Abstract
More than 80 mutations in the skeletal muscle ryanodine receptor gene have been found to be associated with autosomal dominant forms of malignant hyperthermia and central core disease, and with recessive forms of multi-minicore disease. Studies on the functional effects of pathogenic dominant mutations have shown that they mostly affect intracellular Ca2+ homoeostasis, either by rendering the channel hypersensitive to activation (malignant hyperthermia) or by altering the amount of Ca2+ released subsequent to physiological or pharmacological activation (central core disease). In the present paper, we show, for the first time, data on the functional effect of two recently identified recessive ryanodine receptor 1 amino acid substitutions, P3527S and V4849I, as well as that of R999H, another substitution that was identified in two siblings that were affected by multi-minicore disease. We studied the intracellular Ca2+ homoeostasis of EBV (Epstein–Barr virus)-transformed lymphoblastoid cells from the affected patients, their healthy relatives and control individuals. Our results show that the P3527S substitution in the homozygous state affected the amount of Ca2+ released after pharmacological activation with 4-chloro-m-cresol and caffeine, but did not affect the size of the thapsigargin-sensitive Ca2+ stores. The other substitutions had no effect on either the size of the intracellular Ca2+ stores, or on the amount of Ca2+ released after ryanodine receptor activation; however, both the P3527S and V4849I substitutions had a small but significant effect on the resting Ca2+ concentration.
Keywords: calcium channel, central core disease, lymphoblastoid cell line, malignant hyperthermia, multi-minicore disease, ryanodine receptor
Abbreviations: [Ca2+]i, intracellular Ca2+ concentration; CCD, central core disease; 4-cmc, 4-chloro-m-cresol; CT, computerized tomography; EBV, Epstein–Barr virus; MH, malignant hyperthermia; MHS, MH-susceptible; MmD, multi-minicore disease; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase; SR, sarcoplasmic reticulum
INTRODUCTION
In skeletal muscle, Ca2+ is a key second messenger of the excitation–contraction mechanism and the SR (sarcoplasmic reticulum) is the intracellular organelle that is involved in its regulation. The SR is endowed with numerous proteins involved in Ca2+ handling, such as calsequestrin, a low-affinity Ca2+-binding protein which functions as an intracellular Ca2+ pool, the RyR (ryanodine receptor) which functions as the Ca2+-release channel and the SERCA (sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase) which is involved in pumping the released Ca2+ back into the lumen of the SR [1–3]. RyRs play a key role in Ca2+ homoeostasis since they function as Ca2+-release channels through which luminal Ca2+ is released, thereby contributing actively to the elevation of the myoplasmic [Ca2+] (Ca2+ concentration), which is necessary for muscle contraction [4–6]. In view of its important role as a second messenger, the [Ca2+]i (intracellular [Ca2+]) is finely regulated, and any alteration in the proteins that are involved in Ca2+ handling can potentially lead to pathological conditions. Examples of diseases linked to a dysregulation of Ca2+ homoeostasis include MH (malignant hyperthermia) (OMIM #145600), CCD (central core disease) (OMIM #117000), MmD (multi-minicore disease) (OMIM #602771) and Brody's disease (OMIM #601003) [7–9]. The precise mechanism by which genetic alterations of these proteins trigger different pathophysiological patterns remains to be elucidated.
MmD is an autosomal recessive congenital myopathy that is characterized histologically by the presence of multiple cores, which are areas devoid of mitochondria, thus lacking oxidative enzymes, and with highly disorganized sarcomeric structures. Multiple small cores can occur in both type 1 and type 2 fibres [10,11] and do not run the entire length of the muscle fibre. In contrast, the histological pattern observed in biopsies from CCD patients is characterized by fibre-type uniformity, with strong predominance of type 1 fibres, core lesions with clearly defined borders, occurring exclusively in type 1 fibres and running the entire length of the muscle fibre [12–14]. Both MmD and CCD are characterized by hypotonia during infancy, muscle weakness and delayed motor development; however, they differ in their pattern of muscle weakness and modes of inheritance. Clinical and histopathological overlap between CCD and MmD can occur [15,16], and were reported in two families where patients carried recessive homozygous RYR1 (RyR type 1 gene) mutations [17,18].
The RYR1 gene, composed of 106 exons, maps to chromosome locus 19q13.1 and encodes a protein of 5038 amino acids [19,20]. More than 80 mutations in RYR1 have been genetically linked to MH and CCD [21,22]. Disease-causing mutations appear to cluster in three defined regions of RyR1: the cytoplasmic N-terminal domain 1 (Cys35–Arg614; region 1), the cytoplasmic central domain (Asp2129–Arg2458; region 2) and the C-terminal hydrophobic domain (Ile3916–Ala4942; region 3). In MH, a pharmacological disorder that is triggered by exposure to volatile anaesthetics and/or muscle relaxants in genetically predisposed individuals [23–25], disease-linked mutations predominantly cluster in regions 1 and 2, whereas, in CCD, most RyR1 mutations occur in the hydrophobic pore-forming region 3. MmD is genetically heterogeneous, with more than 50% of the cases presenting with the ‘classical’ MmD phenotype harbouring causative mutations in the SEPN1 (selenoprotein N gene) [26]; however, a significant proportion of the remaining cases carry recessive RYR1 mutations, distributed over all the gene.
Previous studies have demonstrated that EBV (Epstein–Barr virus)-immortalized human B-lymphocytes express the skeletal muscle isoform of the RyR [27,28] and that they could be used as a model to test the effect of mutations on RyR function. In order to shed light on the functional impact of RYR1 mutations linked to MmD, in the present study we investigated the influence of naturally occurring RYR1 mutations identified in patients with MmD and/or CCD, on intracellular Ca2+ homoeostasis and pharmacological RyR activation, in EBV-immortalized B-lymphocytes. The genotypes and phenotypes of the two MmD cases have been described previously [17,18] and are linked to the RyR1 substitutions P3527S and V4849I. We also included in the present study the functional effect of the R999H RyR1 substitution, which was identified at the heterozygous state, in a family in which two siblings were affected by MmD/CCD.
EXPERIMENTAL
Materials
Thapsigargin and fura 2/AM (fura 2 acetoxymethyl ester) were from Calbiochem. Caffeine was from Merck (Darmstadt), 4-cmc (4-chloro-m-cresol) was from Fluka Chemicals (Buchs). The ULTRASPEC® RNA isolation system was from Biotecx laboratories. cDNA synthesis kit and Taq polymerase were from Roche Molecular Biochemicals. Tissue culture medium and reagents were from Invitrogen. All other chemicals were reagent or the highest available grade.
Patients
As SEPN1 mutations have been identified in 50% of classical severe MmD cases, haplotyping studies were first performed for every informative family and SEPN1 was clearly excluded as a candidate gene (results not shown).
Family 1
The P3527S substitution resulting from a C>T transition at position 10579 in exon 71 of RYR1 was identified in three siblings from an Algerian consanguineous family [17]. In the present study, we immortalized lymphoblastoid cells from two of the affected daughters carrying the homozygous mutation and their healthy mother, heterozygous for this mutation. This mutation therefore behaved as a recessive trait. In early childhood, minicores were observed in the biopsies of the siblings, but, when the probands were biopsied again during adult life, their muscle showed a typical CCD pattern. Patients were classified as recessive CCD with transient morphological presentation as MmD.
Family 2
The V4849I substitution resulting from a G>A transition at position 14545 in exon 101 of the RYR1 was identified in the homozygous state in the affected daughter and in the heterozygous state in her healthy parents [18]. A muscle biopsy in the proband revealed marked type 1 predominance with the presence of few minicores and central cores. A muscle MRI (magnetic resonance imaging) showed the typical skeletal muscle involvement that has been associated with patients with CCD. A diagnosis of CCD secondary to a recessive RYR1 mutation was made.
