. All PCR reactions displayed efficiencies of between 88 and 100%. The variations in the Ct values, which represent the cycle where a significant increase in amount of PCR product occurs during the various interventions, are summarized in Figure 1. Comparing the median expression values (Ct values) of the housekeeping genes, the variability for housekeeping gene expression was clearly less in the case of the 18 S rRNA as compared with the other genes. Ct values were also expressed as relative expression quantities and analysed using the GeNorm software tool. The results of this analysis are presented as GeNorm expression stability values (or gene-stability measures, M), which are defined as average pairwise variations of a particular gene with all other control genes, and are summarized in Table 2 [26]. Genes with the lowest M values generally have the most stable expression. As a result, the three most stably expressing genes, 18 S rRNA, β2-microglobulin and GAPDH, were subsequently used for normalization of MT expression.
Figure 1. Evaluation of RNA transcription level variation of housekeeping genes.
Variation in real-time PCR-generated Ct values of selected housekeeping genes of HeLa cells treated with rotenone (0–10 μM), CdCl2 (12.5 μM), ZnCl2 (250 μM) and t-BHP (0–1 μM) are shown. Incubations were carried out for 24 h (rotenone, metals) and 3 h (t-BHP). The median values are indicated by small squares, 25–75% percentiles are indicated by the boxes and minimum and maximum values indicated by whiskers. Genes are GAPDH, β-actin, β2-microglobulin (β-2-MG), RNA polymerase II (RP2) and 18 S rRNA.
Table 2. Stability of housekeeping genes expression.
Results are shown as GeNorm expression stability values or M, the internal control gene-stability measure, defined as average pairwise variation of a particular gene with all other control genes [26]. Genes with the lowest M values have the most stable expression.
| Gene | M (24 h) | M (48 h) |
|---|---|---|
| 18 S rRNA | 0.466 | 0.402 |
| β2-Microglobulin | 0.548 | 0.503 |
| GAPDH | 0.575 | 0.506 |
| β-Actin | 0.682 | 0.512 |
| RNA polymerase II | 0.730 | 0.532 |
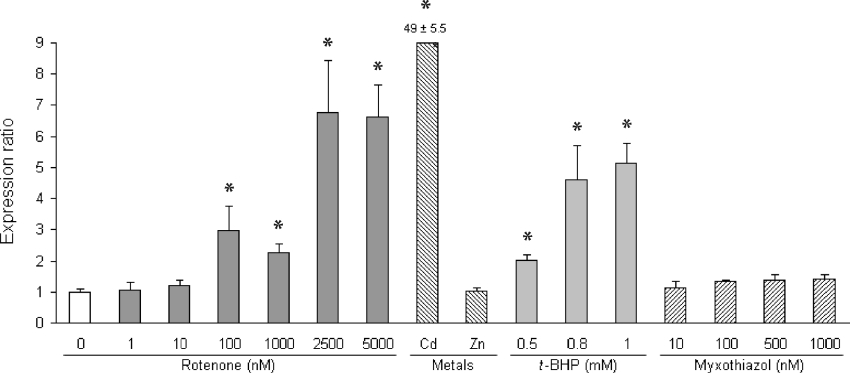
Under the experimental in vitro conditions and interventions performed in the present study, we could not detect any expression of MT1A. In addition, an almost undetectable basal expression of MT1B was observed. From the controls for PCR, using cDNA templates for the various isoforms, we concluded that the PCR was efficient and specific to the isoforms. Treatment of cells with any of the other possible inducers mentioned did not result in detectable changes of expression levels of these common MT1 isoforms either. In contrast, MT2A RNA basal levels in untreated HeLa cells were easily detectable and, as presented in Figure 2, highly inducible by CdCl2 (±50-fold) and t-BHP (up to 5-fold), but not ZnCl2. Myxothiazol treatment up to levels that completely inhibited complex III activity did not result in any significant induction of MT2A expression. Rotenone treatment, however, significantly induced MT2A expression. MT2A levels were slightly elevated with 10 nM rotenone treatment after which expression levels were significantly increased 3-fold upon treatment with 100 nM rotenone. Surprisingly, expression levels at 1000 nM were consistently lower than 100 nM. This result was the same in three independent experiments. This phenomenon was also observed when rotenone treatment was extended to 48 h (results not shown). At rotenone levels higher than 1000 nM, which resulted in almost complete inhibition of rotenone-sensitive complex I activity, MT2A expression was significantly higher and remained constant with higher rotenone levels at approx. 7-fold compared with basal levels. MT protein analysis, which did not enable a distinction between MT1 and MT2 isoforms in cell homogenates, confirmed the increased expression observed with rotenone treatment (Table 3). A slight, albeit significant, dose-dependent increase in MT expression was detected at levels up to 2500 nM. As with RNA expression, expression levels at 2500 nM were notably higher than at 1000 nM rotenone. Treatment with t-BHP also significantly increased expression of MTs on protein level. However, unlike MT2A RNA expression where only Cd-mediated induction was detected (Figure 2), both CdCl2 and ZnCl2 markedly induced MT protein expression.
Figure 2. MT2A RNA expression in rotenone-treated HeLa cells.
MT2A RNA expression and total MT protein levels in cells were determined as described in the Experimental section. Normalized RNA expression levels are expressed as the mean ratio ±S.D. (n=3) relative to untreated cells (open bar). Asterisks indicate statistically significant values (P<0.05) compared with the untreated cells. Expression levels were compared in cells treated with rotenone, metal inducers CdCl2 (12.5 μM) and ZnCl2 (250 μM), t-BHP and myxothiazol as indicated. The value for Cd-induced expression is indicated above the bar.
Table 3. MT protein levels in rotenone-, metal- and t-BHP-treated HeLa cells.
