Abstract
Duchenne muscular dystrophy (DMD) is a lethal disease caused by the lack of the cytoskeletal protein dystrophin. Altered calcium homoeostasis and increased calcium concentrations in dystrophic fibres may be responsible for the degeneration of muscle occurring in DMD. In the present study, we used subsarcolemmal- and mitochondrial-targeted aequorin to study the effect of the antiapoptotic Bcl-2 protein overexpression on carbachol-induced near-plasma membrane and mitochondrial calcium responses in myotubes derived from control C57 and dystrophic (mdx) mice. We show that Bcl-2 overexpression decreases subsarcolemmal and mitochondrial calcium overload that occurs during activation of nicotinic acetylcholine receptors in dystrophic myotubes. Moreover, our results suggest that overexpressed Bcl-2 protein may prevent near-plasma membrane and mitochondrial calcium overload by inhibiting IP3Rs (inositol 1,4,5-trisphosphate receptors), which we have shown previously to be involved in abnormal calcium homoeostasis in dystrophic myotubes. Most likely as a consequence, the inhibition of IP3R function by Bcl-2 also inhibits calcium-dependent apoptosis in these cells.
Keywords: aequorin; apoptosis; Bcl-2; Duchenne muscular dystrophy; inositol 1,4,5-trisphosphate receptor; staurosporine
Abbreviations: 2-APB, 2-aminoethoxydiphenyl borate; CCh, carbachol; DMD, Duchenne muscular dystrophy; IP3, D-myo-inositol 1,4,5-trisphosphate; IP3R, inositol 1,4,5-trisphosphate receptor; m[Ca2+], mitochondrial Ca2+ concentration; nAChR, nicotinic acetylcholine receptor; pm[Ca2+], subsarcolemmal Ca2+ concentration; PSS, physiological salt solution; PTP, permeability transition pore; SNAP-25, 25 kDa synaptosome-associated protein; SOCC, store-operated calcium channel; SR, sarcoplasmic reticulum; STS, staurosporine
INTRODUCTION
Duchenne muscular dystrophy (DMD) is an X-linked neuromuscular disease that affects approx. 1 in 3500 males and which results in progressive muscle degeneration [1]. DMD ultimately leads to premature death by respiratory or cardiac failure during the third decade and is caused by the absence of dystrophin, a 427 kDa protein localized under the plasma membrane [2]. Numerous studies have shown that the absence of dystrophin in DMD impairs the stability of the plasma membrane, resulting in a greater fragility towards mechanical stress and increased permeability to calcium (Ca2+) [3]. Indeed, it has been proposed that an alteration of Ca2+ homoeostasis might be responsible for the muscle degeneration that occurs in muscle fibres from DMD patients or in those of the mouse model of DMD, the mdx mouse [4]. Moreover, elevations of basal Ca2+ concentration in myotubes and skeletal-muscle fibres from DMD patients or mdx mice have been reported [5].
Long-term elevation of cytosolic Ca2+ is linked to apoptosis [6] and mitochondrial Ca2+ overload activates the PTP (permeability transition pore) leading to cell death [7]. Because Ca2+ overload occurs in dystrophic myotubes and fibres, the involvement of apoptosis in these cells has been studied but is still a matter of debate. Indeed, it remains unclear if apoptosis causes or is secondary to muscle degradation [8,9]. Thus some groups have suggested that necrosis only occurs in dystrophin-lacking cells [10]. Other groups have shown that apoptosis occurs in dystrophic cells [11] and finally that both apoptosis and necrosis occur in DMD [12]. Therefore apoptosis, linked to necrosis or not, could be responsible for the muscle degeneration that occurs in mdx cells and DMD patients [13].
Apoptosis is a highly regulated process and numerous studies have shown that the Bcl-2 protein, which belongs to the Bcl-2 family, could regulate apoptosis by modulating the release of mitochondrial apoptogenic factors like cytochrome c or apoptosis-inducing factor that activate proteases such as caspases [14]. Even if the exact role of the Bcl-2 protein is still unclear and debated [15], Bcl-2 could act by decreasing Ca2+ concentration in the SR (sarcoplasmic reticulum) [16] and other cellular compartments such as mitochondria [17]. Recent studies have postulated that Bcl-2 could interact directly with IP3R (inositol 1,4,5-trisphosphate receptor) [18,19]. This receptor is known to be overexpressed in dystrophic myotubes [20]. We have shown recently that IP3R is involved in increased CCh (carbachol)-induced near-plasma membrane Ca2+ responses in dystrophic myotubes [21]. However, at present, the effect of Bcl-2 overexpression on both near-plasma membrane and mitochondrial Ca2+ transients has not been studied in dystrophic myotubes.
In the present paper, we have studied the effect of overexpression of the anti-apoptotic protein Bcl-2 on CCh-induced Ca2+ responses in subcellular compartments. We have also investigated the effect of Bcl-2 overexpression on cell survival and apoptosis of myotubes derived from control C57 and dystrophic mice. We show that Bcl-2 overexpression decreases near-plasma membrane and mitochondrial CCh-induced Ca2+ transients in dystrophic myotubes. We also show that Bcl-2 overexpression prevents Ca2+-dependent apoptosis in dystrophic myotubes and that the beneficial effect of Bcl-2 overexpression may be mediated by a direct Bcl-2-dependent IP3R inhibition.
EXPERIMENTAL
Cell culture
Cultures of purified myoblasts were prepared in Petri dishes (Falcon, Becton Dickinson) and maintained at 37 °C in a water-saturated atmosphere of 95% air/5% CO2. They were obtained as described previously [21].
Cell permeabilization
To permeabilize myotubes, a Ca2+-free PSS (physiological salt solution; 145 mM NaCl, 5 mM KCl, 1 mM MgCl2, 5 mM Hepes and 10 mM glucose, pH 7.6) containing 50 μg/ml saponin (Sigma) was used. Cells were incubated in the presence of saponin for 60 s. Directly after permeabilization, myotubes were perfused with a PSS containing 1.2 mM Ca2+ and either inositol 1,4,5-trisphosphate [50 μM; IP3 (D-myo-inositol 1,4,5-trisphosphate); Calbiochem] or its L-enantiomer (50 μM; L-myo-inositol 1,4,5-trisphosphate; Calbiochem) as a negative control.
Transfection
Control C57 and mdx myoblasts were plated at 15000 cells per cm2 on 13 mm Thermanox coverslips (Nalge Nunc International) in 4-well plates. When 80–90% confluent, growth medium was removed and replaced with a serum-free medium, Optimem 1 (Gibco). Cells were transfected overnight using Lipofectamine™ 2000 (Invitrogen, Life Technologies) at a ratio of 1 μg of DNA per 2 μl of transfection reagent. The DNA–Lipofectamine™ 2000 complex was prepared in Optimem 1 medium. After overnight incubation, this medium was replaced by differentiation medium. Myotubes were used after 3 or 4 days of differentiation.
Plasmids
The aequorin plasmids were gifts from Professor T. Pozzan (University of Padova, Padova, Italy). Cells were transfected with a pcDNAI expression vector containing a cDNA encoding aequorin for Ca2+ measurement, fused with the SNAP-25 (25 kDa synaptosome-associated protein) sequence to measure pm[Ca2+] (subsarcolemmal Ca2+ concentration) [22] or mitochondrial cytochrome c oxidase subunit VIII to measure m[Ca2+] (mitochondrial Ca2+ concentration) [23]. The Bcl-2 plasmid [24] was a gift from Professor Karl Heinz Krause (University of Geneva). The IP3 sponge plasmid was a gift from Dr H. L. Roderick and Dr M. D. Bootman (Calcium Group, Babraham Institute Laboratory of Molecular Signalling, University of Cambridge, U.K.). Cells were transfected with a pdc515 expression vector (Microbix Biosystems) containing a cDNA encoding enhanced green fluorescent protein and the high-affinity IP3 sponge [25].
Immunochemistry
After 3–4 days of differentiation, myotubes were washed with PSS. Then cells were fixed with methanol/acetic acid (95:5, v/v). After saturation with PSS containing 3% (w/v) BSA, antibody raised against the Bcl-2 protein (1:500 dilution; sc-509; Santa Cruz Biotechnology) was added for 1 h at 37 °C and revealed by an Alexa Fluor 488-conjugated anti-mouse antibody (1:1000 dilution; Molecular Probes). Cells were washed four times with PSS after each incubation.
Confocal microscopy
Mdx and control C57 myotubes were grown in plastic culture dishes and transfected with pcDNA3 expression vector containing cDNA encoding Bcl-2. The immunolabelled samples were examined by confocal laser scanning microscopy using a Bio-Rad MRC 1024 ES (Bio-Rad, Hemel Hempstead, U.K.) equipped with an argon–krypton gas laser, 3–4 days after differentiation. The Alexa Fluor fluorochrome was excited with the 488 nm green line and the emission was collected at 520 nm. Mitotracker was excited with the 550 nm red line and the emission was collected at 580 nm. Data were acquired using an inverted microscope (Olympus IX70) and processed with the LaserSharp software (version 3.0; Bio-Rad).
Western blot
Bcl-2 protein content of control C57 and mdx myotubes was determined using Western blotting. Equal amounts of protein extracted were loaded on to SDS/12% PAGE. The relative content of muscle protein in the samples was adjusted according to the actin (antibody dilution 1:500; A2172; Sigma) band. Proteins were transferred to nitrocellulose membranes (Bio-Rad). The Bcl-2 protein was detected using the Bcl-2-specific antibody at a dilution of 1:2500. Subsequently, alkaline phosphatase-conjugated goat anti-mouse (170-6520; Bio-Rad) was used at a dilution of 1:1000. Specific signals were detected with the ECF (enhanced fluorescence) Western blotting detection reagent (Amersham Biosciences).