Family 3
The R999H substitution resulting from a G>A transition at position 2996 in exon 24 of the RYR1 gene was identified in the heterozygous state in a patient classified as MmD, with short cores present only in type 1 fibres. Her brother was mildly affected, also carried the mutation in the heterozygous state and presented short cores in both fibre types. The mother had a normal CT (computerized tomography) scan, no signs of any neuromuscular disorder, but was later found to also harbour the R999H substitution at the heterozygous state.
Lymphoblastoid cell lines
Heparinized peripheral blood was obtained from healthy control individuals, family members of patients and patients with core myopathy carrying proven RYR1 mutations after informed consent. Mononuclear cells were isolated and transformed with EBV according to the protocol of Neitzel [29]. Cells were cultured in RPMI 1640 medium supplemented with 10% foetal bovine serum, 2 mM glutamine, 1 mM sodium pyruvate and 100 units of penicillin and streptomycin.
Mutation screening
Total RNA was isolated using an RNA isolation kit. RNA was reverse-transcribed into cDNA using a commercially available kit following the manufacturer's instructions. Approx. 100 ng of cDNA were used for each PCR amplification using an Applied Biosystems 2720 thermal cycler. The following primers were used to amplify cDNA: 24F, 5′-TGGACCGTCTGGCAGAAAATG-3′, and 24R, 5′GGTCAGGAGGCTCGATGTTGTA-3′; 71F, 5′-TCCGGTGGCTCGGACCAGGAA-3′, and 71R, 5′-TTGGCCAGCGTGATGAGGTCTT-3′; 101F, 5′-ACCTGGCCCCATCCTG-3′, and 101R, 5′-GCTAGGGGAGGGGCTCAC-3′. Amplification conditions were 5 min at 95 °C, followed by 40 cycles of 30 s annealing at 56 °C (24F/R), 71 °C (71F/R) or 60 °C (101F/R), 60 s extension at 72 °C and 30 s denaturation at 94 °C, followed by a final extension for 3 min at 72 °C. The presence of the nucleotide substitutions was detected by restriction enzyme digestion using BstUI, HhaI or AccI. In order to demonstrate that the cDNA preparations used for PCR amplification were not contaminated by genomic DNA, a PCR amplification spanning exons 39–40 was carried out as described previously [28].
Intracellular Ca2+ measurements
Changes in [Ca2+]i of the lymphoblastoid cells were monitored with the fluorescent Ca2+ indicator fura 2. Experiments were carried out on populations of cells, in a PerkinElmer LS-50 spectrofluorimeter as described previously [28,30,31] or at the single-cell level by digital imaging microscopy as described previously [32]. In the latter case, lymphoblastoid cells loaded with 5 μM fura 2 were allowed to attach to poly(L-lysine)-treated glass coverslips for 10 min before the experiments. Individual cells were stimulated with a 12-way 100-mm-diameter quartz micromanifold computer-controlled microperfuser (ALA Scientific) as described in [32]. Online (340 nm, 380 nm and ratio) measurements were recorded using a fluorescent Axiovert S100 TV inverted microscope (Carl Zeiss GmbH) equipped with a 40× oil-immersion Plan-Neofluar® objective [0.17 NA (numerical aperture)] and filters (BP 340/380, FT 425, BP 500/530). The cells were analysed using an Openlab imaging system, and the average pixel value for each cell was measured at excitation wavelengths of 340 and 380 nm.
Statistical analysis
Statistical analysis was performed using Student's t test for paired samples or using ANOVA when more than two groups were compared. Origin computer program (Microcal Software) was used for statistical analysis and dose–response curve generation. The EC50 and Rmax values were calculated using the Origin program from sigmoidal curve fitting of all of the data points.
RESULTS
RYR1 mutation analysis
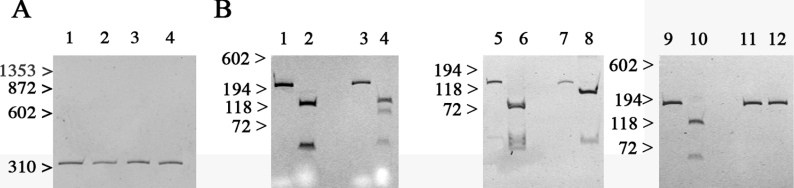
The EBV-immortalized lymphoblastoid cells were analysed by reverse transcription–PCR to verify the presence of the different mutations at the transcriptional level. Figure 1 shows the results after digestion of the PCR-amplified cDNA obtained from the lymphoblastoid cell lines from controls or three probands, each carrying one of the identified mutations. The primers used to amplify the cDNA fragment depicted in Figure 1(A) span exons 39–40, and the size of the amplified band (338 bp) corresponds to that of the cDNA, since the corresponding genomic sequence would encompass intron 39 and yield a fragment of 1400 bp. The 2996G>A substitution in exon 24 abolishes a BstUI restriction site, resulting in the presence of four bands of 127, 93, 47 and 46 bp in the heterozygous state and three bands of 127, 47 and 46 bp in controls (Figure 1B, lanes 1–4). The 10579C>T substitution in exon 71 abolishes a HhaI restriction site, resulting in the presence of two bands of 118 and 38 bp after digestion of the amplified cDNA from the homozygous proband with HhaI and of three bands of 86, 38 and 32 bp after digestion of the amplified cDNA from controls (Figure 1B, lanes 5–8). The 14545G>A substitution in exon 101 abolishes an AccI restriction site. After digestion, the amplified cDNA from the homozygous carrier remains uncut (176 bp fragment), whereas digestion of the amplified cDNA from controls yields two bands of 118 and 58 bp (Figure 1B, lanes 9–12).
Figure 1. EBV-immortalized B-cells from patients express a mutated RyR.
Polyacrylamide gels showing PCR-amplification of cDNA using the primer pairs indicated in the Experimental section. (A) Amplification of cDNA spanning RYR1 exons 38–40 from control (lane 1), R999H (lane 2), P3527S (lane 3) and V4849I (lane 4) yields a band of 338 bp corresponding to the expected cDNA product, and none from genomic DNA amplification. (B) Lanes 1–4, amplification of exon 24 of the RYR1 gene for the R999H substitution: lanes 1 and 3, undigested cDNA; lanes 2 and 4, cDNA after digestion with BstU I; lanes 1 and 2, control; lanes 3 and 4, patient harbouring the heterozygous R999H substitution. Lanes 5–8, amplification of exon 71 of the RYR1 gene for the P3527S substitution: lanes 5 and 7, undigested cDNA; lanes 6 and 8, cDNA after digestion with HhaI; lanes 5 and 6, control; lanes 7 and 8, patient harbouring the homozygous P3527S substitution. Lanes 9–12, amplification of exon 101 for V4849I substitution: lanes 9 and 11, undigested cDNA; lanes 10 and 12, cDNA after digestion with AccI; lanes 9 and 10, control; lanes 11 and 12, patient harbouring the homozygous V4849I substitution.
