Abstract
Enterocytes are responsible for the absorption of dietary lipids, which involves TRL [TG (triacylglycerol)-rich lipoprotein] assembly and secretion. In the present study, we analysed the effect on TRL secretion of Caco-2 enterocyte adaptation to a differential glucose supply. We showed that TG secretion in cells adapted to a low glucose supply for 2 weeks after confluence was double that of control cells maintained in high-glucose-containing medium, whereas the level of TG synthesis remained similar in both conditions. This increased secretion resulted mainly from an enlargement of the mean size of the secreted TRL. The increased TG availability for TRL assembly and secretion was not due to an increase in the MTP (microsomal TG transfer protein) activity that is required for lipid droplet biogenesis in the ER (endoplasmic reticulum) lumen, or to the channelling of absorbed fatty acids towards the monoacylglycerol pathway for TG synthesis. Interestingly, by electron microscopy and subcellular fractionation studies, we observed, in the low glucose condition, an increase in the TG content available for lipoprotein assembly in the ER lumen, with the cytosolic/microsomal TG levels being verapamil-sensitive. Overall, we demonstrate that Caco-2 enterocytes modulate TRL secretion through TG partitioning between the cytosol and the ER lumen according to the glucose supply. Our model will help in identifying the proteins involved in the control of the balance between TRL assembly and cytosolic lipid storage. This mechanism may be a way for enterocytes to regulate TRL secretion after a meal, and thus impact on our understanding of post-prandial hypertriglyceridaemia.
Keywords: apolipoprotein B, Caco-2 cell, cytosolic lipid droplet, enterocyte, lipoprotein secretion, microsomal triacylglycerol transfer protein (MTP)
Abbreviations: ApoB, apolipoprotein B; BA, batyl alcohol; DGAT, diacylglycerol acyltransferase; DGE, diacylglyceryl ether; DMEM, Dulbecco's modified Eagle's medium; ER, endoplasmic reticulum; FCS, foetal calf serum; GPAT, glycerolphosphate acyltransferase; LPC, L-α-lysophosphatidylcholine; MG, monoacylglycerol; MGAT, MG acyltransferase; 2-MO, 2-mono-oleoylglycerol; TG, triacylglycerol; MTP, microsomal TG transfer protein; OA, oleic acid; PDI, protein disulphide-isomerase; TRL, TG-rich lipoprotein
INTRODUCTION
Enterocytes are polarized absorptive cells responsible for the transepithelial transfer of nutrients from the luminal side of the intestine to the bloodstream. During a meal, the different nutrients are assimilated simultaneously and then processed through their own metabolic pathway, while modulating the metabolic pathways of other nutrients (for a review, see [1]). Whereas monosaccharides are transferred from the luminal to the basolateral pole of the enterocyte, fatty acids are processed in a complex way by synthesis of TG (triacylglycerol) and its assembly into TRLs (TG-rich lipoproteins), which are finally secreted at the basolateral pole [2]. A cross-talk between lipid and glucose metabolism has been reported for numerous tissues, especially adipose tissue and liver (for reviews, see [3,4]). In these tissues, glucose and lipid metabolism are tightly linked at the level of glycerol-3-phosphate, from which TG synthesis is initiated from the glycerol backbone provided through glycolysis [5,6]. In the small intestine, TG synthesis mainly occurs through the same glycerol-3-phosphate pathway during the inter-prandial period [2]. In contrast, during the post-prandial period, up to 80% of TGs are newly synthesized through the MG (monoacylglycerol) pathway using as substrates the large amounts of 2-MG and fatty acids released by the digestion of dietary lipids in the duodenal lumen [7,8]. TGs are then assembled and secreted as chylomicrons, the intestinal form of TRL. TRL consists of a core of neutral lipids surrounded by a monolayer of amphipathic lipids and apolipoproteins. ApoB (apolipoprotein B) plays a major role in TRL assembly and is required for their secretion [9]. TRL assembly has mostly been characterized in hepatocytes as a two-step process, consisting of the formation of a lipid-poor ApoB particle followed by its fusion with an independently formed TG-rich ApoB-free lipid droplet. MTP (microsomal TG transfer protein) is necessary for this process (for reviews, see [10–12]).