Total metallothionein (MT1+MT2) levels are expressed relative to total protein content in cell homogenates (10 mM Tris/HCl, pH 8.0). Mean values are shown with ranges in parentheses (n=2, except metal controls where n=1).
| Treatment | MT (ng/mg) |
|---|---|
| Untreated | 34.7 (32.3–37.1) |
| Rotenone | |
| 100 nM | 47.0 (44.1–49.8) |
| 500 nM | 52.1 (51.3–52.8) |
| 1000 nM | 53.8 (53.1–54.4) |
| 2500 nM | 71.7 (71.1–72.4) |
| Metal controls | |
| CdCl2 (12.5 μM) | 875 |
| ZnCl2 (250 μM) | 481 |
| t-BHP | |
| 0.5 mM | 56.2 (47.7–64.5) |
| 0.8 mM | 61.9 (57.6–66.2) |
| 1.0 mM | 91.5 (86.1–96.8) |
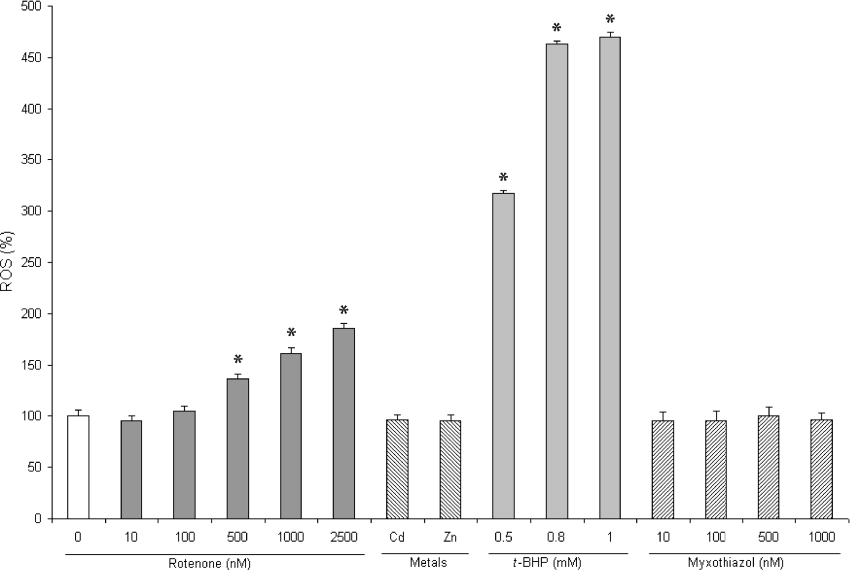
Treatment of cells with rotenone elevated ROS levels only significantly at levels higher than 100 nM (±36% residual activity) (Figure 3). At complete inhibition of complex I activity (2500 nM), ROS levels increased approx. 2-fold compared with untreated cells. As expected, t-BHP treatment had a marked effect on ROS levels up to almost 5-fold at 1 mM. Although a complete inhibition of complex III with myxothiazol was demonstrated (Table 1), surprisingly, no significant effect on ROS production could be observed in HeLa cells.
Figure 3. ROS production in rotenone-treated HeLa cells.
ROS production was measured fluorimetrically in cells treated with rotenone, metal inducers CdCl2 (12.5 μM) and ZnCl2 (250 μM), t-BHP and myxothiazol using the ROS-sensitive probe, DCFDA, and normalized to protein content. Values are means±S.D. (n=8) and expressed as percentage relative to untreated cells.
MT-overexpressing HeLa cells
HeLa cells were transfected with plasmid constructs containing either the pIRESneo2 base vector (control cells), MT1B or MT2A plasmid constructs. After confirming stable transfer of plasmid DNA, normalized expression ratios of MT1B and MT2A of the various transfected cell lines were determined as summarized in Table 4. Basal MT1B expression in control cells was barely detectable (Ct values similar to negative controls), similar to a previous report [19]. Consequently, a significantly higher MT1B expression ratio in MT1B-overexpressing cells was calculated. Comparatively, on the background of a relatively high basal expression level of MT2A in control cells (Ct values±18), the MT2A-overexpressing cells resulted in only a 2-fold increase in expression of MT2A. The MT expression levels in transfected cell lines were measured on separate occasions and remained similar to those indicated in Table 4 over the time period of the investigation. The total MT protein content, which represents combined MT1 and MT2 levels, however, was similar in both MT-overexpressing cell lines and was approx. 20% higher than control cells. From this we concluded that the additional levels of expression of either MT1B or MT2A in the respective MT-overexpressing cell lines were similar and were suitable for use in comparative studies.
Table 4. MT RNA and protein expression in recombinant MT-overexpressing HeLa cell lines.
RNA expression of MT1B or MT2A in cell lines was analysed by real-time PCR and normalized relative to the expression of GAPDH and β2-microglobulin. RNA expression values represent the expression ratios relative to pIRESneo2-transfected HeLa cells (control). Total MT protein levels were quantified in homogenates (PBS containing 1% Tween 20) using an RIA and expressed relative to protein content. All values are means±S.D. (n=3, *P<0.05).
| RNA expression ratio | Protein (ng/mg) | ||
|---|---|---|---|
| Cell line | MT1B | MT2A | MT |
| Control | 1.0±1.6 | 1.0±0.3 | 151±6.6 |
| MT1B-HeLa | 133000±38000* | 1.1±0.3 | 188±8.8* |
| MT2A-HeLa | 1.2±0.8 | 2.2±0.2* | 186±7.6* |
ATP analyses in MT-overexpressing cells
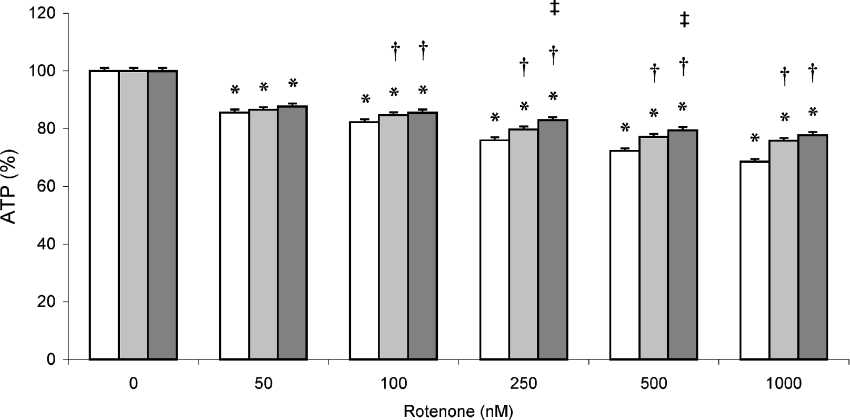
Rotenone treatment of control and MT-overexpressing cell lines resulted in a dose-dependent decrease in ATP levels (Figure 4). With complete inhibition (1000 nM rotenone), ATP levels decreased to 68% in control cells. Comparatively, ATP levels were 76 and 78% in MT1B- and MT2A-overexpressing cells respectively. A clear and significant variance in response to increasing rotenone treatment occurred between the cell lines, with ATP levels in both MT-overexpressing cell lines decreasing notably more slowly to the levels mentioned above, compared with the control cell line.