Resting [Ca2+]i and status of the intracellular Ca2+ stores in lymphoblastoid cells carrying RyR1 mutations
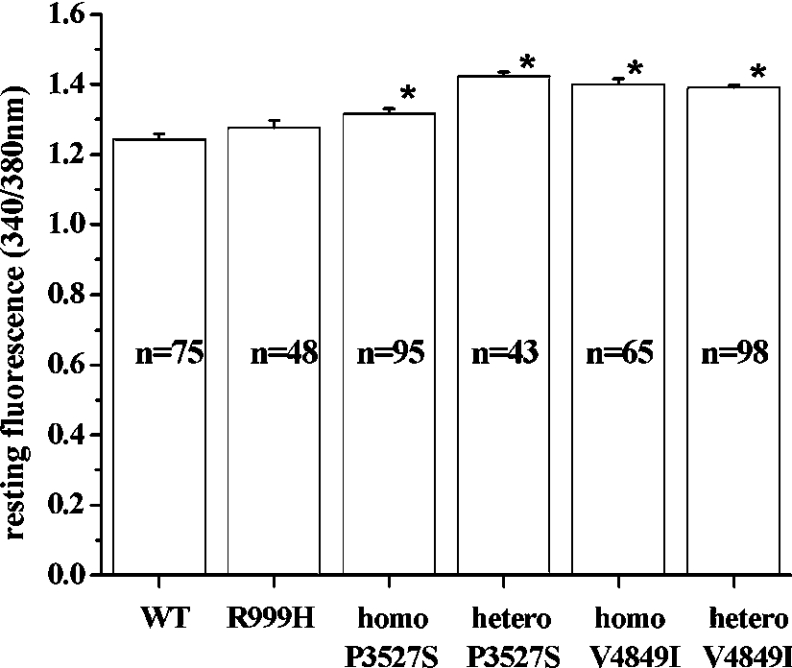
The aim of the present study was to establish the characteristics of the intracellular Ca2+ pools of lymphoblastoid cells carrying the RyR1 substitutions R999H, P3527S and V4849I. To this end, we first analysed the resting [Ca2+]i of lymphoblastoid cells from mutation carriers and compared it with that observed in cells from control individuals (Figure 2). The resting fluorescence intensity observed in cells from patients harbouring the R999H substitution was not significantly different from that observed in control cells, while the presence of the other mutations caused a small, but significant, increase in the fluorescence intensity ratio (P<0.04; ANOVA). In terms of [Ca2+], the observed increases are of the order 10–30 nM.
Figure 2. Resting [Ca2+] of EBV-immortalized lymphoblastoid cells from control individuals and individuals bearing different RyR1 mutations.
Average resting myoplasmic [Ca2+] of cells from controls and individuals bearing the indicated RyR1 mutations. Values are means±S.E.M. (n=43–98). Data were determined in fura-2-loaded lymphoblastoid cells (1×106 cells/ml) in nominally Ca2+-free Krebs–Ringer solution supplemented with 0.5 mM EGTA. [Ca2+]i was measured using the fluorescent Ca2+ indicator fura 2. *, P<0.04. homo, homozygous; hetero, heterozygous; WT, wild-type.
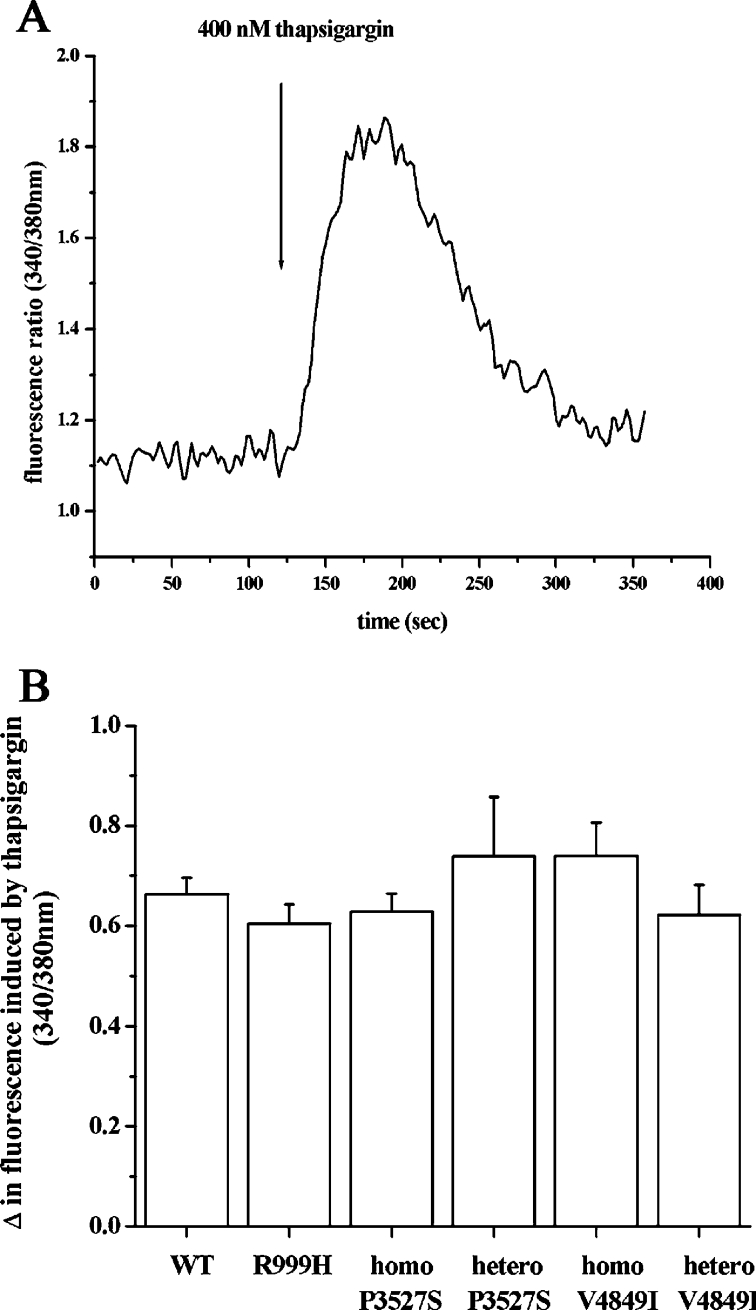
We next examined the status of the intracellular Ca2+ pools by comparing the peak [Ca2+] obtained after addition of the SERCA inhibitor thapsigargin, in the absence of external Ca2+ (Figure 3A). This treatment causes the release of the Ca2+ present in the intracellular pool into the cytoplasm; since Ca2+ cannot be pumped back into the endoplasmic reticulum and no Ca2+ is present in the extracellular medium, the peak fluorescence obtained reflects the size of the rapidly releasable intracellular Ca2+ stores [27,30,31]. When cells from controls or mutation-bearing individuals were treated with 400 nM thapsigargin, no significant differences were observed in the amount of Ca2+ released, suggesting that none of the mutations affect the size of the thapsigargin-sensitive intracellular stores (Figure 3B).
Figure 3. Thapsigargin-sensitive [Ca2+]i stores of EBV-immortalized lymphoblastoid cells from control individuals and RyR1-mutated patients are not significantly different.
(A) Representative trace of the effect of 400 nM thapsigargin on the [Ca2+]i of lymphoblastoid cells from a control individual. Conditions as described in Figure 2. Once the steady state was obtained, 400 nM thapsigargin was added where indicated by the arrow. (B) The mean increase in [Ca2+]i (=peak ratio 340/380 nm−resting ratio 340/380 nm) induced by the addition of 400 nM thapsigargin was calculated. Results are means±S.E.M. (n=6–23). WT, wild-type; homo, homozygous; hetero, heterozygous.