Intracellular calcium measurements
After 3–4 days of differentiation, Ca2+ concentration was determined in a population of myotubes as described previously [21]. Briefly, aequorins were reconstituted in a PSS containing 5 μM coelenterazine (Calbiochem) for 1 h before the experiment. Particular conditions are needed for the subsarcolemmal-targeted aequorin because the removal of intracellular Ca2+ is required for the complete aequorin reconstitution [26]. Thus SNAP-25–aequorin was reconstituted in a Ca2+-free PSS containing 0.1 mM EGTA in order to decrease the [Ca2+] within the cell. Mitochondrial-targeted aequorin was reconstituted in 1.2 mM Ca2+. Cells were perfused at a rate of 1 ml/min in a custom made 0.5 ml chamber thermostatically maintained at 37 °C (MecaTest, Geneva, Switzerland). Emitted luminescence was detected at 466 nm with a photomultiplier apparatus (EMI 9789A; Electron Tubes, U.K.) and recorded every 1 s using a computer photon-counting board (EMI C660) as described previously [27]. The relationship between recorded emitted light and [Ca2+] was calculated using a previously described equation [28]. Total light output was obtained by exposing cells to 10 mM CaCl2 after permeabilization with 100 μM digitonin to consume all the aequorin.
Survival assay and apoptosis
The enzymatic colorimetric measurement of the phosphatase acid activity was used to determine the effect of Bcl-2 overexpression on the survival of the myotubes. Myotubes were cultured in 24-well plates coated with collagen. Briefly, after 3–4 days of differentiation, cells were washed twice for 5 min with 500 μl of PBS (0.2 g/l KCl, 0.2 g/l KH2PO4, 8 g/l NaCl and 1.15 g/l NaH2PO4, pH 7.2). Then 250 μl of substrate solution containing citrate buffer aqueous solution (0.1 M, pH 5.5), 0.1% Triton X-100 and 1 mg/ml p-nitrophenyl phosphate substrate was added to each well for 90 min. The reaction was stopped by adding 25 μl of 1 M NaOH. Absorbance was measured at 405 nm.
The APO percentage apoptosis assay (Biocolor, Newtownabbey, U.K.) was used to assess the effect of Bcl-2 overexpression on apoptosis. Cellular dye uptake was analysed according to the manufacturer's method modified for our myotubes that were cultured in 24-well plates coated with collagen. Briefly, after a 90 min incubation at 37 °C with APODye reagent (dilution 1:20), cells were washed with PSS to remove extracellular dye. Subsequently, APO dye release reagent was applied for 10 min and absorbance of the released dye was measured at 550 nm with a plate reader (Fluostar Galaxy; BMG Labtechnologies).
Data analysis
Data analysis was performed using the software GraphPad Prism 4 (GraphPad Software, San Diego, CA, U.S.A.) and Matlab 7 SP1 (The MathWorks, Natick, MA, U.S.A.). Results are expressed as means±S.E.M. Statistical significance of differences between the values was assessed with the unpaired Student's t test and one-way ANOVA followed by Dunnett post-hoc test when necessary. P values ≤0.05 were considered significant.
RESULTS
Bcl-2 localization and overexpression
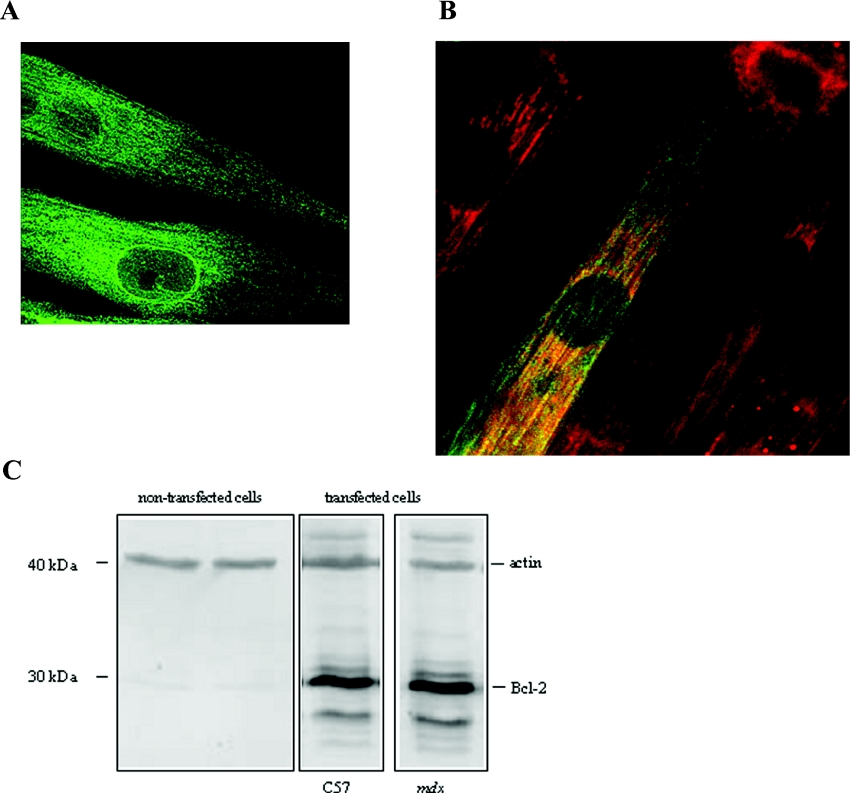
In order to investigate the localization of the Bcl-2 protein, myoblasts were transfected with cDNA encoding Bcl-2. Localization of Bcl-2 was assessed using immunochemistry and confocal imaging. Figure 1 shows representative confocal sections of dystrophic myotubes after 3 days of differentiation. The green fluorescence alone corresponds to overexpressed Bcl-2 protein (Figure 1A), as no fluorescence was detected in non-transfected myotubes (results not shown). Overexpressed Bcl-2 protein appears to be located on membranes which could be SR membranes and also in the perinuclear area. Double staining of Bcl-2 protein and mitochondria (with mitotracker, red fluorescence) indicates that overexpressed Bcl-2 proteins were located in part in mitochondria (yellow fluorescence, Figure 1B). Similar results were obtained with control C57 myotubes (results not shown). Bcl-2 overexpression was confirmed by Western-blot analysis of both control C57 and mdx myotubes (Figure 1C). Indeed, Bcl-2 protein was only detected in transfected myotubes. Moreover, results shown in Figure 1(C) indicate that Bcl-2 was similarly expressed in both cell types.
Figure 1. Bcl-2 localization and overexpression.
(A) Confocal section of mdx myotubes labelled with anti-Bcl-2 antibody for protein localization (green fluorescence). (B) Mitotracker (red fluorescence) and co-localization of mitochondria and Bcl-2 (yellow fluorescence). (C) Western-blot analysis of cell lysates from non-transfected and transfected control C57 and mdx myotubes. The blot was probed with anti-Bcl-2 antibody. Actin was used as control.