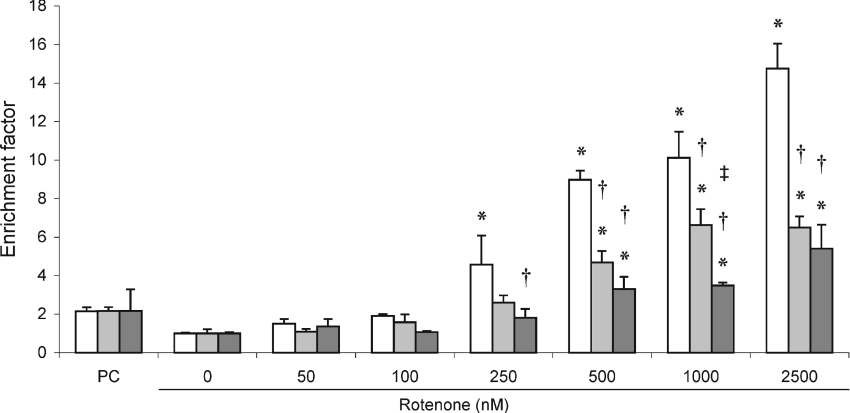
Figure 4. ATP levels in rotenone-treated MT-overexpressing HeLa cells.
Total cellular ATP content was measured in rotenone-treated MT1B- (pIRESneo2-MT1B-transfected; light grey bars), MT2A-overexpressing (pIRESneo2-MT2A-transfected; dark grey bars) and control (pIRESneo2-transfected; open bars) HeLa cells as described in the Experimental section. Values were normalized to protein content and expressed as a mean percentage of untreated cells (±S.D., n=4). *P<0.05 when compared with untreated cells of the same cell line; †P<0.05 when compared with control cell line at the same treatment; ‡P<0.05 when comparing MT2A- with MT1B-overexpressing cell line at the same treatment.
Rotenone- and t-BHP-induced ROS production
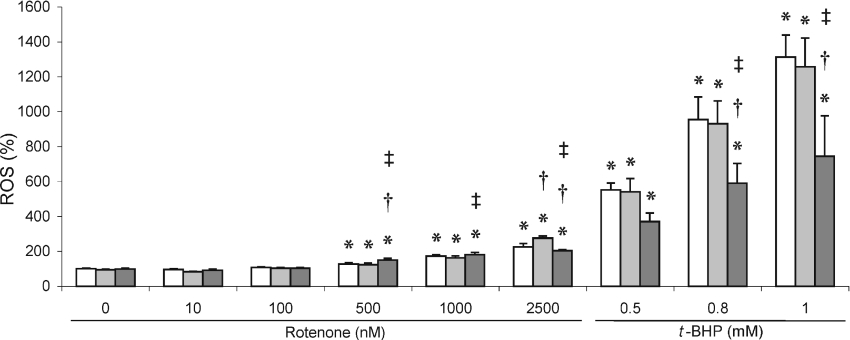
As with HeLa cells that were not genetically modified (Figure 3), total cellular ROS production in genetically modified HeLa cells increased significantly only after treatment with rotenone levels higher than 100 nM (Figure 5), which represents approx. 33% residual complex I activity. As before, ROS levels further increased dose-dependently only to levels approx. 2-fold higher at 2500 nM rotenone. At this concentration, ROS levels in MT2A, which were higher at 500 nM, were significantly, albeit only slightly, lower compared with the other two cell lines. ROS production could be further and significantly induced using t-BHP (Figure 5). A clear variance in response to this treatment could be observed with MT2A-overexpressing cells, which had significantly lower ROS levels (±40%) compared with control and MT1B-overexpressing cells.
Figure 5. ROS production in rotenone- and t-BHP-treated MT-overexpressing HeLa cells.
ROS production was measured in control (open bars), MT1B- (light grey bars) and MT2A- (dark grey bars) overexpressing cell lines treated with rotenone or t-BHP. Values, which were normalized to protein content, are means±S.D. (n=8) and expressed as percentage relative to untreated cells. *P<0.05 when compared with untreated cells of the same cell line; †P<0.05 when compared with control cell line at the same treatment; ‡P<0.05 when comparing MT2A- with MT1B-overexpressing cell line at the same treatment.