Sensitivity of lymphoblastoid cell lines to pharmacological activation with RyR agonists
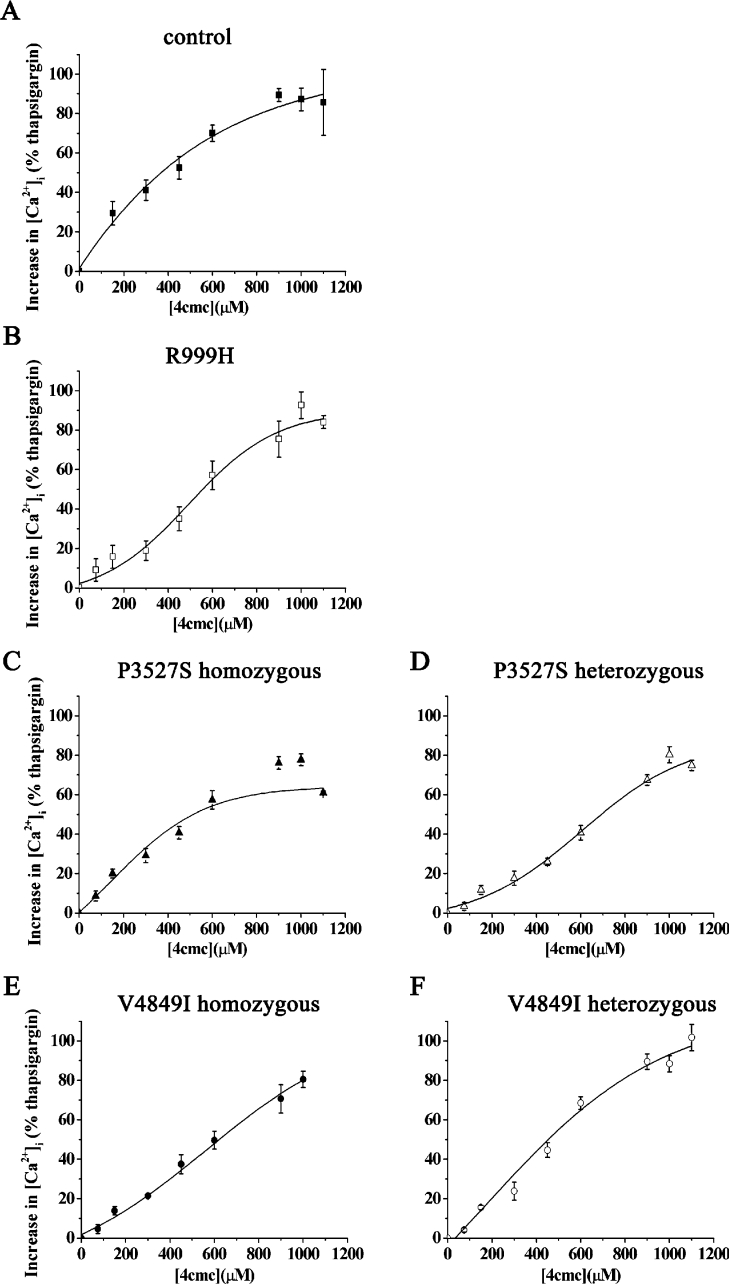
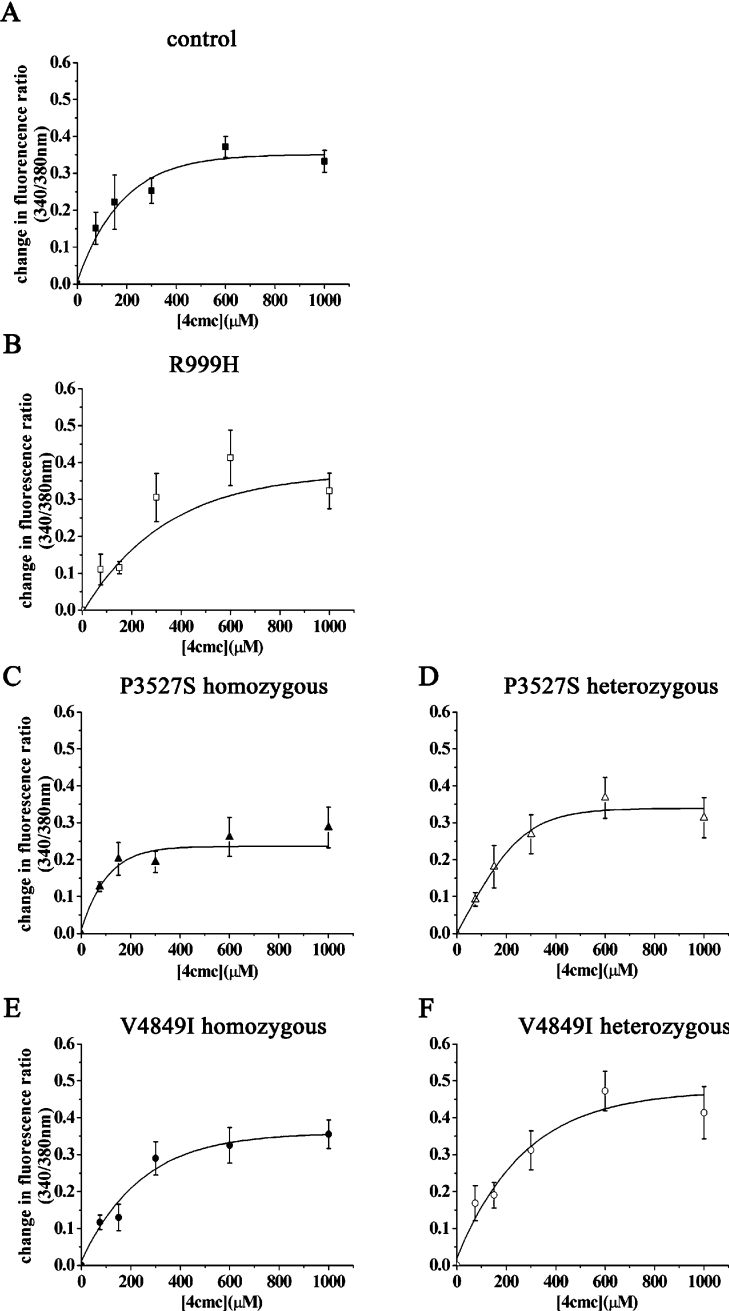
In the next set of experiments, we tested the sensitivities of lymphoblastoid cells to the RyR1 agonists 4-cmc and caffeine. We first created dose–response curves to 4-cmc by studying changes in [Ca2+]i in cell populations in Ca2+-free Krebs–Ringer solution and expressed these as percentage release with respect to the thapsigargin-sensitive pool (set as 100%). 4-cmc is a specific activator of the RyR1 and can be used pharmacologically to discriminate between MHN (MH-normal) and MHS (MH-susceptible) [33–35]; when RYR1 mutations are associated with the MHS phenotype, the sensitivity of the receptor to 4-cmc is increased [28,34]. Figure 4 shows the dose–response curves to 4-cmc in lymphoblastoid cells from control individuals, from individuals harbouring the R999H substitution and from individuals harbouring the P3527S and V4849I substitutions in the homozygous and heterozygous states. None of the mutations caused a significant shift in the dose–response curve to lower agonist concentration, while the P3527S substitution in the homozygous state, caused a small, but significant, reduction in the amount of Ca2+ released by 4-cmc (the percentage maximal releases were 77.6±3.0 and 89.3±3.3 for homozygous P3527S and controls respectively. P<0.02; Student's t test).
Figure 4. Dose-dependent 4-cmc-induced changes in [Ca2+]i in EBV-immortalized lymphoblastoid cells from control individuals and patients carrying different RyR1 mutations.
The increase in [Ca2+]i induced by the indicated concentrations of 4-cmc were calculated as a percentage of the maximal amount which could be released by 400 nM thapsigargin (which was set at 100%). Results are means±S.E.M. of the change in fluorescence (n=4–13). Sigmoidal dose–response curves were generated using the Origin software. (A) Control; (B) heterozygous R999H RyR1 substitution; (C and D) P3527S substitution, in (C) two homozygous daughters and (D) heterozygous mother; (E and F) V4849I substitution, in (E) homozygous daughter and (F) heterozygous parents.