Effect of Bcl-2 overexpression on pm[Ca2+]
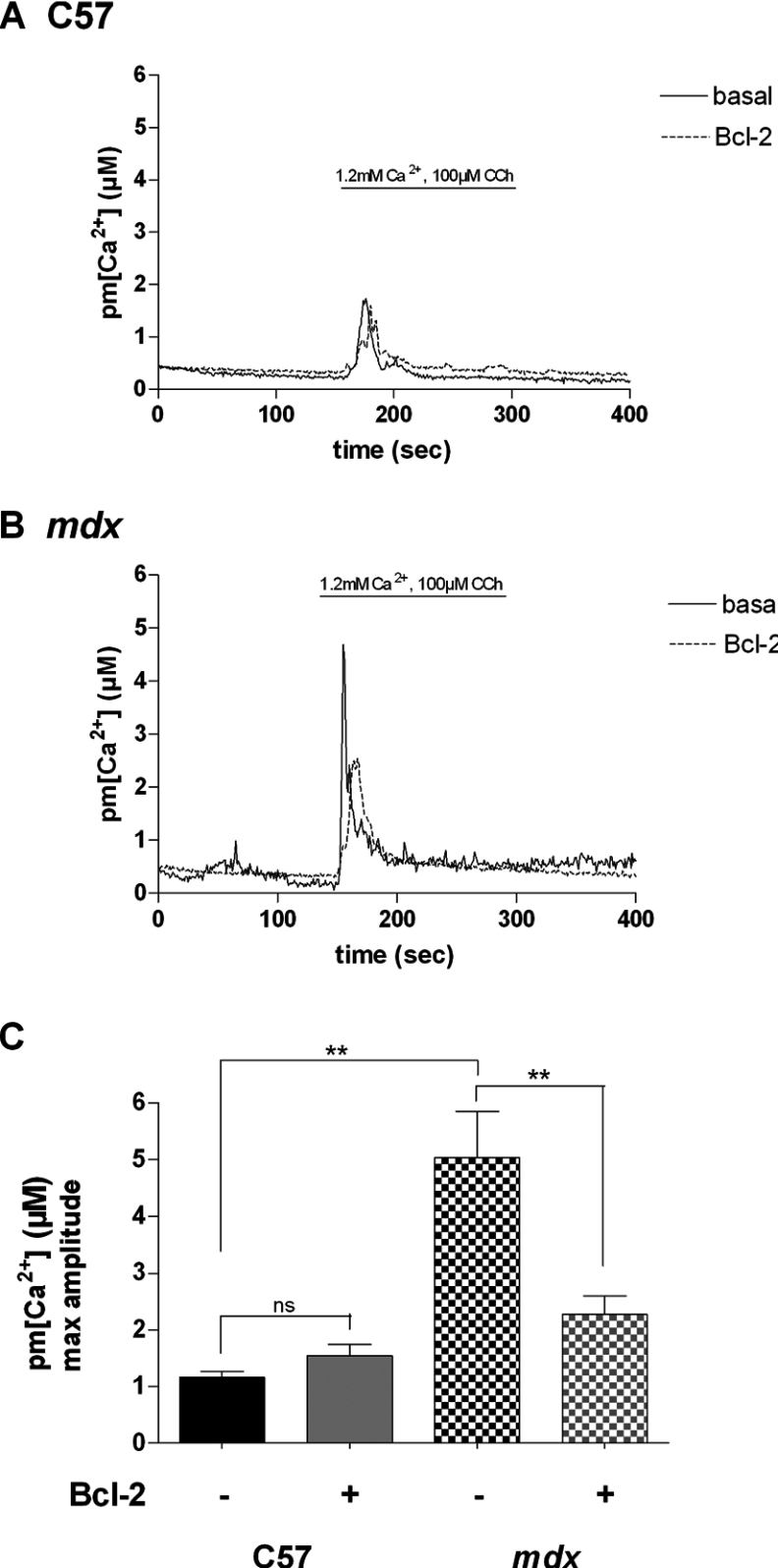
In order to study the effect of Bcl-2 overexpression on Ca2+ homoeostasis in both control C57 and mdx myotubes, we investigated increases in pm[Ca2+] and m[Ca2+] triggered by nAChR (nicotinic acetylcholine receptor) stimulation using CCh. CCh-induced pm[Ca2+] increases were measured with plasma membrane-targeted aequorin as previously described [21]. Due to very high [Ca2+] in this compartment, aequorin was reconstituted in Ca2+-free PSS containing 0.1 mM EGTA for 1 h before the experiment in order to decrease the [Ca2+] within the cell. This allows complete reconstitution of aequorin [26]. Stimulation of myotubes with CCh triggered near-plasma membrane Ca2+ increases with average amplitudes of 1.10±0.11 and 5.04±0.81 μM for control C57 and dystrophic myotubes respectively. Bcl-2 overexpression did not affect significantly the CCh-induced near-plasma membrane Ca2+ increases in control C57 myotubes but decreased this response in dystrophic myotubes approx. 2-fold (to 2.35±0.35 μM; Figure 2). These results demonstrate that the CCh-induced Ca2+ increase is five times higher in dystrophic myotubes as compared with control C57 cells. These results also show that Bcl-2 overexpression decreases CCh-induced near-plasma membrane Ca2+ increases only in dystrophic myotubes. Thus Bcl-2 overexpression appears to correct exaggerated nearplasma membrane Ca2+ transients that occurred during stimulation of nAChRs in dystrophic myotubes. We have previously shown that the increased CCh-induced Ca2+ response in dystrophic myotubes involves Ca2+ release through IP3Rs [21], which have been shown to be overexpressed in dystrophic myotubes [20].
Figure 2. Effect of Bcl-2 overexpression on pm[Ca2+].
Representative traces of pm[Ca2+] increases in a population of (A) control C57 and (B) mdx myotubes showing the effect of nAChR stimulation and Bcl-2 overexpression on the pm[Ca2+]. (C) Summarizing histogram of CCh-induced subsarcolemmal Ca2+ responses measured in control C57 (n≥4) and mdx (n≥14) myotubes. The bar graphs correspond to mean pm[Ca2+] values±S.E.M. ns, not significant (P>0.05); ** corresponds to a significant inhibition (0.01<P<0.001) using unpaired Student's t test.
Effect of IP3R inhibitors on the near-plasma membrane Ca2+ response in dystrophic myotubes that overexpress Bcl-2
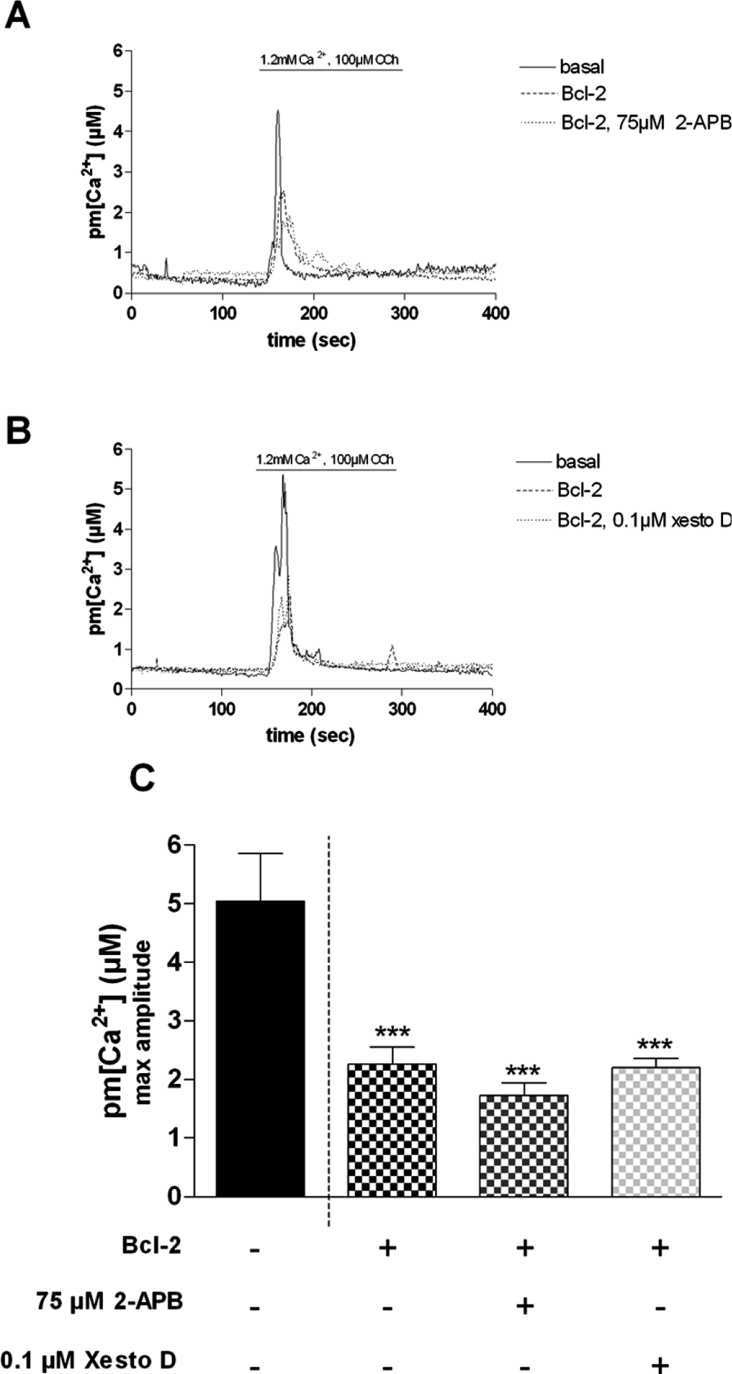
As Bcl-2 protein has been shown to interact directly with IP3R and to inhibit its opening [18], we next investigated the effect of potent IP3R inhibitors on CCh-induced near-plasma membrane Ca2+ increases in dystrophic myotubes. As shown in Figure 3, incubation of dystrophic myotubes that overexpress Bcl-2 with either 2-APB (2-aminoethoxydiphenyl borate; 75 μM) [29] or xestospongin D (0.1 μM) [30] for 10 min did not affect the CCh-induced Ca2+ response when compared with non-treated cells (2.32±0.30 and 1.63±0.21 μM respectively for 2-APB; 2.32±0.30 and 2.20±0.15 μM respectively for xestospongin D) (Figure 3C). In contrast, in dystrophic myotubes that do not overexpress Bcl-2, the two IP3R inhibitors have been shown to strongly reduce CCh-induced Ca2+ responses [21]. Altogether, these results suggest that Bcl-2, when overexpressed in dystrophic myotubes, may reduce CCh-induced Ca2+ responses by inhibiting the IP3R, as shown in other cell types [18].
Figure 3. Effect of IP3R inhibitors on Bcl-2-transfected dystrophic myotubes.
Representative traces of Ca2+ increases in a population of mdx myotubes showing the effect of Bcl-2 overexpression alone and Bcl-2 overexpression together with (A) 75 μM 2-APB or (B) xestospongin D (xesto D; 0.1 μM) on the CCh-induced Ca2+ response. (C) Summarizing histogram. The bar graphs correspond to mean pm[Ca2+] values±S.E.M.; n≥8; *** corresponds to a significant inhibition (P<0.001) using one-way ANOVA followed by a Dunnett post-hoc test when compared with the control bar, e.g. 1.2 mM Ca2+, 100 μM CCh, no Bcl-2 overexpression.