We have previously shown in Caco-2 enterocytes that an apical supply of complex lipid micelles, whose composition is close to that of the content of the human duodenal lumen after a lipid-rich meal, induces a chase of apically expressed ApoB [13] and the secretion of TRL [14]. Although fatty acid supply and ApoB availability were not limiting for TRL secretion, an accumulation of large cytosolic lipid droplets was observed in these cells [14]. These results led us to suggest that the targeting of de novo synthesized TG towards the lumen of the ER (endoplasmic reticulum) represents a key step in TRL secretion. In vivo, a transient accumulation of cytosolic lipid droplets in enterocytes is observed during the post-prandial period [15–18], which may result from an overflow in the capacity of the ER to release lipid droplets into its lumen. However, the molecular mechanisms governing TG partitioning between entry into the secretory pathway and storage as cytosolic droplets are still poorly understood [19,20].
In liver, it is well documented that glycolytic and lipogenic gene expression is modulated by a high-carbohydrate diet (reviewed in [3,4]). In intestine, adaptation to a high-carbohydrate diet was shown to increase the secretion of intestinal TRL [21].
The purpose of the present study was to analyse TRL assembly and secretion by Caco-2 enterocytes when adapted to differential glucose supply. We compared Caco-2 cells cultured in high-glucose-containing medium (25 mM in the apical and basal compartments) with Caco-2 cells adapted to a lower glucose concentration (i.e. 5 mM in the basal compartment and no apical glucose supply). By biochemical and microscopy analyses, we demonstrate that Caco-2 cells adapted to a low glucose concentration secrete more TRLs than cells cultured at a high glucose concentration, and that this results from an increase in the TG available for lipoprotein assembly in the ER lumen.
MATERIALS AND METHODS
Materials
Cell culture reagents were obtained from Invitrogen (Cergy-Pontoise, France) and FCS (foetal calf serum) from AbCys Biowest (Paris, France). Microporous polyethylene terephthalate membrane inserts were from Becton Dickinson (Le Pont de Claix, France) (23.1 mm diameter, 1 μm pore size) or from Corning (Avon, France) (75 mm diameter, 3 μm pore size). [1-14C]OA (where OA is oleic acid; 56 mCi/mmol) and [35S]methionine/cysteine mixture (EasyTag™ express protein labelling mix) were obtained from PerkinElmer Life Sciences (Courtaboeuf, France).
Verapamil (Isoptine®) was obtained from Abbott France (Rungis, France). Desqualenized shark liver oil containing a high proportion of glyceryl ethers [22,23] was kindly provided by Professor A. Legrand (Rennes University, Rennes, France). The rabbit antibody against MTP/PDI (protein disulphide-isomerase) was generously provided by Dr C. Shoulders (Hammersmith Hospital, London, U.K.).
Preparation of lipid micelles
Stock solutions (100 mM) of OA, LPC (L-α-lysophosphatidylcholine), BA (batyl alcohol; 1-O-octadecyl-rac-glycerol) and 2-MO (2-mono-oleoylglycerol), and of 25 mM cholesterol (all from Sigma–Aldrich, Saint-Quentin Fallavier, France) were prepared in chloroform/methanol (2:1, v/v). To prepare 1 ml of lipid micelles, OA (6 μl) was mixed with other lipids (2 μl) as stated, in a sterile glass tube. The mixture was dried under a stream of nitrogen gas, the residue dissolved in 83 μl of a sterile solution of 24 mM sodium taurocholate in serum-free medium and the volume brought up to 1 ml with serum-free medium. The final lipid micelle preparation therefore consisted of serum-free medium containing sodium taurocholate (2 mM), OA (0.6 mM), LPC (0.2 mM) and cholesterol (0.05 mM) with or without 2-MO (0.2 mM) or BA (0.2 mM). For analysis of the newly synthesized lipids, we added [1-14C]OA (1 μCi per ml of final medium) to lipids before evaporation under nitrogen and the completion of micelle preparation.