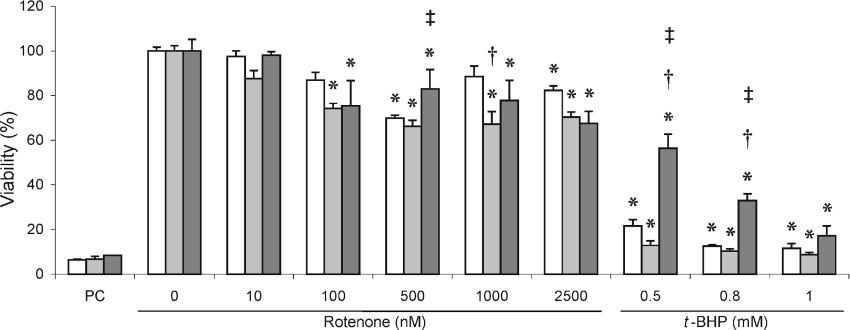
Cell viability
Rotenone treatment of the cell lines resulted in a general decrease in cell viability as determined by the MTT test (Figure 6). The cell lines, however, showed no consistent variation in cell viability across the range of rotenone concentrations, i.e. compared with MT1B, MT2A-overexpressing cells had significantly higher viability at 500 nM rotenone with lower viability at 2500 nM rotenone. As with ROS production, a more pronounced response of cell lines was observed when t-BHP was used to increase ROS production and consequently lowering cell viability to less than 20%. Also, in this case, a clear and statistically significant variation in response to t-BHP treatment occurred in the MT2A-overexpressing cells compared with both control and MT1B-overexpressing cells. This is strikingly obvious at 0.5 and 0.8 nM t-BHP, where MT2A-overexpressing cells had significantly higher viability. Unlike the responses in ROS levels (compare Figure 5), at 1 mM t-BHP, viability of all three cell lines was similar at levels lower than 20% of the untreated cells.
Figure 6. Cell viability in rotenone- and t-BHP-treated MT-overexpressing HeLa cells.
Cell viability was measured using the MTT test in MT-overexpressing (MT1B, light grey bars; MT2A, dark grey bars) and control cell lines (open bars) treated with rotenone or t-BHP as described in the Experimental section. Values, which were normalized to protein content, are means±S.D. (n=3) and expressed as percentage viability relative to untreated cells. Acetic acid (6%) was used as positive control (PC). *P<0.05 when compared with untreated cells of the same cell line; †P<0.05 when compared with control cell line at the same treatment; ‡P<0.05 when comparing MT2A- with MT1B-overexpressing cell line at the same treatment.
Mitochondrial membrane potential
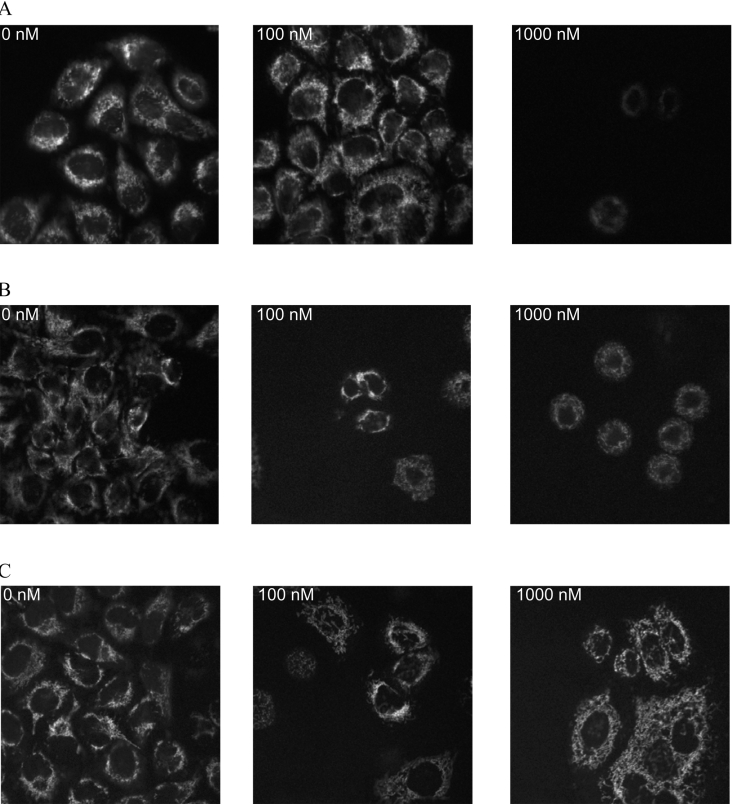
The membrane potential was visualized with confocal microscopy using TMRM staining. Fluorescence from TMRM generally co-localized with green fluorescence from mitochondrial staining (results not shown). The control cell line (Figure 7A) showed a clear decrease in membrane potential with increasing rotenone concentration, having almost no visual membrane potential when treated with 1000 nM rotenone. Both MT-overexpressing cell lines had visibly more membrane potential remaining when treated with rotenone (Figures 7B and 7C). Compared with MT1B, MT2A-overexpressing cells (Figure 7C) had a visibly smaller decrease in membrane potential with increased rotenone treatment.
Figure 7. Assessment of mitochondrial membrane potential in rotenone-treated MT-overexpressing cells.
Membrane potential of control (A), MT1B- (B) and MT2A-overexpressing (C) HeLa cells treated with 0, 100 and 1000 nM rotenone was visualized by confocal microscopy after TMRM staining.
Apoptosis
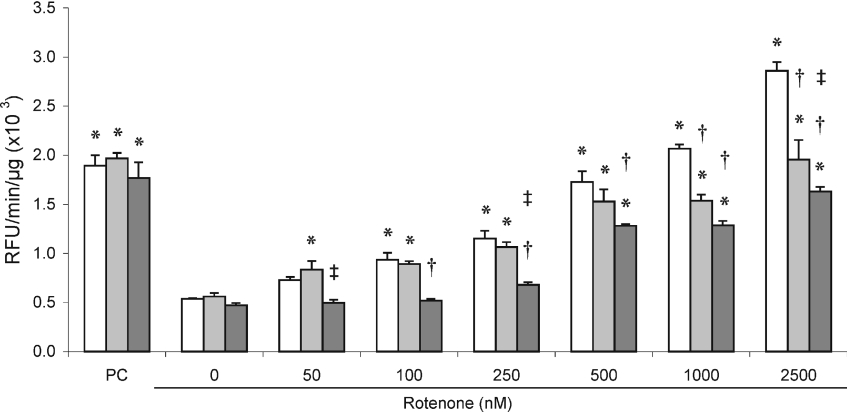
Staurosporine treatment of all cell lines resulted in the induction of similar caspase 3/7 activities, indicating that MT overexpression in these cell lines did not result in changes in protein kinase-mediated caspase activation (Figure 8). In all cell lines, caspase 3/7 activity increased approximately in a dose-dependent way over the full range of rotenone incubations used. Both MT-overexpressing cell lines had a significant variation in response to rotenone treatment. In MT1B-overexpressing cells, caspase activity increased much slower to similar activities of control cells at 250 nM rotenone. At higher rotenone levels, caspase activity in this cell line was significantly lower than control cells and, at 2500 nM rotenone, was ±35% lower than control cells. Induction of caspase 3/7 activity in MT2A-overexpressing cells was significantly lower than both control and MT1B-overexpressing cells with complete inhibition of complex I. Activities remained relatively constant up to 250 nM rotenone and, up to 2500 nM, increased only to levels of ±45% lower than the control cells.