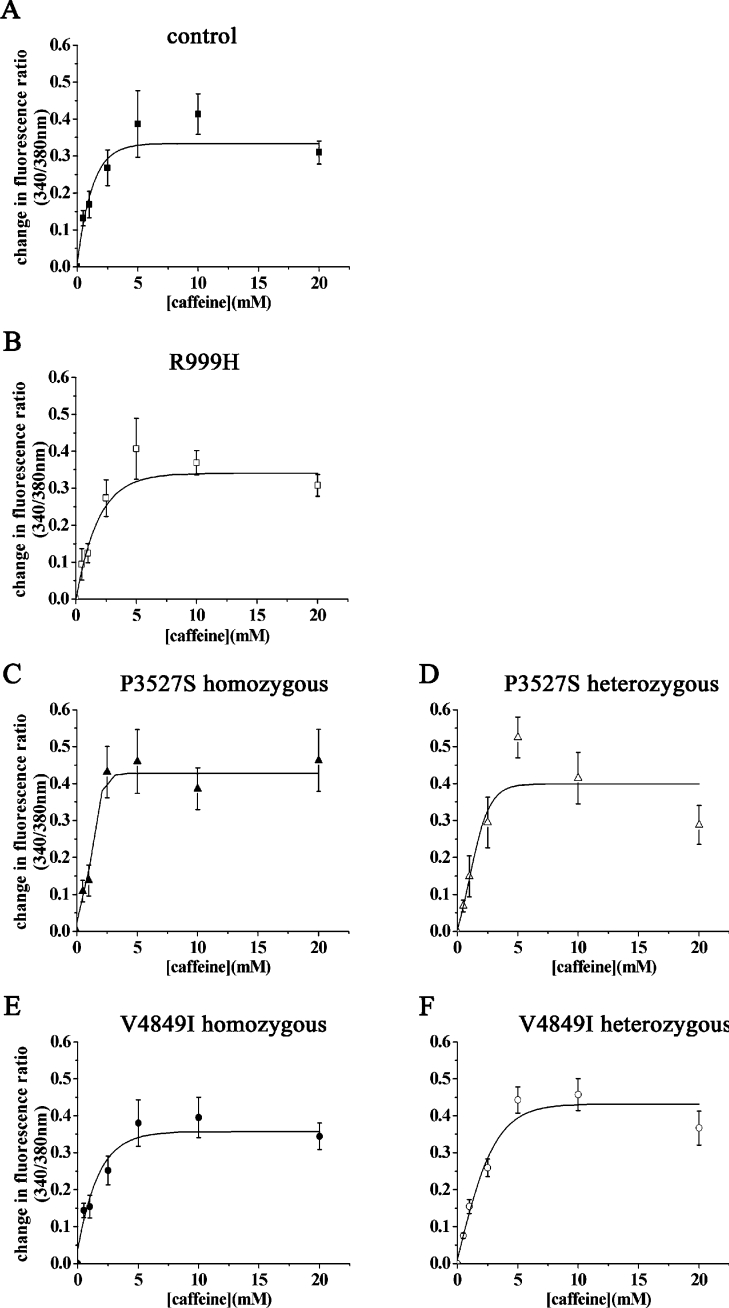
In order to gain more information on how different RYR1 mutations may affect the response to different pharmacological activators, and since, under our experimental conditions in cell populations, we were unable to detect caffeine-induced changes in [Ca2+]i, we next examined the Ca2+ response of lymphoblastoid cells at the single-cell level. Individual cells were stimulated by a pressure pulse of either 4-cmc or caffeine and the release of Ca2+ was assessed by Ca2+ imaging. This technique is more sensitive and allowed us to detect changes in the [Ca2+]i even after addition of caffeine (Figure 5); it also helped us monitor the proportion of cells responding to a particular concentration of agonist. Figures 6 and 7 show the 4-cmc and caffeine dose–response curves obtained from control cells and from cells from mutation-bearing individuals. These curves were generated taking into consideration only cells responding to the added agonist: using these values, only the P3527S homozygous substitution caused a reduction in the amount of Ca2+ released by 4-cmc, while none of the mutations caused a significant change in the maximal amount of Ca2+ released by caffeine (Figures 6 and 7, compare curve in panels A with those in panels B–F). The sensitivities to 4-cmc and caffeine and maximal change in [Ca2+]i induced in lymphoblastoid cells bearing the different mutations are shown in Table 1. The maximal fluorescence change (Rmax) and EC50 were calculated from the data shown in Figures 6 and 7 and the values given were calculated using the Origin program for sigmoidal curve generation. The results show: (i) that none of the mutations significantly reduced the sensitivity of RyR1 to activation by both caffeine and 4-cmc, while, in some cases, there was a shift to higher agonist concentrations; (ii) the Rmax value for 4-cmc-induced Ca2+ release was only different for the P3527S homozygous carriers, and (iii) the Rmax values obtained for caffeine and 4-cmc were of comparable magnitude. Table 2 compares the percentage of responding cells at each concentration of 4-cmc and caffeine in lymphoblastoid cells carrying different mutations. It is apparent that (i) the percentage of cells responding to 4-cmc was always higher than that responding to caffeine, and (ii) the number of cells harbouring the homozygous substitution P3527S responding to any concentration of caffeine was always lower than that from controls or from carriers of the two other substitutions. When the cumulative change in fluorescence of all analysed cells was taken into account (i.e. responding and non-responding cells), only the P3527S cells bearing the homozygous mutations showed a significantly lower peak fluorescence change in response to 4-cmc and caffeine (means±S.E.M. were 0.11±0.03 and 0.05±0.02 for P3527S homozygous carriers compared with 0.23±0.04 and 0.12±0.03 for controls respectively. P<0.05; Student's t test).
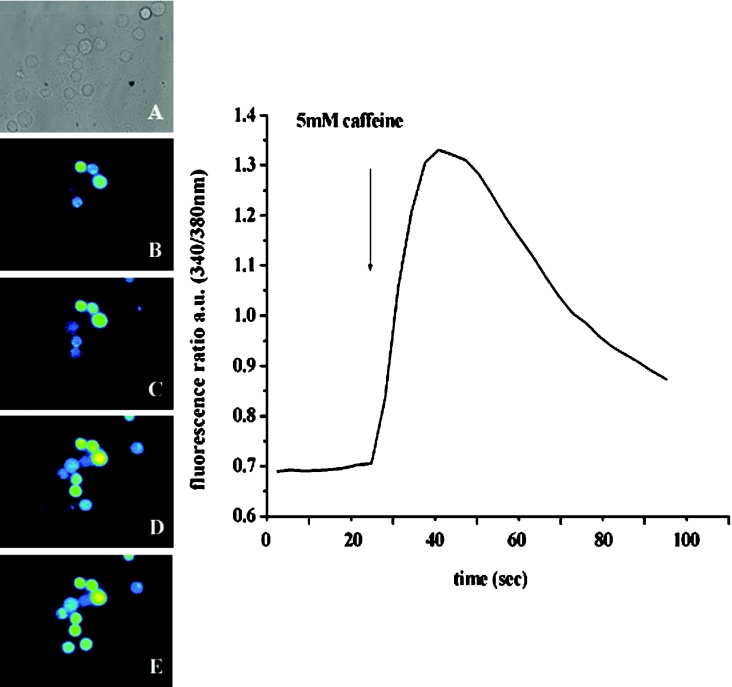
Figure 5. Ca2+ release stimulated by caffeine in individual EBV-immortalized lymphoblastoid cells from a control individual.
(A) Phase-contrast; (B–E), single-cell [Ca2+]i measurements of fura-2-loaded lymphoblastoid cells from control individuals: (B) resting [Ca2+]I, (C) 36 s, (D) 50 s and (E) 77 s after the application of 10 mM caffeine. Cells were individually stimulated by addition of caffeine in Krebs–Ringer solution containing 1 mM CaCl2. The trace in the right-hand panel is a representative trace obtained after stimulation of a single cell with 5 mM caffeine (arrow).
Figure 6. Changes in [Ca2+]i induced by 4-cmc in EBV-immortalized lymphoblastoid cells from control individuals and from patients harbouring different RyR1 mutations.
Single-cell [Ca2+]i measurements of fura-2-loaded cells were measured before and after the addition of the indicated concentration of 4-cmc. The curves show the 4-cmc-dependent change in [Ca2+]i, expressed as change in fluorescence ratio (peak ratio 340/380 nm−resting ratio 340/380 nm). Results are means±S.E.M. of the change in fluorescence (n=4–19). The curves were generated using a sigmoidal dose–response curve function included in the Origin software.