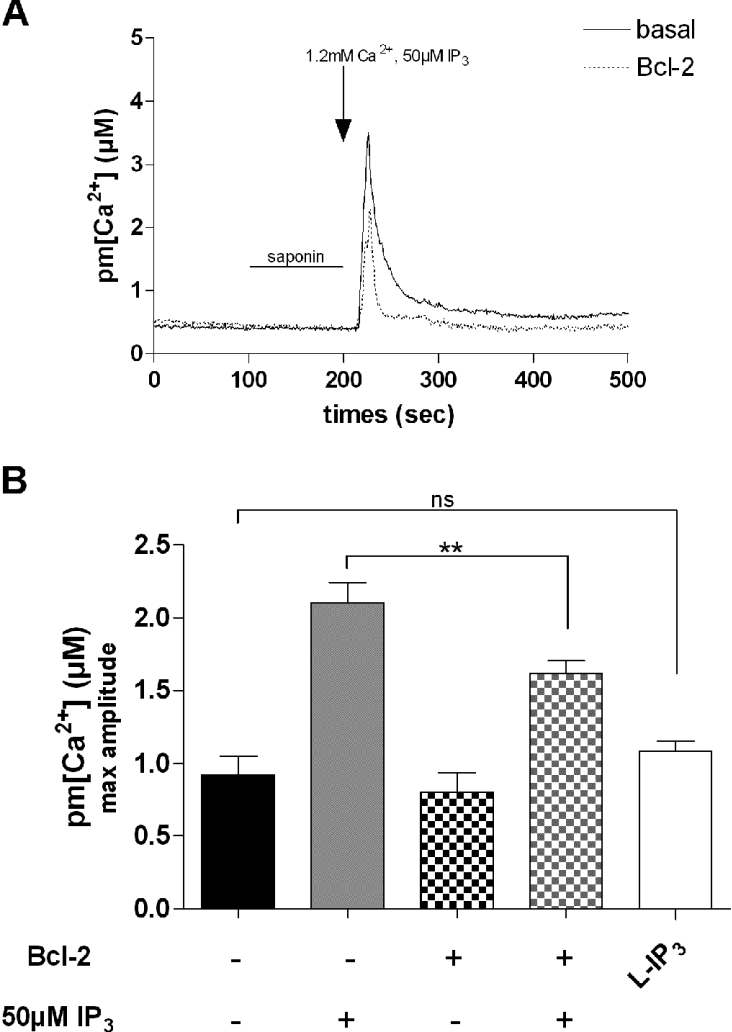
To confirm that Bcl-2 could act by inhibiting IP3R, we used saponin-permeabilized dystrophic myotubes perfused with 50 μM IP3. As shown in Figure 4, perfusion with this second messenger induced a subsarcolemmal Ca2+ increase with a maximal amplitude of 2.10±0.14 μM, whereas Ca2+ readdition alone triggered a maximal Ca2+ transient of 0.92±0.13 μM. In myotubes overexpressing Bcl-2, the IP3-induced Ca2+ response was significantly decreased when compared with myotubes not overexpressing the anti-apoptotic protein (from 2.10±0.14 to 1.62±0.09 μM). Perfusion with the L-enantiomer of IP3 (50 μM), known to have very little affinity for IP3R, did not significantly change the subsarcolemmal Ca2+ response when compared with Ca2+ readdition (1.08±0.07 and 0.92±0.13 μM respectively). Thus Ca2+ transients induced by both CCh, which triggers an IP3-dependent Ca2+ response [21], and IP3, which acts directly on IP3R, are inhibited by Bcl-2 overexpression. However, Bcl-2 overexpression has also been shown to affect SR Ca2+ content in some cases [16]. To test this hypothesis, we investigated the effect of caffeine (20 mM), an activator of the ryanodine receptor [31], on pm[Ca2+]. Caffeineinduced near-plasma membrane Ca2+ increases were not significantly affected in dystrophic myotubes overexpressing Bcl-2 when compared with myotubes not overexpressing the anti-apoptotic protein (results not shown). This suggests that Bcl-2 overexpression does not correct the CCh-induced Ca2+ response by reducing the Ca2+ content of SR Ca2+ stores. Altogether, these results therefore suggest that Bcl-2 overexpression is likely to inhibit IP3R-dependent Ca2+ release in dystrophic myotubes, thus leading to a reduced near-plasma membrane Ca2+ transient during activation with CCh.
Figure 4. IP3-induced Ca2+ response is modulated by Bcl-2 overexpression.
(A) Representative trace of pm[Ca2+] increases in a population of saponin-permeabilized mdx myotubes showing the effect of Bcl-2 overexpression on the IP3 (50 μM)-induced pm[Ca2+] response. (B) Summarizing histogram. The L-enantiomer of IP3 (L-IP3;50 μM) is used as a negative control. The bar graphs correspond to mean pm[Ca2+] values ±S.E.M.; n≥6; ns, not significant (P>0.05); ** corresponds to a significant inhibition (0.01<P<0.001) using unpaired Student's t test.
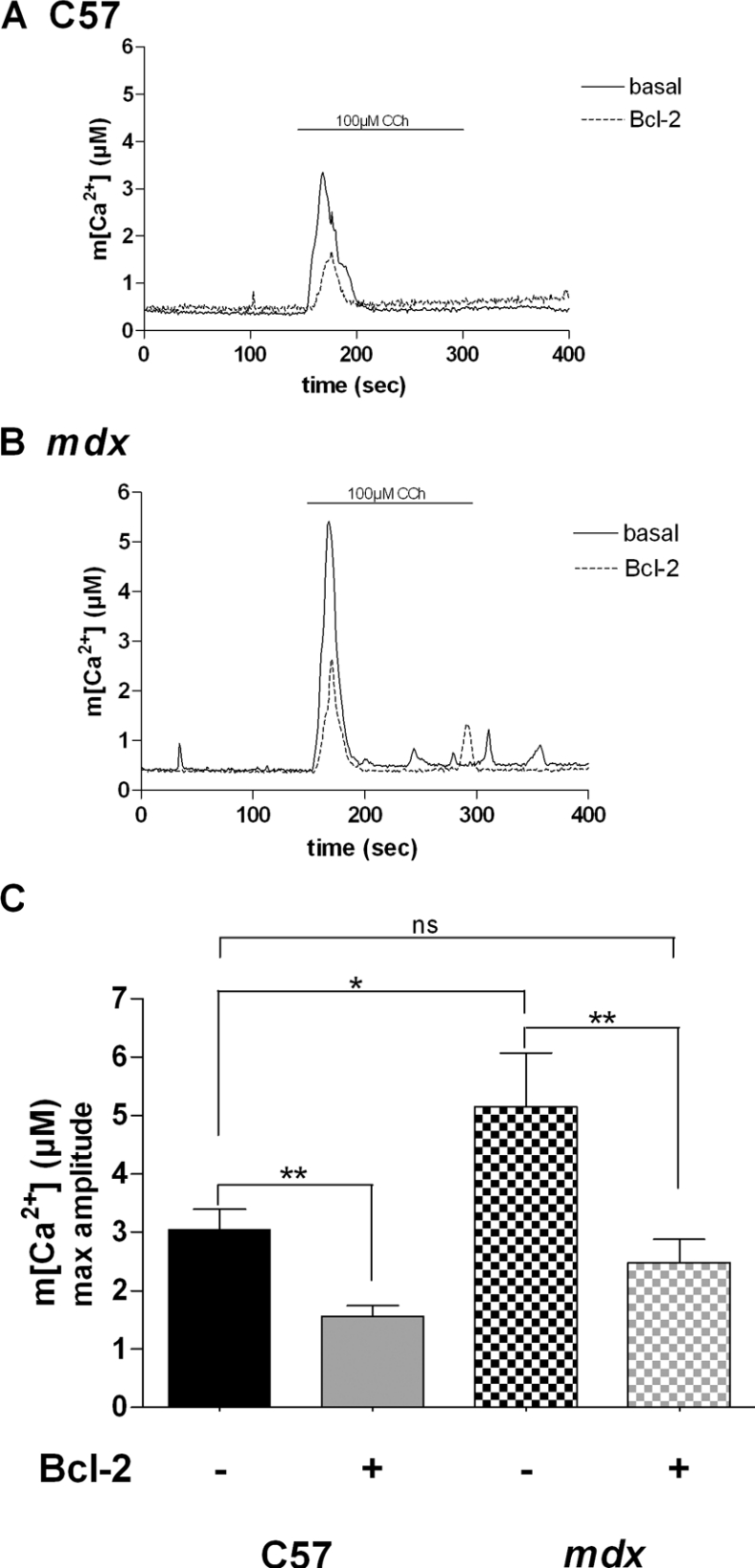
Effect of Bcl-2 overexpression on m[Ca2+]
It has been shown that a tight coupling between SR Ca2+ release channels (IP3Rs and ryanodine receptors) and mitochondrial Ca2+ uptake sites operates in various types of cells [32]. CCh-induced m[Ca2+] increases were measured with aequorin targeted to mitochondria. As shown in Figure 5, the amplitudes of CCh-induced Ca2+ response were 3.05±0.34 and 5.15±0.92 μM in control C57 and dystrophic myotubes respectively. In myotubes overexpressing Bcl-2, CCh-induced mitochondrial Ca2+ increases were significantly decreased in both control C57 and mdx myotubes (1.56±0.18 and 2.47±0.4 μM respectively). These results show that CCh-induced mitochondrial Ca2+ transients are approx. 1.5-fold higher in dystrophic myotubes than in control C57 cells. This demonstrates that elevated mitochondrial Ca2+ responses occur in dystrophic myotubes during nicotinic activation and also that Bcl-2 overexpression decreases these transients to a value close to control C57 levels. Altogether these results suggest that Bcl-2 overexpression decreases exaggerated subsarcolemmal and mitochondrial Ca2+ responses during physiological activation of dystrophic myotubes.
Figure 5. Effect of the Bcl-2 overexpression on m[Ca2+].