Figure 8. Caspase 3/7 activation in rotenone-treated MT-overexpressing HeLa cells.
Caspase 3/7 activity was measured in MT-overexpressing (MT1B, light grey bars; MT2A, dark grey bars) and control cell lines (open bars) treated with rotenone as described in the Experimental section. Reaction velocities are indicated as change in relative fluorescence units (RFU) per μg of total protein. Staurosporine treatment (1 μg/ml for 2 h) was the positive control (PC) for caspase activation. Values are means±S.D. (n=3). *P<0.05 when compared with untreated cells of the same cell line; †P<0.05 when compared with control cell line at the same treatment; ‡P<0.05 when comparing MT2A- with MT1B-overexpressing cell line at the same treatment.
Over the incubation period used in the present study (24 h), visible DNA laddering was not detected using gel-electrophoresis analysis (results not shown). However, cytosolic nucleosome enrichment which is a result of DNA degradation could be detected with rotenone treatment in all three cell lines using an immunological assay (Figure 9). In view of the caspase data, the generally slower onset of DNA fragmentation can also be seen in the comparatively lower increase in nucleosome formation with staurosporine treatment. In the rotenone-treated cells, nucleosome formation increased significantly only at levels higher than 100 nM. For control cell lines, the nucleosome formation increased up to 14-fold at 2500 nM rotenone. Comparatively, in MT1B- and MT2A-overexpressing cell lines, nucleosome formation increased significantly more slowly at rotenone levels higher than 100 nM (36% of residual complex I activity) to reach levels of approx. 6- and 5-fold respectively at 2500 nM compared with baseline activity. Thus, as with caspase activation, a significant variance in response to increasing rotenone treatment occurred between the cell lines. In addition, nucleosome formation in rotenone-treated MT2A-overexpressing cells increased more slowly than in MT1B-overexpressing cells and was significantly lower at the higher levels of rotenone treatment.
Figure 9. Cytosolic nucleosome enrichment in rotenone-treated MT-overexpressing HeLa cells.
Cytosolic nucleosome enrichment was determined in MT1B- (light grey bars), MT2A- (dark grey bars) overexpressing and control (open bars) cells treated with rotenone for 24 h or staurosporine (1 μg/ml for 2 h) as positive control (PC). Values, normalized relative to protein content, are indicated as a mean fold change (±S.D., n=3) relative to untreated cells. *P<0.05 when compared with untreated cells of the same cell line; †P<0.05 when compared with control cell line at the same treatment; ‡P<0.05 when comparing MT2A with MT1B-overexpressing cell line at the same treatment.
DISCUSSION
The contribution of nuclear and mitochondrial gene expression in the pathophysiology of mitochondrial disorders has been recognized and investigated over the past decade [9–11,32]. One of the model systems often used to investigate disorders associated with the mitochondrial respiratory chain includes the inhibition of complex I with irreversible inhibitors such as rotenone. Among the diversity of genes differentially expressed in inherited and induced complex I-deficient cell lines, the marked overexpression of MTs occurred in complex I-deficient fibroblasts when mitochondrial energy metabolism was challenged by changing the carbon source of the medium. It was hypothesized that MT expression points to a possible beneficial adaptive response under such conditions [9]. It is conceivable that the expression and function of MTs are related to disorders of the respiratory chain as these proteins are not only induced by ROS, which is a common feature in such disorders, but scavenges hydroxyl radicals [13,14,33]. Evidence linking MT expression responsiveness to oxidative stress and associated protection against oxidative stress in in vitro and in vivo models is mounting [13,14,20,34]. These include reports of MT1-mediated protection of key mitochondria-associated functions, such as apoptosis, coenzyme Q10 synthesis and mitochondrial genome integrity, against neurotoxin-treated murine neuronal cells [35].
We demonstrated that MT2A expression in HeLa cells is highly inducible with rotenone treatment. This is in contrast with a previous report that MT1 RNA expression is decreased in the striatum cells of rats treated with MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) [36], which also binds and inhibits complex I [37]. Our results show a significant increase in expression of MT2A to occur only after residual complex I activity was inhibited to levels below 50%. Expression of MT2A also did not increase in a co-ordinate way relative to rotenone concentration, and a clear biphasic expression pattern was observed. The reason for this phenomenon is not clear and has to be investigated further. Some clues as to the mechanism of rotenone-induced MT expression may, however, be revealed by the lack of both MT expression and ROS production by treatment with the complex III inhibitor, myxothiazol. It was evident that both MT2A expression and elevated ROS production occurred at residual complex I activities lower than 50%, which was not the case with complete inhibition of complex III activity. This result supports a ROS-related mechanism of rotenone-induced MT2A expression. Lack of myxothiazol-induced ROS production was an unexpected result, as the associated electron-transfer-inhibitory effect of myxothiazol has been well documented [38]. The reason for this lack of detectable ROS production is not clear, although ROS production as a result of deficient electron transport chain in the predominantly glycolytic HeLa cells may, however, in general be less pronounced than in cell lines that rely more on OXPHOS. This is evident from the limited ROS production in addition to the limited decrease in ATP levels induced by rotenone treatment.