Figure 7. Changes in [Ca2+]i induced by caffeine in EBV-immortalized lymphoblastoid cells from control individuals and from patients harbouring different RyR1 mutations.
Single-cell [Ca2+]i measurements of fura-2-loaded cells were measured before and after the addition of the indicated concentrations of caffeine. The curves show the caffeine-dependent change in [Ca2+]i, expressed as change in fluorescence ratio (peak ratio 340/380 nm−resting ratio 340/380 nm). Each point represents the mean±S.E.M. of the change fluorescence (n=4–15). The curves were generated using a sigmoidal dose–response curve function included in the Origin software.
Table 1. Rmax and EC50 for 4-cmc and caffeine activation of calcium release from control and RyR1 carrying the indicated amino acid substitutions.
Results are means±S.E.M. (n=4–19 for 4-cmc measurements or 4–15 for caffeine measurements).
| Cell type | Rmax 4-cmc | EC50 4-cmc (μM) | Rmax caffeine | EC50 caffeine (mM) |
|---|---|---|---|---|
| WT | 0.36±0.03 | 136±33 | 0.33±0.03 | 1.08±0.33 |
| Heterozygous R999H | 0.37±0.14 | 191±27 | 0.34±0.02 | 1.40±0.27 |
| Homozygous P3527S | 0.23±0.04* | 75±18 | 0.43±0.04 | 1.21±0.18 |
| Heterozygous P3527S | 0.34±0.04 | 157±21 | 0.40±0.03 | 1.56±0.47 |
| Homozygous V4849I | 0.36±0.05 | 184±23 | 0.36±0.03 | 1.39±0.31 |
| Heterozygous V4849I | 0.47±0.05 | 204±36 | 0.43±0.03 | 1.94±0.29 |
*, P<0.009 (Student's t test).
Table 2. Number of lymphoblastoid cells carrying RyR1 substitutions, responding to different concentrations of caffeine and 4-cmc.
The number of cells analysed was in the range 15–302.
| (a) | |||||
|---|---|---|---|---|---|
| Responding cells (% of total) | |||||
| [Caffeine] (mM)… | 0.5 | 2.5 | 5 | 10 | 20 |
| Control | 21.6 | 21.3 | 25 | 29.2 | 22.9 |
| R999H heterozygous | 18.1 | 21.7 | 25 | 14.3 | 17 |
| P3527S homozygous | 11.6 | 14.9 | 10.6 | 10 | 17.6 |
| P3527S heterozygous | 20.1 | 33 | 22.4 | 20 | 18.4 |
| V4849I homozygous | 26.3 | 43.8 | 40 | 18.3 | 17.8 |
| V4849I heterozygous | 20.2 | 35.6 | 14.6 | 18.9 | 19.2 |
| (b) | |||||
| Responding cells (% of total) | |||||
| [4-cmc] (μM)… | 75 | 150 | 300 | 600 | 1000 |
| Control | 31.6 | 40 | 39.3 | 61.3 | 29.7 |
| R999H heterozygous | 20.7 | 26.7 | 47.4 | 30.3 | 32 |
| P3527S homozygous | 41.7 | 42.9 | 38.5 | 50 | 39.3 |
| P3527S heterozygous | 29.4 | 29.1 | 53.8 | 50 | 22.5 |
| V4849I homozygous | 35.7 | 40 | 35 | 26.1 | 34.8 |
| V4849I heterozygous | 38.9 | 47.6 | 60 | 57.9 | 60 |
DISCUSSION
During the last decade, a number of reports dealing with mutations in the skeletal muscle RyR1 Ca2+ channel, their genetic association with neuromuscular disorders and impact on protein function have appeared [36–39]. While it is well established that most MH-causing mutations affect the RyR1 by making it hypersensitive to activating substances, and CCD-causing mutations affect the amount of Ca2 released after activation, not much is known about the functional effect of RYR1 mutations leading to MmD. In the present study, we investigated the functional effect of three amino acid substitutions linked to a mixed MmD/CCD phenotype [17,18] by studying the Ca2+ homoeostasis of lymphoblastoid cells established from the patients. Two of these were clearly inherited as recessive mutations. Under our experimental conditions, only the P3527S substitution in the homozygous state significantly affected intracellular Ca2+ homoeostasis. Our results show that, unlike many CCD-linked mutations, the presence of this mutation did not affect the size of the intracellular Ca2+ pools, but rather affected the total amount of Ca2+ released by pharmacological activation of the RyR. This finding implies that (i) the presence of the P3527S substitution on one allele alone is not sufficient to significantly alter the functional properties of the RyR, (ii) the homozygous mutation does not diminish the size of the Ca2+ stores and therefore does not cause the channel to become leaky, and (iii) the presence of the P3527S substitution causes a decrease in the maximal amount of Ca2+ released by 4-cmc and caffeine. In view of the fact that the mutated residue lies in the vicinity of binding sites for calmodulin and S100 [40,41], two proteins which enhance channel opening and promote Ca2+ release [42,43], and based on the fact that proline, an amino acid known to disrupt α-helices, is substituted by a residue having a polar uncharged side chain, one may hypothesize that the P3527S substitution interferes with the binding of accessory proteins involved in stabilizing the RyR in the open state in the native tetrameric (in cells heterozygous for the mutation) conformation and that the presence of wild-type channels within the tetramer are sufficient to bind regulatory proteins and therefore allow ‘normal’ Ca2+ release after activation. Alternatively, the decrease in Ca2+ released after the addition of 4-cmc may reflect the fact that the mutation lies in proximity to the binding site for this agonist [44].
As for the other two substitutions, under our experimental conditions, we found that channels carrying the R999H substitution behave like their wild-type counterpart both in terms of resting [Ca2+], and total amount of Ca2+ released after RyR1 activation. We would like to point out that genetic investigation into the family carrying the R999H substitution revealed that also the unaffected mother (with normal clinical examination and muscle CT scan) was a carrier of this mutation. Since the entire RYR1 of the proband was sequenced and found to contain no other amino acid substitutions, it is unlikely that this mutation alone is pathological in the heterozygous state and mutations in other genes must be responsible for the MmD/CCD phenotype. As far as the V4849I substitution is concerned, lymphoblastoid cells carrying this mutation in the homozygous and heterozygous states behaved like wild-type cells in terms of Ca2+ store content and total amount of Ca2+ released after pharmacological RyR activation; however, they exhibited a small, but significant, increase in their resting [Ca2+]. Sambuughin et al. [45] recently reported that the V4849I substitution may be causative of MH since it was found in one proband with a very strong IVCT (in vitro muscle contracture test). On the other hand, Monnier et al. [46] reported that this same mutation was also found in a control individual. Our data show that cells carrying the V4849I substitution do not show an increased sensitivity to either 4-cmc or caffeine, thus it is unlikely to be causative of MH when present (alone) in either the heterozygous or homozygous state. We are aware that lymphoblastoid cells do not express all of the proteins which interact with the RyR1 in the SR, and we do not think that Val4849 is involved in protein–protein interaction, since it is predicted to be within a hydrophobic transmembrane domain (TM8) [19,47]. However, since the conserved mutation of this residue (V4849I) affects the resting [Ca2+], it may influence channel function in a way that could not be detected by our system.
An interesting observation emerging from the present study concerns the response of the lymphoblastoid cells to caffeine. We had attempted previously to obtain changes in [Ca2+] in response to caffeine in populations of lymphoblastoid cells; however, the results were not reproducible. In the light of the results obtained on single-cell measurements, we suggest that only a proportion of lymphoblastoid cells respond to caffeine; since spectrofluorimetric measurements average changes in [Ca2+] occurring in millions of cells, if fewer than 50% of the cells respond synchronously, no increase in fluorescence can be observed. Imaging analysis revealed that the number of cells responding to different concentrations of caffeine was <50%, while that responding to 4-cmc was always higher, even though the cells were viable and fluorescent. Furthermore, both agonists induced comparable global increases in [Ca2+] in responding cells. We do not know the reason for this but it may reflect the quenching effect of caffeine on fura 2 fluorescence as well as the fact that the lymphoblastoid cell lines are polyclonal in nature. The results obtained averaging all cells stimulated with 4-cmc and caffeine are consistent, and demonstrate that only the P3527S substitution causes significant alterations in Ca2+ homoeostasis. The decrease in the maximal amount of Ca2+ released after pharmacological activation was observed in cell lines established from two individuals carrying the same homozygous mutation and in none of the other cell lines, thus we think that it is unlikely to reflect the selection of particular clones of cells.