Representative traces of m[Ca2+] increases in a population of (A) control C57 and (B) mdx myotubes showing the effect of nAChR stimulation and Bcl-2 overexpression on m[Ca2+]. (C) Summarizing histogram of CCh-induced m[Ca2+] responses measured in control C57 (n=5) and mdx myotubes (n=14). The bar graphs correspond to mean m[Ca2+] values±S.E.M. ns, not significant (P>0.05); * and ** correspond to a significant inhibition (P<0.05 and 0.01<P<0.001 respectively) using unpaired Student's t test.
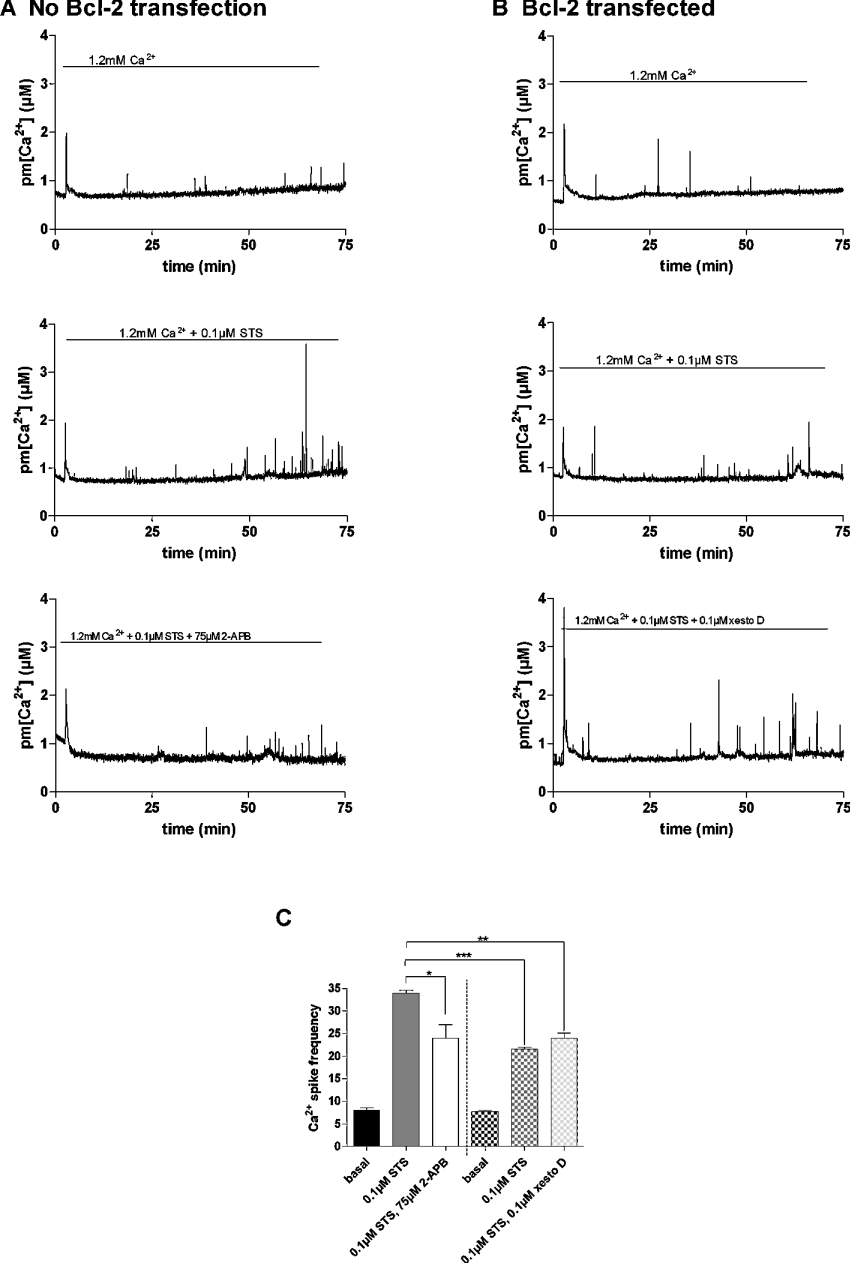
Effect of Bcl-2 overexpression on near-plasma membrane STS (staurosporine)-dependent Ca2+ responses
As Bcl-2 is known to have anti-apoptotic effects mediated by modifications of Ca2+ homoeostasis in various cell types [33], we investigated the effect of Bcl-2 overexpression on Ca2+ responses triggered by the pro-apoptotic agent STS. STS is known to trigger Ca2+-dependent apoptosis [34]. To investigate the effect of STS on the Ca2+ homoeostasis in our dystrophic myotubes, we measured pm[Ca2+] during 75 min under basal conditions (1.2 mM Ca2+) and in the presence of 0.1 μM STS. This limited recording time was chosen to prevent elevated aequorin consumption that may disturb calibration at the end of the experiment. Dystrophic myotubes were transfected with the plasma membrane-targeted aequorin for control experiments and with both Bcl-2- and plasma membrane-targeted aequorin to study the effect of the anti-apoptotic protein. When perfused with a PSS containing 1.2 mM Ca2+, STS induced a 3.5-fold increase in near-plasma membrane Ca2+ spike frequency as compared with the frequency occurring in these myotubes when perfused with 1.2 mM Ca2+ alone (Figures 6A and 6C). Moreover, when the IP3R inhibitor 2-APB (75 μM) was perfused together with STS, the Ca2+ spike frequency decreased approx. 1.5-fold (Figures 6A and 6C). This suggests that the increased Ca2+ spike frequency is due to Ca2+ release through IP3Rs. In Bcl-2-overexpressing myotubes, near-plasma membrane Ca2+ spike frequency was only increased 2.5-fold in the presence of STS (Figures 6B and 6C), which is consistent with Bcl-2 overexpression inhibiting Ca2+ spikes triggered by STS. The addition of the IP3R inhibitor xestospongin D did not further increase the inhibition already triggered by Bcl-2 overexpression. Moreover, Ca2+ spike frequency in Bcl-2-overexpressing dystrophic myotubes was similar to that recorded in dystrophic myotubes perfused with 2-APB but which do not overexpress Bcl-2. Altogether, these results show that STS increases near-plasma membrane Ca2+ spike frequency in dystrophic myotubes and that overexpression of Bcl-2 counteracts the effect of STS. Moreover, near-plasma membrane Ca2+ spike frequencies are reduced in myotubes by Bcl-2 or by 2-APB, suggesting that the decrease in Ca2+ spike frequency may be dependent on reduction of IP3R-dependent Ca2+ release.
Figure 6. Effects of STS on plasma membrane Ca2+ spikes in dystrophic myotubes.
Representative trace of the effect of STS (0.1 μM) in the presence or absence of IP3R inhibitors on the pm[Ca2+] spikes in (A) control dystrophic myotubes and (B) in dystrophic myotubes that overexpress Bcl-2. (C) Summarizing histogram of the mean plasma membrane Ca2+ spike frequency (n≥3). Frequency was calculated manually and represents spikes that point out from the basal pm[Ca2+]. Plain columns represent dystrophic myotubes that were not transfected with Bcl-2. Hatched columns represent Bcl-2-overexpressing dystrophic myotubes. *, ** and *** correspond to a significant decrease (P<0.05, 0.01<P<0.001 and P<0.001 respectively) using Student's t test. xesto D, xestospongin D.
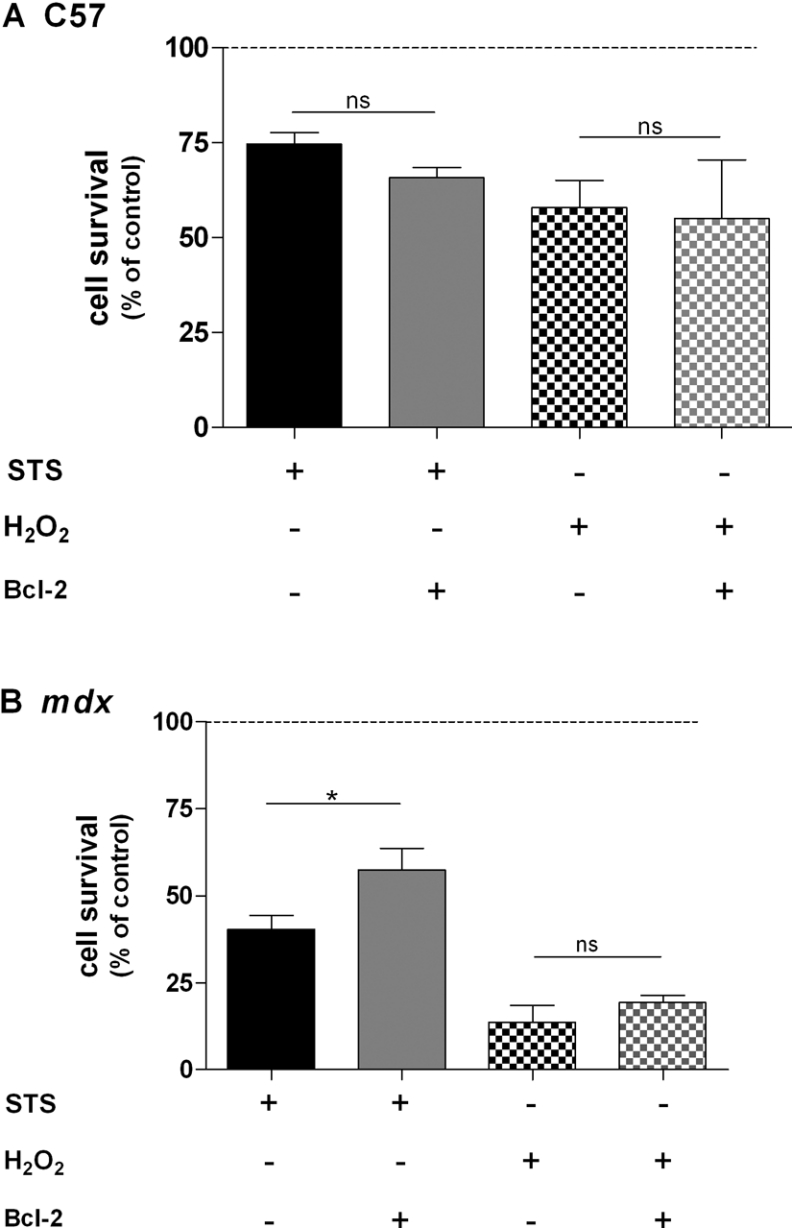
Effect of Bcl-2 overexpression on cell survival and apoptosis
We next investigated the effect of Bcl-2 overexpression on cell survival. In addition to STS, we used H2O2, which induces reactive oxygen species formation in similar cultures [35]. Control C57 and dystrophic myotubes were exposed for 18 h to H2O2 (1 mM) or STS (0.1 μM) followed by cell survival assay using acid phosphatase activity. As shown in Figure 7, both compounds decreased cell survival of control C57 and mdx myotubes. Indeed, in the presence of STS, cell survival was 74.64±3.07% for control C57 cells and 40.26±4.12% for mdx myotubes. In the presence of H2O2, cell survival was 57.85±7.20% for control C57 cells and 13.73±4.80% for mdx myotubes. These results show that dystrophic myotubes are more sensitive to H2O2 and STS compared with control C57 myotubes. However, when myotubes were treated with STS, cell survival of Bcl-2-overexpressing myotubes was significantly increased (from 40.26±4.12 to 57.50±6.17%) only in dystrophic myotubes (Figure 7B). In contrast, cell survival of control C57 myotubes was not affected by Bcl-2 overexpression (Figure 7A). When myotubes were treated with H2O2, Bcl-2 overexpression had no effect on cell survival in both cell types (Figures 7A and 7B). It is therefore likely that Bcl-2 overexpression protects only dystrophic myotubes against cell death triggered by STS.