MT2A expression was also inducible by Cd, but not Zn, both of which modulate MT expression by a different mechanism from oxidative-stress-related inducers [14,39,40]. Both Cd and Zn induced combined MT1/MT2 protein expression. Although Cd and H2O2 share a common MTF-1 activation pathway in HeLa cells that is dependent on the release of Zn from MT-bound Zn [41], it is not clear why ZnCl2 treatment had no effect on MT2A expression. Similar observations were also made in human proximal tubule cells [42]. In addition, surprisingly, neither MT1A nor MT1B RNA expression induction was detected with any of the inducers used. Lack of MT1B expression in HeLa cells was previously reported [19]. Other MT1 isoforms in HeLa cells may be expressed that could explain the observed Zn induction of MT protein expression.
The effects of overexpressed MT2A on ATP and ROS levels, cell viability and apoptosis in rotenone-treated cells were evaluated in parallel to MT1B overexpression, which, as mentioned above, were not induced by any of the possible MT inducers used in the present study. ATP levels, which were only decreased to ±70% in control cells with rotenone treatment, remained slightly, albeit significantly, higher in MT-overexpressing cells. ROS production in MT1B- and MT2A-overexpressing HeLa cells was similar to control cells when treated with rotenone up to levels where almost no rotenone-sensitive complex I activity could be measured (1000 nM). As mentioned above and evident from our results, HeLa cells rely mainly on glycolytic ATP production. ROS production via inhibition of the OXPHOS system in HeLa cells may therefore be limited compared with cells that have a greater dependence on ATP produced from OXPHOS [5,43]. With the induction of more ROS, i.e. by treatment with t-BHP, which generates ROS via microsomal cytochrome P450 activity [44] in addition to opening the mtPTP (mitochondrial permeability transition pore) [45], a markedly higher level of ROS could be induced. ROS levels under these conditions were significantly less and cell viability was significantly higher in MT2A-overexpressing HeLa cells compared with control and MT1B-overexpressing cells.
Increased production of ROS has been found to relate quantitatively to apoptosis induction in cells treated with rotenone [4,5]. The mechanism of rotenone-induced cell death in HeLa cells has previously been investigated to a limited extent only. Apoptotic cell death predominantly occurs in cells such as HeLa cells, which are less dependent on pyruvate/malate-supported ATP production [5,43]. Apoptosis induction, in addition to caspase activation, also results in the opening of the mtPTP [46], which results in a breakdown of mitochondrial membrane potential [47]. The qualitative results obtained with 100 and 1000 nM rotenone treatment of all three cell lines indicated that sustaining of membrane potential was improved in MT-overexpressing cells, but more so for MT2A, compared with control cells. Similar to previous observations, activation of caspase 3/7 and subsequent nucleosome formation occurred at much lower rotenone levels than the levels needed to induce cellular toxicity [4]. Rotenone-induced caspase 3/7 activation increased dose-dependently after a 24 h period in control cells to levels similar to that induced by staurosporine in 2 h. Over the rotenone concentration range, caspase 3/7 activity was clearly significantly lower in MT2A-overexpressing cells and, to a lesser extent, also in MT1B-overexpressing cells. This protective delaying effect of MT expression on apoptosis was strongly supported by the significantly lower nucleosome formation in MT2A- and, to a lesser extent, MT1B-overexpressing cells, compared with control cells.
Studies on the function of MT are not conclusive as to its role in the prevention of oxidative stress. It was recently reported that MTs present in the intermembrane space of liver mitochondria could inhibit mitochondrial respiratory-chain complexes I and III, through transfer of Zn to the complexes [48–50]. However, this phenomenon did not occur in heart muscle mitochondria [48,51]. In general, studies show that MT expression is associated with a protective effect against interventions leading to oxidative stress. Our results have shown that in rotenone-induced complex I-deficient HeLa cells, overexpressing MT2A indeed had a lowering effect on oxidative stress and increased cell viability, which were especially clear when further challenged with t-BHP treatment. Furthermore, MT2A overexpression had a preventative or delaying effect on rotenone-induced apoptosis in HeLa cells. MT1B, at similar overexpressed levels, in general did not show the same responsiveness as MT2A.
To conclude, recent interest in the downstream adaptive responses to deficiencies of the OXPHOS system has revealed that, via differential gene expression, several genes may be involved in novel responses apart from those already associated with the deficiency, such as induction of apoptosis and changes in redox status [9–11]. Of these responses, little, if any, have been further investigated or reported. We have investigated the expression and role of MTs in an in vitro complex I deficiency model and concluded that the induced expression of MTs, and specifically MT2A, has a protective effect against death-causing cellular consequences of rotenone-treated HeLa cells. Although our results support a ROS-related mechanism, it remains to be determined what the mechanistic properties of this expression are, and if MT expression is functionally relevant to complex I and other inherited OXPHOS deficiencies in vivo. Our results are comparable with current literature reports on the functional properties associated with MT expression, but specifically reveal MT2A expression to be a beneficial downstream adaptive response in complex I-deficient cells.
Acknowledgments
We are grateful for the financial support from the National Research Foundation (South Africa) and the Spanish Ministerio de Ciencia y Tecnología and Feder SAF 2002-01268 and Direcció General de Recerca 2001SGR 00203. We also thank Lissinda du Plessis and Leigh Cooper for technical assistance, Dr Wayne Towers (Centre for Genome Research, North-West University, Pretoria, South Africa) for reading through the paper before submission, and Dr Suria Ellis (Statistical Consultation Service of North-West University, Potchefstroom, South Africa) for consultation on statistical matters.