One of the questions remaining to be answered is how RYR1 mutations differently modify intracellular Ca2+ dynamics and contribute to the pathophysiology of MH, CCD and MmD. In MHS individuals, most mutations shift the sensitivity of the receptor to lower agonist concentration, and the clinical signs translate to a hypermetabolic state triggered by a hyperactive RyR. In CCD, the function of the RyR Ca2+ channel is altered either because mutations cause the channels to become excessively leaky or because they become unable to transport Ca2+ efficiently [36,37,39]. The present results suggest that at least as far as the MmD-linked P3527S recessive substitution is concerned, the RyR channels do not become leakier, but rather transport less Ca2+ upon activation. Single-channel recording experiments will be important in order to confirm whether this mutation renders the channel unstable in the open state.
In conclusion, the present data strongly support a causative role for the P3527S RyR1 substitution at the homozygous level for the CCD/MmD phenotype; on the other hand, our results show that the functional properties of RyR1 carrying the R999H substitution are not different from controls and are most likely not causative of CCD/MmD. As to the V4849I substitution, our results show that its presence does not cause a shift in the sensitivity to pharmacological activation of the RyR, or cause alterations in the amount of thapsigargin, 4-cmc and caffeine-induced Ca2+ release. However, since its presence affected the resting Ca2+ concentration, the V4849I substitution may perturb the function of the RyR channel in a way which was not discernable in the present study. Finally, these results confirm that lymphoblastoid cells can be used as a tool to study the effects of causative mutations, compared with polymorphisms, among RYR1 mutations; however, one must keep in mind that these cells do not express all the proteins of the skeletal muscle SR involved in Ca2+ homoeostasis.
Acknowledgments
This work was supported by grants from the Swiss National Science Foundation SNF No. 3200-063959.00 and 3200-067820.02 and from the Department of Anaesthesia, Basel University Hospital, from a grant from the Association Française contre les Myopathies. The financial support of the Muscular Dystrophy Campaign grant is also gratefully acknowledged. C.R.M. wishes to acknowledge the support by the German MD-Net [BMBF (Bundesministerium für Bildung und Forschung) grant 01GM302].
References
- 1.Endo M. Calcium release from the sarcoplasmic reticulum. Physiol. Rev. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- 2.Fleischer S., Inui M. Biochemistry and biophysics of excitation–contraction coupling. Annu. Rev. Biophys. Biophys. Chem. 1989;18:333–364. doi: 10.1146/annurev.bb.18.060189.002001. [DOI] [PubMed] [Google Scholar]
- 3.Franzini-Armstrong C., Jorgensen A. O. Structure and development of E–C coupling units in skeletal muscle. Annu. Rev. Physiol. 1994;56:509–534. doi: 10.1146/annurev.ph.56.030194.002453. [DOI] [PubMed] [Google Scholar]
- 4.Franzini-Armstrong C., Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- 5.Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu. Rev. Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- 6.Zucchi R., Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol. Rev. 1997;49:1–51. [PubMed] [Google Scholar]
- 7.MacLennan D. H., Phillips M. S. Malignant hyperthermia. Science. 1992;256:789–794. doi: 10.1126/science.1589759. [DOI] [PubMed] [Google Scholar]
- 8.Loke J., MacLennan D. H. Malignant hyperthermia and central core disease: disorders of Ca2+ release channels. Am. J. Med. 1998;104:470–486. doi: 10.1016/s0002-9343(98)00108-9. [DOI] [PubMed] [Google Scholar]
- 9.MacLennan D. H. Ca2+ signalling and muscle disease. Eur. J. Biochem. 2000;267:5291–5297. doi: 10.1046/j.1432-1327.2000.01566.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferreiro A., Estournet B., Chateau D., Romero N. B., Laroche C., Odent S., Toutain A., Cabello A., Fontan D., dos Santos H. G., et al. Multi-minicore disease – searching for boundaries: phenotype analysis of 38 cases. Ann. Neurol. 2000;48:745–757. [PubMed] [Google Scholar]
- 11.Ferreiro A., Fardeau M. 80th ENMC International Workshop on Multi-Minicore Disease: 1st International MmD Workshop. 12–13th May, 2000, Soestduinen, The Netherlands. Neuromuscul. Disord. 2002;12:60–68. doi: 10.1016/s0960-8966(01)00237-1. [DOI] [PubMed] [Google Scholar]
- 12.Shuaib R. T., Paasuke Brownell K. W. Central core disease: clinical features in 13 patients. J. Comp. Pathol. 1987;97:597–600. [PubMed] [Google Scholar]
- 13.Greenfield J. G., Cornman T., Shy G. M. The prognostic value of the muscle biopsy in the floppy infant. Brain. 1958;81:461–484. doi: 10.1093/brain/81.4.461. [DOI] [PubMed] [Google Scholar]
- 14.Sewry C. A., Müller C., Davis M., Dwyer J. S., Dove J., Evans G., Schroder R., Furst D., Helliwell T., Laing N., Quinlivan R. C. The spectrum of pathology in central core disease. Neuromuscul. Disord. 2002;12:930–938. doi: 10.1016/s0960-8966(02)00135-9. [DOI] [PubMed] [Google Scholar]
- 15.Bethlem J., Arts W. F., Dingemans K. P. Common origin of rods, cores, miniature cores, and focal loss of cross-striations. Arch. Neurol. 1978;35:555–566. doi: 10.1001/archneur.1978.00500330003002. [DOI] [PubMed] [Google Scholar]
- 16.Vallat J. M., de Lumley L., Loubet A., Leboutet M. J., Corvisier N., Umdenstock R. Coexistence of minicores, cores, and rods in the same muscle biopsy: a new example of mixed congenital myopathy. Acta Neuropathol. 1982;58:229–232. doi: 10.1007/BF00690806. [DOI] [PubMed] [Google Scholar]
- 17.Ferreiro A., Monnier N., Romero N. B., Leroy J. P., Bonnemann C., Haenggeli C. A., Straub V., Voss W. D., Nivoche Y., Jungbluth H., et al. A recessive form of central core disease, transiently presenting as multi-minicore disease, is associated with a homozygous mutation in the ryanodine receptor type 1 gene. Ann. Neurol. 2002;51:750–759. doi: 10.1002/ana.10231. [DOI] [PubMed] [Google Scholar]
- 18.Jungbluth H., Müller C. R., Halliger-Keller B., Brockington M., Brown S. C., Feng L., Chattopadhyay A., Mercuri E., Manzur A. Y., Ferreiro A., et al. Autosomal recessive inheritance of RYR1 mutations in a congenital myopathy with cores. Neurology. 2002;59:284–287. doi: 10.1212/wnl.59.2.284. [DOI] [PubMed] [Google Scholar]
- 19.Zorzato F., Fujii J., Otsu K., Phillips M., Green N. M., Lai F. A., Meissner G., MacLennan D. H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1990;265:2244–2256. [PubMed] [Google Scholar]
- 20.Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T., et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature (London) 1989;339:439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- 21.Jurkat-Rott K., McCarthy T., Lehmann-Horn F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve. 2000;23:4–17. doi: 10.1002/(sici)1097-4598(200001)23:1<4::aid-mus3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy T. V., Quane K. A., Lynch P. J. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum. Mutat. 2000;15:410–417. doi: 10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 23.Denborough M. A., Lovell R. R. H. Anesthetic deaths in a family. Lancet. 1960;2:45. [Google Scholar]