Figure 7. Effects of STS and H2O2 on cell survival of control C57 and mdx myotubes.
Summarizing histogram of the sensitivity of control C57 (A) and mdx (B) myotubes to STS (0.1 μM; plain bar) and H2O2 (1 mM; hatched bar) combined with the effect of Bcl-2 overexpression. STS and H2O2 were applied for 18 h. The bar graphs correspond to mean cell survival ±S.E.M.; n≥6; ns, not significant (P>0.05); * corresponds to a significant increase (P<0.05) using unpaired Student's t test.
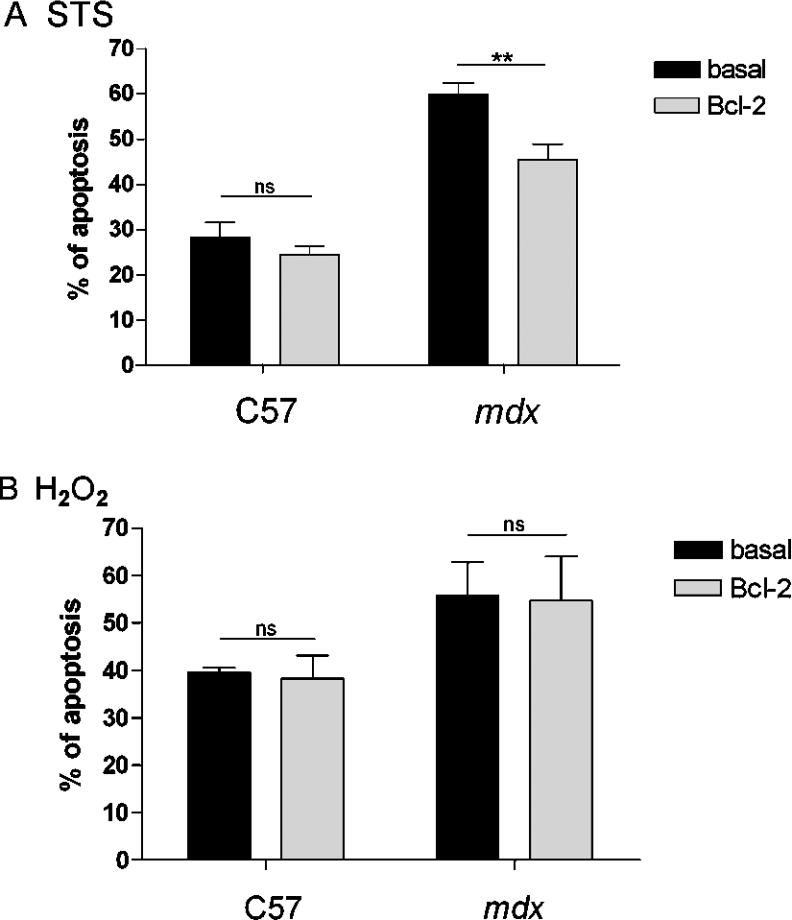
As acid phosphatase measurement does not discriminate between necrosis and apoptosis, we used a specific apoptosis assay. When myotubes were treated with STS, 28.35±3.30% of the control C57 cells and 59.91±2.56% of the dystrophic cells were apoptotic (Figure 8A), confirming the fact that dystrophic myotubes are more sensitive to STS than control C57 cells. When Bcl-2 was overexpressed in these myotubes, the number of apoptotic cells decreased in dystrophic cells only (from 59.91±2.56 to 45.55±3.35%), showing that Bcl-2 overexpression does not protect control C57 myotubes. When cells were treated with H2O2, Bcl-2 overexpression did not change the apoptotic rate significantly in both control C57 and dystrophic myotubes (Figure 8B). These results show that Bcl-2 overexpression counteracts the STS-induced apoptosis in dystrophic myotubes only and that H2O2-induced apoptosis remains unchanged in Bcl-2-overexpressing myotubes.
Figure 8. Apoptosis of control C57 and mdx myotubes overexpressing Bcl-2.
(A) Summarizing histogram of the sensitivity of control C57 (n≥6) and mdx (n≥15) myotubes to STS (0.1 μM). (B) Summarizing histogram of the sensitivity of control C57 (n≥3) and mdx (n≥5) myotubes to H2O2 (1 mM). The bar graphs correspond to mean apoptosis ±S.E.M. ns, not significant (P>0.05); ** corresponds to a significant decrease (0.01<P<0.001) using Student's t test.
IP3 sponge protects dystrophic myotubes from STS-induced apoptosis
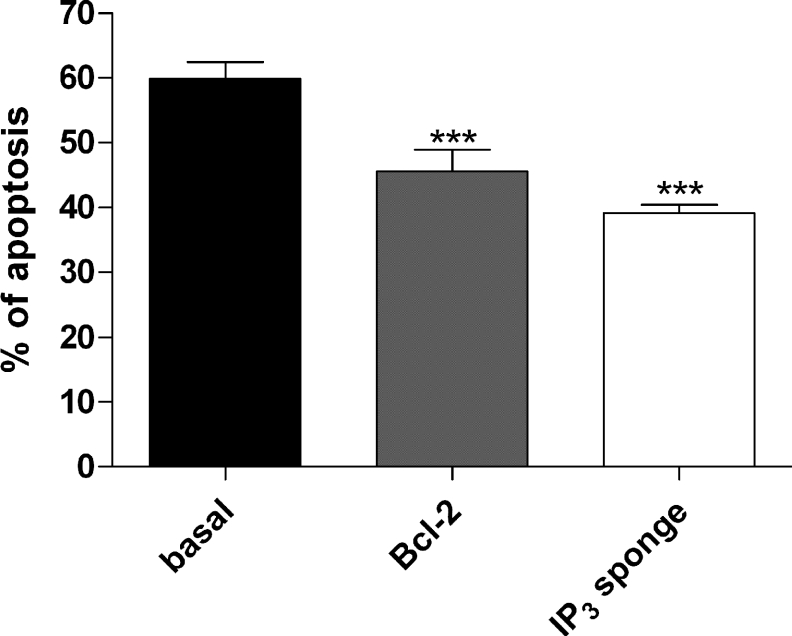
Dystrophic myotubes were transfected with a plasmid containing either Bcl-2 or the IP3 sponge. IP3 sponge has been shown to behave as a very specific IP3 chelator and to inhibit IP3-mediated Ca2+ responses [25]. After 3–4 days of differentiation, cells were treated with STS (0.1 μM) for 18 h. As shown above (Figure 8A), Bcl-2 decreased STS-induced apoptosis in dystrophic myotubes. More interestingly, apoptosis was also decreased from 59.91±2.56 to 39.09±1.32% in myotubes transfected with the IP3 sponge (Figure 9). The decrease observed in Bcl-2-overexpressing myotubes was not statistically different from the value obtained with the IP3 sponge. This result strongly suggests that the IP3 pathway is involved in STS-induced apoptosis in dystrophic myotubes.
Figure 9. IP3 sponge protects cells against STS-induced apoptosis.
Histogram showing the effects of Bcl-2 overexpression and IP3 sponge on apoptosis induced by STS in dystrophic myotubes. The bar graphs correspond to mean apoptosis rate±S.E.M.; n≥15; *** corresponds to a significant decrease (P<0.001) using one-way ANOVA followed by a Dunnett post-hoc test when compared with the control bar. The Bcl-2 bar and IP3 sponge bar were not significantly different (using Student's t test).