References
- 1.Carroll J., Shannon R. J., Fearnley I. M., Walker J. E., Hirst J. Definition of the nuclear encoded protein composition of bovine heart mitochondrial complex I: identification of two new subunits. J. Biol. Chem. 2002;277:50311–50317. doi: 10.1074/jbc.M209166200. [DOI] [PubMed] [Google Scholar]
- 2.Loeffen J. L., Smeitink J. A., Trijbels J. M., Janssen A. J., Triepels R. H., Sengers R. C., van den Heuvel L. P. Isolated complex I deficiency in children: clinical, biochemical and genetic aspects. Hum. Mutat. 2000;15:123–134. doi: 10.1002/(SICI)1098-1004(200002)15:2<123::AID-HUMU1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Smeitink J. A., van den Heuvel L., DiMauro S. The genetics and pathology of oxidative phosphorylation. Nat. Rev. Genet. 2001;5:342–352. doi: 10.1038/35072063. [DOI] [PubMed] [Google Scholar]
- 4.Barrientos A., Moraes C. T. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J. Biol. Chem. 1999;274:16188–16197. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- 5.Li N., Ragheb K., Lawler G., Sturgis J., Rajwa B., Melendez J. A., Robinson J. P. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J. Biol. Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- 6.Vazquez-Memije M. E., Shanske S., Santorelli F. M., Kranz-Eble P., Davidson E., DeVivo D. C., DiMauro S. Comparative biochemical studies in fibroblasts from patients with different forms of Leigh syndrome. J. Inherit. Metab. Dis. 1996;19:43–50. doi: 10.1007/BF01799347. [DOI] [PubMed] [Google Scholar]
- 7.Visch H. J., Rutter G. A., Koopman W. J. H., Koenderink J. B, Verkaart S., de Groot T., Varadi A., Mitchell K. J., van den Heuvel L. P., Smeitink J. A. M., et al. Inhibition of mitochondrial Na+–Ca2+ exchange restores agonist-induced ATP production and Ca2+ handling in human complex I deficiency. J. Biol. Chem. 2004;279:40328–40336. doi: 10.1074/jbc.M408068200. [DOI] [PubMed] [Google Scholar]
- 8.Moudy A. M., Handran S. D., Goldberg M. P., Ruffin N., Karl I., Kranz-Eble P., DeVivo D. C., Rothman S. M. Abnormal calcium homeostasis and mitochondrial polarization in human encephalomyopathy. Proc. Natl. Acad. Sci. U.S.A. 1995;92:729–733. doi: 10.1073/pnas.92.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Westhuizen F. H., van den Heuvel L. P., Smeets R., Veltman J. A., Pfundt R., van Kessel A. G., Ursing B. M., Smeitink J. A. M. Human mitochondrial complex I deficiency: investigating transcriptional responses by microarray. Neuropediatrics. 2003;34:14–22. doi: 10.1055/s-2003-38618. [DOI] [PubMed] [Google Scholar]
- 10.Heddi A., Stepien G., Benke P. J., Wallace D. C. Coordinate induction of gene expression in tissues of mitochondrial disease. J. Biol. Chem. 1999;274:22968–22976. doi: 10.1074/jbc.274.33.22968. [DOI] [PubMed] [Google Scholar]
- 11.Collombet J. M., Faure-Vigny H., Mandon G., Dumoulin R., Boissier S., Bernard S., Mousson B., Stepien G. Expression of oxidative phosphorylation genes in muscle cell cultures from patients with mitochondrial myopathies. Mol. Cell. Biochem. 1997;168:73–85. doi: 10.1023/a:1006830807107. [DOI] [PubMed] [Google Scholar]
- 12.Kägi J. H. R., Himmelhoch S. R., Whanger P. D., Bethune J. L., Vallee B. L. Equine hepatic and renal metallothioneins. J. Biol. Chem. 1974;249:3537–3542. [PubMed] [Google Scholar]
- 13.Thornalley P. J., Vašák M. Possible role for metallothionein in protection against radiation-induced oxidative stress: kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim. Biophys. Acta. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- 14.Andrews G. K. Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem. Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 15.Haq F., Mahoney M., Koropatnick J. Signalling events for metallothionein induction. Mutat. Res. 2003;533:211–216. doi: 10.1016/j.mrfmmm.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 16.Palmiter R. D., Findley S. D., Whitmore T. E., Durnam D. M. MT-III, a brain-specific member of the metallothionein gene family. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quiafe C. J., Findley S. D., Erickson J. C., Froelick G. J., Kelly E. J., Zambrowicz B. P., Palmiter R. D. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994;33:7250–7259. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- 18.Hidalgo J., Aschner M., Zatta P., Vašák M. Roles of the metallothionein family of proteins in the central nervous system. Brain Res. Bull. 2001;55:133–145. doi: 10.1016/s0361-9230(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 19.Heguy A., West A., Richards R. I., Karin M. Structure and tissue-specific expression of the human metallothionein 1B gene. Mol. Cell. Biol. 1986;6:2149–2157. doi: 10.1128/mcb.6.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebadi M., Brown-Borg H., El Refaey H., Singh B. B., Garrett S., Shavali S., Sharma S. K. Metallothionein-mediated neuroprotection in genetically engineered mouse models of Parkinson's disease. Mol. Brain Res. 2005;134:67–75. doi: 10.1016/j.molbrainres.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman S., Blok R. B., Dahl H. H., Danks O. M., Kirby D. M., Chow C. W., Christodoulou J., Thorburn D. R. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann. Neurol. 1996;39:343–351. doi: 10.1002/ana.410390311. [DOI] [PubMed] [Google Scholar]
- 22.Robinson J. B., Jr, Brent L. G., Sumegi B., Srere P. A. An enzymatic approach to the study of the Krebs tricarboxylic acid cycle. In: Darley-Usmar V. M., Rickwood D., Wilson M. T., editors. Mitochondria: A Practical Approach. Oxford, U.K.: IRL Press; 1987. pp. 160–161. [Google Scholar]