- 24.Denborough M. Mailgnant hyperthermia. Lancet. 1998;352:1131–1136. doi: 10.1016/S0140-6736(98)03078-5. [DOI] [PubMed] [Google Scholar]
- 25.Litman R. I., Rosenberg H. Mailgnant Hyperthermia: update on susceptibility testing. JAMA, J. Am. Med. Assoc. 2005;293:2918–2924. doi: 10.1001/jama.293.23.2918. [DOI] [PubMed] [Google Scholar]
- 26.Ferreiro A., Quijano-Roy S., Pichereau C., Moghadaszadeh B., Goemans N., Bonnemann C., Jungbluth H., Straub V., Villanova M., Leroy J. P., et al. Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am. J. Hum. Genet. 2002;71:739–749. doi: 10.1086/342719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavagna D., Zorzato F., Babini E., Prestipino G., Treves S. Methyl p-hydroxybenzoate (E-218) a preservative for drugs and food is an activator of the ryanodine receptor Ca2+ release channel. Br. J. Pharmacol. 2000;131:335–341. doi: 10.1038/sj.bjp.0703571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girard T., Cavagna D., Padovan E., Spagnoli G., Urwyler A., Zorzato F., Treves S. B-lymphocytes from malignant hyperthermia susceptible patients have an increased sensitivity to skeletal muscle ryanodine receptor activators. J. Biol. Chem. 2002;276:48077–48082. doi: 10.1074/jbc.M107134200. [DOI] [PubMed] [Google Scholar]
- 29.Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum. Genet. 1986;73:320–326. doi: 10.1007/BF00279094. [DOI] [PubMed] [Google Scholar]
- 30.Tilgen N., Zorzato F., Halliger-Keller B., Muntoni F., Sewry C., Palmucci L. M., Schneider C., Hauser E., Lehmann-Horn F., Müller C. R., Treves S. Identification of four novel mutations in the C-terminal membrane spanning domain of the ryanodine receptor 1: association with central core disease and alteration of calcium homeostasis. Hum. Mol. Genet. 2001;10:2879–2887. doi: 10.1093/hmg/10.25.2879. [DOI] [PubMed] [Google Scholar]
- 31.Zorzato F., Yamaguchi N., Xu L., Meissner G., Müller C. R., Pouliquin P., Muntoni F., Sewry C., Girard T., Treves S. Clinical and functional effects of a deletion in a COOH-terminal lumenal loop of the skeletal muscle ryanodine receptor. Hum. Mol. Genet. 2003;12:379–388. doi: 10.1093/hmg/ddg032. [DOI] [PubMed] [Google Scholar]
- 32.Ducreux S., Zorzato F., Müller C., Sewry C., Muntoni F., Quinlivan R., Restagno G., Girard T., Treves S. Effect of ryanodine receptor mutations on interleukin-6 release and intracellular calcium homeostasis in human myotubes from malignant hyperthermia-susceptible individuals and patients affected by central core disease. J. Biol. Chem. 2004;279:43838–43446. doi: 10.1074/jbc.M403612200. [DOI] [PubMed] [Google Scholar]
- 33.Zorzato F., Scutari E., Tegazzin V., Clementi E., Treves S. Chlorocresol: an activator of ryanodine receptor-mediated Ca2+ release. Mol. Pharmacol. 1993;44:1192–1201. [PubMed] [Google Scholar]
- 34.Tegazzin F., Scutari E., Treves S., Zorzato F. Chlorocresol, an additive to commercial succinylcholine induces contracture of human malignant hyperthermia susceptible muscles via activation of the ryanodine receptor Ca2+ channel. Anesthesiology. 1996;84:1380–1385. doi: 10.1097/00000542-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Herrmann-Frank A., Richter M., Sarkozi S., Mohr U., Lehmann-Horn F. 4-Chloro-m-cresol, a potent and specific activator of the skeletal muscle ryanodine receptor. Biochim. Biophys. Acta. 1996;1289:1–40. doi: 10.1016/0304-4165(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 36.Lyfenko A. D., Sanjeewa A., Goonasekera A., Dirksen R. T. Dynamic alterations in myoplasmic Ca2+ in malignant hyperthermia and central core disease. Biochem. Biophys. Res. Commun. 2004;322:1256–1266. doi: 10.1016/j.bbrc.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 37.Mathews K. Multiminicore myopathy, central core disease, malignant hyperthermia susceptibility, and RYR1 mutations. Arch. Neurol. 2004;61:27–28. doi: 10.1001/archneur.61.1.27. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd S., Ellis F., Halsall J., Hopkins P., Robinson R. RYR1 mutations in UK central core disease patients: more than just the C-terminal transmembrane region of the RYR1 gene. J. Med. Genet. 2004;41:1–7. doi: 10.1136/jmg.2003.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treves S., Anderson A. A., Ducreux S., Divet A., Bleunven C., Grasso C., Paesante S., Zorzato F. Ryanodine receptor 1 mutations, dysregulation of calcium homeostasis and neuromuscular disorders. Neuromuscul. Disord. 2005;15:57–587. doi: 10.1016/j.nmd.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Yamaguchi N., Xin C., Meissner G. Identification of apocalmodulin and Ca2+-calmodulin regulatory domain in skeletal muscle Ca2+ release channel, ryanodine receptor. J. Biol. Chem. 2001;276:22579–22585. doi: 10.1074/jbc.M102729200. [DOI] [PubMed] [Google Scholar]
- 41.Treves S., Scutari E., Robert M., Groh S., Ottolia M., Prestipino G., Ronjat M., Zorzato F. Interaction of S100A1 with the Ca2+ release channel (ryanodine receptor) of skeletal muscle. Biochemistry. 1997;36:1496–11503. doi: 10.1021/bi970160w. [DOI] [PubMed] [Google Scholar]
- 42.Rodney G. G., Williams B. Y., Strasburg G. M., Beckingham K., Hamilton S. L. Regulation of RYR1 activity by Ca2+ and calmodulin. Biochemistry. 2000;39:7807–7812. doi: 10.1021/bi0005660. [DOI] [PubMed] [Google Scholar]
- 43.Balshaw D. M., Yamaguchi N., Meissner G. Modulation of intracellular calcium-release channels by calmodulin. J. Membr. Biol. 2002;181:1–8. doi: 10.1007/s00232-001-0111-4. [DOI] [PubMed] [Google Scholar]
- 44.Treves S., Pouliquin P., Moccagatta L., Zorzato F. Functional properties of EGFP-tagged skeletal muscle calcium release channel (ryanodine receptor) expressed in COS-7 cells: sensitivity to caffeine and 4-chloro-m-cresol. Cell Calcium. 2002;31:1–12. doi: 10.1054/ceca.2001.0252. [DOI] [PubMed] [Google Scholar]
- 45.Sambuughin N., Holley H., Muldoon S., Brandom B., Bantel A. M., Tobin J. R., Nelson T. E., Goldfarb L. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the North American population. Anesthesiology. 2005;102:515–521. doi: 10.1097/00000542-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Monnier N., Romero N. B., Lereale J., Landrieu P., Nivoche Y., Fardeau M., Lunardi J. Familial and sporadic forms of central core disease are associated with mutations in the C-terminal domain of the skeletal muscle ryanodine receptor. Hum. Mol. Genet. 2001;10:2581–2592. doi: 10.1093/hmg/10.22.2581. [DOI] [PubMed] [Google Scholar]
- 47.Du G. G., Sandhu B., Khanna V. J., Guo X. H., MacLennan D. H. Topology of the Ca2+ release channel of the skeletal muscle sarcoplasmic reticulum (RyR1) Proc. Natl. Acad. Sci. U.S.A. 2002;99:16725–16730. doi: 10.1073/pnas.012688999. [DOI] [PMC free article] [PubMed] [Google Scholar]