DISCUSSION
In these experiments we have investigated the effect of Bcl-2 overexpression on Ca2+ homoeostasis and apoptosis in both control C57 and dystrophic myotubes. Plasma membrane- and mitochondrial-targeted aequorin fusion proteins have been used to study the effect of Bcl-2 overexpression on pm[Ca2+] and m[Ca2+] following nAChR stimulation of both types of myotubes.
First, we found that CCh-induced near-plasma membrane Ca2+ responses are 4.5 times higher in dystrophic myotubes compared with control C57 cells, indicating that exaggerated near-plasma membrane Ca2+ responses occur in dystrophic myotubes during physiological activation. These results are in agreement with previous reports on dystrophic myotubes and fibres showing that a subsarcolemmal Ca2+ overload occurs in these cells [23,36]. Secondly, we show here for the first time that Bcl-2 overexpression counteracts the elevated Ca2+ transients upon physiological activation. On the other hand, Bcl-2 overexpression had no effect in control C57 myotubes, indicating that overexpressed Bcl-2 protein reduces CCh-induced near-plasma membrane Ca2+ responses in dystrophic myotubes by a mechanism that is present or visible only in these cells. We have previously shown that Ca2+ release mechanisms are different between control C57 and dystrophic myotubes [21]. Indeed, CCh-induced near-plasma membrane Ca2+ responses in dystrophic myotubes appear to selectively involve Ca2+ release through IP3Rs as demonstrated with blockers of this receptor (2-APB, xestospongin D or by chelating IP3 with an IP3 sponge), or by reducing the SR Ca2+ store content with the SERCA (sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase) inhibitor thapsigargin [21]. These findings suggest that IP3Rs are likely to be very close to the plasma membrane but also that SOCCs (store-operated calcium channels) which could be controlled by IP3R [37] may contribute to CCh-induced near-plasma membrane Ca2+ increases. In the present paper, we show that in dystrophic myotubes overexpressing Bcl-2, IP3R blockers did not affect CCh-induced near-plasma membrane Ca2+ responses, suggesting that Bcl-2 may reduce CCh-induced Ca2+ responses by inhibiting IP3R-dependent Ca2+ release, as proposed in other cell types [18]. Indeed, it has been shown that Bcl-2 protein interacts allosterically with the C-terminal part of the channel and in this way decreases its open probability [18]. Our results indicate that such an inhibition may operate in our dystrophic myotubes as IP3-induced near-plasma membrane Ca2+ responses were reduced by Bcl-2 overexpression. However, Bcl-2 overexpression has also been shown to reduce SR Ca2+ content in some cell types [16]. In our experiments, such an effect of Bcl-2 overexpression on SR Ca2+ content is unlikely, as the amount of releasable Ca2+ from the SR (estimated using caffeine-induced near-plasma membrane Ca2+ responses) was not significantly different in dystrophic myotubes overexpressing Bcl-2 or not. Our results therefore suggest that the Bcl-2 protein, when overexpressed in dystrophic myotubes, is likely to inhibit IP3R-dependent Ca2+ release and possibly subsequent IP3R-dependent SOCC-dependent influx after CCh stimulation.
We also found that CCh induced mitochondrial Ca2+ increases in both control C57 and dystrophic myotubes, suggesting that mitochondrial Ca2+ uptake sites are closely linked to SR in our myotubes, as demonstrated in other cell types [32]. Moreover, we show that CCh-induced mitochondrial Ca2+ responses are 1.5 times higher in dystrophic myotubes as compared with control C57 cells, indicating that transient mitochondrial Ca2+ overload occurred during nicotinic activation of dystrophic myotubes, similar to what was observed by Robert et al. [23] during highpotassium stimulations. In both control C57 and dystrophic myotubes, Bcl-2 overexpression decreased CCh-induced mitochondrial Ca2+ responses. In dystrophic myotubes overexpressing Bcl-2, reduced mitochondrial Ca2+ responses may be linked to reduced SR Ca2+ release through IP3R, as CCh-induced near-plasma membrane Ca2+ responses were decreased. Links between IP3R or SOCC and mitochondria have been demonstrated [38,39]. In our dystrophic myotubes overexpressing Bcl-2, reduced mitochondrial Ca2+ uptake during CCh stimulation may be explained by an inhibitory effect of Bcl-2 on IP3R-dependent Ca2+ release and subsequent SOCC-dependent influx. However, in control C57 myotubes overexpressing Bcl-2, reduced mitochondrial Ca2+ responses cannot be explained by reduced SR Ca2+ release, as CCh-induced near-plasma membrane Ca2+ increases were not affected by Bcl-2 overexpression. Bcl-2 proteins, when overexpressed in these control C57 myotubes, are likely to act on mitochondria by a direct mechanism such as inhibition of cytochrome c release [40] or by decreasing Ca2+ influx into mitochondria through the Ca2+ uniporter [41], which is consistent with the co-localization of Bcl-2 and mitochondria observed in our myotubes.
Ca2+ overload is known to play a key role in apoptosis by activating a cascade of proteolytic enzymes [42]. In particular, mitochondrial Ca2+ overload can induce PTP opening, resulting in the release of pro-apoptotic factors such as cytochrome c [40]. Moreover, cytochrome c has been shown to bind to IP3R and to induce Ca2+ release from the endoplasmic reticulum, amplifying Ca2+-dependent apoptosis [43]. As Bcl-2 overexpression counteracted transient near-plasma membrane and mitochondrial Ca2+ overload, we have investigated the effect of Bcl-2 overexpression on cell survival and apoptosis in our myotubes. When myotubes were exposed to two types of stress, both control C57 and dystrophic myotubes displayed increased apoptosis and decreased cell survival. However, dystrophic myotubes were more sensitive to H2O2- and STS-induced stresses. These results are in agreement with previous studies indicating that dystrophin-deficient cells are more susceptible to free radicals [35]. However, Bcl-2 overexpression was unable to protect both control C57 and dystrophic myotubes against reactive oxygen species produced by H2O2. On the other hand, Bcl-2 overexpression decreased STS-induced apoptosis selectively in dystrophic myotubes. As STS increased the frequency of near-plasma membrane Ca2+ spikes in dystrophic myotubes, our results suggest that STS is likely to trigger Ca2+-dependent apoptosis, as shown previously [34]. Recently, it was proposed that once apoptosis is induced by STS in HeLa cells, caspase 3 can remove the IP3-binding site and most of the regulatory domain of type 1 IP3R, thus triggering formation of a ‘channel only’ domain that remains constitutively open and which leads to Ca2+ leak in the cytosol [44]. Our results show that both an IP3R inhibitor (2-APB) and Bcl-2 overexpression reduced the STS effect on Ca2+ spike frequency, which suggests that leaky IP3R may be involved in Ca2+ spikes and subsequent Ca2+ overload triggered by STS.
Overall, our results suggest that the anti-apoptotic effect of Bcl-2 may be explained by its inhibitory effect on STS-triggered Ca2+ overload, possibly by a direct inhibition of IP3R [18]. Moreover, the fact that the transiently transfected IP3 sponge inhibited STS-dependent apoptosis indicates that the IP3 pathway is involved in this form of cell death. Overexpressed Bcl-2 proteins may therefore exert their anti-apoptotic effect by inhibiting IP3Rs, which are overexpressed in dystrophic myotubes [20] and could lead to abnormal Ca2+ homoeostasis near the plasma membrane (Ca2+ leak) and in mitochondria.
To conclude, our results indicate that Bcl-2 overexpression reduces both near-plasma membrane and mitochondrial Ca2+ responses occurring during nicotinic activation of dystrophic myotubes, most probably by inhibiting IP3R. Our results also indicate that Bcl-2 overexpression can prevent STS-induced apoptosis selectively in dystrophic myotubes, presumably by reducing near-plasma membrane Ca2+ overload. As the IP3 pathway is involved in CCh-induced Ca2+ transients and in STS-induced apoptosis, the beneficial effect of Bcl-2 overexpression in dystrophic myotubes may be explained by a direct inhibition of IP3Rs, which are indeed involved in abnormal Ca2+ homoeostasis in these cells. Thus overexpression of Bcl-2 or other anti-apoptotic proteins could represent an interesting approach to prevent muscle damage occurring in DMD.
Acknowledgments
We thank Professor P. Poindron (University of Strasbourg, Strasbourg, France), Dr O. M. Dorchies (Laboratory of Pharmacology, University of Geneva) and Dr S. Wagner (Neurofit S.A., Strasbourg, France) for the initiation of cell culture, and Professor T. Pozzan (University of Padova), Professor Karl Heinz Krause (University of Geneva), Dr H. L. Roderick and Dr M. D. Bootman for their gift of plasmids. This work was supported by grants from the Swiss National Science Foundation (31-68315.02), the Swiss Foundation for Research on Muscular Diseases, the ‘Association Française contre les Myopathies’ and the Leenaards Foundation.