- 23.Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 24.Radonic A., Thulke S., Mackay I. M., Landt O., Siegert W., Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffl M. W., Horgan G. W., Dempfle L. Relative expression software tool (REST) for groupwise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasull T., Rebollo D. V., Romero B., Hidalgo J. Development of a competitive double antibody radioimmunoassay for rat metallothionein. J. Immunoassay. 1993;14:209–225. doi: 10.1080/15321819308019851. [DOI] [PubMed] [Google Scholar]
- 28.Maniatis T., Fritsch E. F., Sambrook J. Plainview: Cold Spring Harbor Laboratory Press; 1982. Molecular Cloning: A Laboratory Manual; p. 280. [Google Scholar]
- 29.Wang H., Joseph J. A. Quantifying cellular oxidative stress by dichorofluorescein assay using microplate reader. Free Radical Biol. Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 30.Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. J. Immunol. Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 31.Koopman W. J. H., Verkaart S., Visch H. J., van der Westhuizen F. H., Murphy M. P., van den Heuvel L. W., Smeitink J. A., Willems P. H. G. M. Inhibition of complex I of the electron transport chain causes oxygen radical-mediated mitochondrial outgrowth. Am. J. Physiol. Cell. Physiol. 2005;288:C1440–C1450. doi: 10.1152/ajpcell.00607.2004. [DOI] [PubMed] [Google Scholar]
- 32.Miranda S., Foncea R., Guerrero J., Leighton F. Oxidative stress and upregulation of mitochondrial biogenesis genes in mitochondrial DNA-depleted HeLa cells. Biochem. Biophys. Res. Commun. 1999;258:44–49. doi: 10.1006/bbrc.1999.0580. [DOI] [PubMed] [Google Scholar]
- 33.Thomas J. P., Bachowski G. J., Girotti A. W. Inhibition of cell membrane lipid peroxidation by cadmium- and zinc-metallothioneins. Biochim. Biophys. Acta. 1986;884:448–461. doi: 10.1016/0304-4165(86)90195-9. [DOI] [PubMed] [Google Scholar]
- 34.Kumari M. V., Hiramatsu M., Ebadi M. Free radical scavenging actions of metallothionein isoforms I and II. Free Radical Res. 1998;29:93–101. doi: 10.1080/10715769800300111. [DOI] [PubMed] [Google Scholar]
- 35.Sharma S. K., Ebadi M. Metallothionein attenuates 3-morpholinosydnonimine (SIN-1)-induced oxidative stress in dopaminergic neurons. Antioxid. Redox Signal. 2003;5:251–264. doi: 10.1089/152308603322110832. [DOI] [PubMed] [Google Scholar]
- 36.Rojas P., Rojas-Castaneda J., Vigueras R. M., Habeebu S. S., Rojas C., Rios C., Ebadi M. MPTP decreases MT-1 mRNA in mouse striatum. Neurochem. Res. 2000;25:503–509. doi: 10.1023/a:1007564126478. [DOI] [PubMed] [Google Scholar]
- 37.Ramsay R. R., Krueger M. J., Youngster S. K., Gluck M. R., Casida J. E., Singer T. P. Interaction of 1-methyl-4-phenylpyridinium ion (MPP+) and its analogs with the rotenone/piericidin binding site of NADH dehydrogenase. J. Neurochem. 1991;56:1184–1190. doi: 10.1111/j.1471-4159.1991.tb11409.x. [DOI] [PubMed] [Google Scholar]
- 38.Turrens J. F. Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 39.Saydam N., Adams T. K., Steiner F., Schaffner W., Freedman J. H. Regulation of metallothionein transcription by the metal-responsive transcription factor MTF-1. J. Biol. Chem. 2002;277:20438–20445. doi: 10.1074/jbc.M110631200. [DOI] [PubMed] [Google Scholar]
- 40.LaRochelle O., Gagne V., Charron J., Soh J. W., Seguin C. Phosphorylation is involved in the activation of metal-regulatory transcription factor 1 in response to metal ions. J. Biol. Chem. 2001;276:41879–41888. doi: 10.1074/jbc.M108313200. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B., Georgiev O., Hagmann M., Günes Ç., Cramer M., Faller P., Vasák M., Schaffner W. Activity of metal-responsive transcription factor I by toxic heavy metals and H2O2 in vitro is modulated by metallothionein. Mol. Cell. Biol. 2003;23:8471–8485. doi: 10.1128/MCB.23.23.8471-8485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garrett S. H., Somji S., Todd J. H., Sens D. A. Exposure of human proximal tubule cells to Cd2+, Zn2+, and Cu2+ induces metallothionein protein accumulation but not metallothionein isoform 2 mRNA. Environ. Health Perspect. 1998;106:587–595. doi: 10.1289/ehp.98106587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vrbacky M., Krijt J., Drahota Z., Melkova Z. Inhibitory effects of Bcl-2 on mitochondrial respiration. Physiol. Res. 2003;52:545–554. [PubMed] [Google Scholar]
- 44.Davies M. J. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with rat liver microsomal fractions. Biochem. J. 1989;257:603–606. doi: 10.1042/bj2570603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieminen A. L., Saylor A. K., Tesfai S. A., Herman B., Lemasters J. J. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem. J. 1995;307:99–106. doi: 10.1042/bj3070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isenberg J. S., Klaunig J. E. Role of the mitochondrial membrane permeability transition (MPT) in rotenone-induced apoptosis in liver cells. Toxicol. Sci. 2000;53:340–351. doi: 10.1093/toxsci/53.2.340. [DOI] [PubMed] [Google Scholar]
- 47.Zoratti M., Szabo I. The mitochondrial permeability transition. Biochem. Biophys. Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 48.Ye B., Maret W., Vallee B. L. Zinc metallothionein imported into liver mitochondria modulates respiration. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2317–2322. doi: 10.1073/pnas.041619198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simpkins C. O., Zhao H. L., Torrence C. A. Effect of metallothionein I on mitochondrial oxygen consumption. Life Sci. 1994;55:221–226. doi: 10.1016/0024-3205(94)00883-3. [DOI] [PubMed] [Google Scholar]
- 50.Simpkins C., Balderman S., Mensah E. Mitochondrial oxygen consumption is synergistically inhibited by metallothionein and calcium. J. Surg. Res. 1998;80:16–21. doi: 10.1006/jsre.1998.5383. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Z., Kang Y. K. Immunocytochemical localization of metallothionein and its relation to doxorubicin toxicity in transgenic mouse heart. Am. J. Pathol. 2000;156:1653–1662. doi: 10.1016/S0002-9440(10)65036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]