References
- 1.Emery A. E. Population frequencies of inherited neuromuscular diseases – a world survey. Neuromuscul. Disord. 1991;1:19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 2.Ahn A. H., Kunkel L. M. The structural and functional diversity of dystrophin. Nat. Genet. 1993;3:283–291. doi: 10.1038/ng0493-283. [DOI] [PubMed] [Google Scholar]
- 3.Gillis J. M. Membrane abnormalities and Ca2+ homeostasis in muscles of the mdx mouse, an animal model of the Duchenne muscular dystrophy: a review. Acta Physiol. Scand. 1996;156:397–406. doi: 10.1046/j.1365-201X.1996.201000.x. [DOI] [PubMed] [Google Scholar]
- 4.Gailly P. New aspects of calcium signaling in skeletal muscle cells: implications in Duchenne muscular dystrophy. Biochim. Biophys. Acta. 2002;1600:38–44. doi: 10.1016/s1570-9639(02)00442-9. [DOI] [PubMed] [Google Scholar]
- 5.Imbert N., Cognard C., Duport G., Guillou C., Raymond G. Abnormal calcium homeostasis in Duchenne muscular dystrophy myotubes contracting in vitro. Cell Calcium. 1995;18:177–186. doi: 10.1016/0143-4160(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 6.Demaurex N., Distelhorst C. Apoptosis – the calcium connection. Science. 2003;300:65–67. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- 7.Duchen M. R. Mitochondria and calcium: from cell signalling to cell death. J. Physiol. (Cambridge, U.K.) 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda R., Nishikawa A., Tanaka H. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J. Biochem. (Tokyo) 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- 9.Spencer M. J., Walsh C. M., Dorshkind K. A., Rodriguez E. M., Tidball J. G. Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J. Clin. Invest. 1997;99:2745–2751. doi: 10.1172/JCI119464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida M., Matsuzaki T., Date M., Wada K. Skeletal muscle fiber degeneration in mdx mice induced by electrical stimulation. Muscle Nerve. 1997;20:1422–1432. doi: 10.1002/(sici)1097-4598(199711)20:11<1422::aid-mus10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Sandri M., Minetti C., Pedemonte M., Carraro U. Apoptotic myonuclei in human Duchenne muscular dystrophy. Lab. Invest. 1998;78:1005–1016. [PubMed] [Google Scholar]
- 12.Tidball J., Albrecht D., Lokensgard B., Spencer M. Apoptosis precedes necrosis of dystrophin-deficient muscle. J. Cell Sci. 1995;108:2197–2204. doi: 10.1242/jcs.108.6.2197. [DOI] [PubMed] [Google Scholar]
- 13.De Luca A., Pierno S., Liantonio A., Cetrone M., Camerino C., Fraysse B., Mirabella M., Servidei S., Ruegg U. T., Conte Camerino D. Enhanced dystrophic progression in mdx mice by exercise and beneficial effects of taurine and insulin-like growth factor-1. J. Pharmacol. Exp. Ther. 2003;304:453–463. doi: 10.1124/jpet.102.041343. [DOI] [PubMed] [Google Scholar]
- 14.Tsujimoto Y., Shimizu S. Bcl-2 family: life-or-death switch. FEBS Lett. 2000;466:6–10. doi: 10.1016/s0014-5793(99)01761-5. [DOI] [PubMed] [Google Scholar]
- 15.Distelhorst C., Shore G. C. Bcl-2 and calcium: controversy beneath the surface. Oncogene. 2004;23:2875–2880. doi: 10.1038/sj.onc.1207519. [DOI] [PubMed] [Google Scholar]
- 16.Oakes S. A., Opferman J. T., Pozzan T., Korsmeyer S. J., Scorrano L. Regulation of endoplasmic reticulum Ca2+ dynamics by proapoptotic Bcl-2 family members. Biochem. Pharmacol. 2003;66:1335–1340. doi: 10.1016/s0006-2952(03)00482-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L., Ling S., Yu X.-D., Venkatesh L. K., Subramanian T., Chinnadurai G., Kuo T. H. Modulation of mitochondrial Ca2+ homeostasis by Bcl-2. J. Biol. Chem. 1999;274:33267–33273. doi: 10.1074/jbc.274.47.33267. [DOI] [PubMed] [Google Scholar]
- 18.Chen R., Valencia I., Zhong F., McColl K. S., Roderick H. L., Bootman M. D., Berridge M. J., Conway S. J., Holmes A. B., Mignery G. A., et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson C. J., Bootman M. D., Roderick H. L. Cell signalling: IP3 receptors channel calcium into cell death. Curr. Biol. 2004;14:R933–R935. doi: 10.1016/j.cub.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Liberona J. L., Powell J. A., Shenoi S., Petherbridge L., Caviedes R., Jaimovich E. Differences in both inositol 1,4,5-trisphosphate mass and inositol 1,4,5-trisphosphate receptors between normal and dystrophic skeletal muscle cell lines. Muscle Nerve. 1998;21:902–909. doi: 10.1002/(sici)1097-4598(199807)21:7<902::aid-mus8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Basset O., Boittin F.-X., Dorchies O. M., Chatton J.-Y., van Breemen C., Ruegg U. T. Involvement of inositol 1,4,5-trisphosphate in nicotinic calcium responses in dystrophic myotubes assessed by near-plasma membrane calcium measurement. J. Biol. Chem. 2004;279:47092–47100. doi: 10.1074/jbc.M405054200. [DOI] [PubMed] [Google Scholar]
- 22.Brini M., Pinton P., Pozzan T., Rizzuto R. Targeted recombinant aequorins: tools for monitoring [Ca2+] in the various compartments of a living cell. Microsc. Res. Tech. 1999;46:380–389. doi: 10.1002/(SICI)1097-0029(19990915)46:6<380::AID-JEMT6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 23.Robert V., Massimino M. L., Tosello V., Marsault R., Cantini M., Sorrentino V., Pozzan T. Alteration in calcium handling at the subcellular level in mdx myotubes. J. Biol. Chem. 2001;276:4647–4651. doi: 10.1074/jbc.M006337200. [DOI] [PubMed] [Google Scholar]
- 24.Cleary M., Smith S., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid Bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell (Cambridge, Mass.) 1986;47:19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- 25.Uchiyama T., Yoshikawa F., Hishida A., Furuichi T., Mikoshiba K. A novel recombinant hyperaffinity inositol 1,4,5-trisphosphate (IP3) absorbent traps IP3, resulting in specific inhibition of IP3-mediated calcium signaling. J. Biol. Chem. 2002;277:8106–8113. doi: 10.1074/jbc.M108337200. [DOI] [PubMed] [Google Scholar]
- 26.Marsault R., Murgia M., Pozzan T., Rizzuto R. Domains of high Ca2+ beneath the plasma membrane of living A7r5 cells. EMBO J. 1997;16:1575–1581. doi: 10.1093/emboj/16.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy E. D., Rizzuto R., Theler J. M., Pralong W. F., Bastianutto C., Pozzan T., Wollheim C. B. Glucose-stimulated insulin secretion correlates with changes in mitochondrial and cytosolic Ca2+ in aequorin-expressing INS-1 cells. J. Clin. Invest. 1996;98:2524–2538. doi: 10.1172/JCI119071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez J., Montero M. Measuring [Ca2+] in the endoplasmic reticulum with aequorin. Cell Calcium. 2002;32:251–260. doi: 10.1016/s0143416002001860. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama T., Kanaji T., Nakade S., Kanno T., Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. (Tokyo) 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 30.Gafni J., Munsch J., Lam T., Catlin M., Costa L., Molinski T., Pessah I. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 31.Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J. Biol. Chem. 1986;261:6300–6306. [PubMed] [Google Scholar]
- 32.Rizzuto R., Duchen M. R., Pozzan T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Science STKE 2004. 2004:re1. doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q.-H., Sheng H.-P., Loh T.-T. Bcl-2 protects HL-60 cells from apoptosis by stabilizing their intracellular calcium pools. Life Sci. 2001;68:2873–2883. doi: 10.1016/s0024-3205(01)01073-6. [DOI] [PubMed] [Google Scholar]
- 34.Kruman I., Guo Q., Mattson M. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J. Neurosci. Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 35.Rando T. A., Disatnik M.-H., Yu Y., Franco A. Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromuscul. Disord. 1998;8:14–21. doi: 10.1016/s0960-8966(97)00124-7. [DOI] [PubMed] [Google Scholar]
- 36.Mallouk N., Jacquemond V., Allard B. Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibers detected with Ca2+-activated K+ channels. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4950–4955. doi: 10.1073/pnas.97.9.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Launikonis B. S., Barnes M., Stephenson D. G. Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2941–2944. doi: 10.1073/pnas.0536227100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandebrouck A., Ducret T., Basset O., Sebille S., Raymond G., Ruegg U., Gailly P., Cognard C., Constantin B. Regulation of store-operated calcium entries and mitochondrial uptake by minidystrophin expression in cultured myotubes. FASEB J. 2006;20:136–138. doi: 10.1096/fj.04-3633fje. [DOI] [PubMed] [Google Scholar]
- 39.Pacher P., Csordas G., Schneider T. G., Hajnoczky G. Quantification of calcium signal transmission from sarco-endoplasmic reticulum to the mitochondria. J. Physiol. (Cambridge, U.K.) 2000;529:553–564. doi: 10.1111/j.1469-7793.2000.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kluck R., Bossy-Wetzel E., Green D., Newmeyer D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 41.Gunter T. E., Buntinas L., Sparagna G., Eliseev R., Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28:285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 42.Orrenius S., Zhivotovsky B., Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 43.Boehning D., Patterson R., Sedaghat L., Glebova N., Kurosaki T., Snyder S. H. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 44.Nakayama T., Hattori M., Uchida K., Nakamura T., Tateishi Y., Bannai H., Iwai M., Michikawa T., Inoue T., Mikoshiba K. The regulatory domain of the inositol 1,4,5-trisphosphate receptor is necessary to keep the channel domain closed: possible physiological significance of specific cleavage by caspase 3. Biochem. J. 2004;377:299–307. doi: 10.1042/BJ20030599. [DOI] [PMC free article] [PubMed] [Google Scholar]