Cell culture and incubations
Caco-2 cells (between passages 42 and 55) were plated in inserts at a density of 95×103 cells/cm2 and grown until confluence in DMEM (Dulbecco's modified Eagle's medium) containing 25 mM glucose and Glutamax, supplemented with penicillin (100 i.u./ml) and streptomycin (100 μg/ml), 1% non-essential amino acids and 20% (v/v) heat-inactivated FCS. Cells were then cultured for 2 weeks in media containing FCS only in the lower compartment and in the presence of various concentrations of glucose. For the high glucose concentration condition (referred to as 25 mM/25 mM), cells were cultured with 25 mM glucose medium in both compartments, whereas for the low glucose concentration condition (referred to as 0 mM/5 mM), cells were cultured with 5 mM glucose in the lower compartment and no glucose in the upper compartment. The glucose concentration in media was adjusted by adding appropriate amounts of a sterile 2.5 M glucose solution to DMEM devoid of glucose. Media were changed every day. For incubations with lipid micelles, fresh complete medium (2.5 ml for 4.2 cm2 inserts and 12 ml for 44.1 cm2 inserts) was added to the lower compartment and lipid micelles (1.5 ml for 4.2 cm2 inserts and 8 ml for 44.1 cm2 inserts) were added to the upper compartment for the times indicated.
[35S]Cys/Met labelling experiments were performed as described previously [14]. When used, verapamil (25 μM) was added in both compartments.
Recovery of cellular material
After incubations, media were immediately collected and processed for Western blotting, lipoprotein fractionation or lipid extraction as described below. Cell layers were briefly rinsed twice with ice-cold PBS (Invitrogen), scraped into 0.5 ml of lysis buffer (1% Triton X-100 and 5 mM EDTA in PBS) supplemented with 2% (v/v) protease inhibitor cocktail (P8340; Sigma–Aldrich), and immediately frozen and kept at −20 °C until analysis.
Subcellular fractionation
Caco-2 cells collected from three 44.1 cm2 inserts were scraped into 25 mM Tris/HCl (pH 7.4) containing 100 mM KCl, 1 mM EDTA, 5 mM EGTA and a protease inhibitor cocktail (one tablet per 25 ml of buffer; Roche, Meylan, France), and lysed using a cell disruption bomb (Parr Instrument Company, Moline, IL, U.S.A.) at 8280 kPa twice for 10 min. Cell lysates were adjusted to 0.25 M sucrose, and centrifuged for 10 min at 800 g and then for 10 min at 9000 g at 4 °C to remove cell debris and large granules. The supernatant was put aside and the pellet was resuspended in buffer containing 0.25 M sucrose and centrifuged for 10 min at 9000 g. The two supernatants were pooled and the concentration of KCl was adjusted to 0.5 M in order to release any cytosolic TG associated with the membranes, as previously described [24]. After recovery of cytosolic TG by centrifugation (150000 g for 2 h at 4 °C) in a Beckman SW41 rotor through a discontinuous sucrose gradient ranging from 0.25 to 0 M [25], the microsomal pellet was washed with PBS and resuspended in 10 mM Tris/HCl (pH 7.4) containing 0.25 M sucrose and protease inhibitors. The protein concentration was measured using the Bio-Rad DC (detergent compatible) protein assay with BSA as the standard. The luminal content of whole microsomes (100 μg of proteins) was released by treatment with 0.1 M sodium carbonate (pH 11) for 1 h 30 min on ice [24] and separated from membranes by centrifugation through buffer (150000 g for 1 h 30 min at 4 °C). Lipids were extracted from the different recovered fractions and were analysed as described below in the Lipid analysis section.
Western blotting
Cell lysates were sonicated and the protein concentration was determined using the Bio-Rad DC protein assay with BSA as the standard. Cell lysates (4 μg of protein) were fractionated by SDS/7.5% PAGE and proteins were transferred to a nitrocellulose membrane. After an overnight incubation at 4 °C in TBS-T (20 mM Tris/HCl, pH 7.6, 137 mM NaCl and 0.1% Tween 20) supplemented with 10% (w/v) non-fat milk powder, blots were probed with a rabbit antibody against MTP/PDI (1:1000 dilution for 1 h in TBS-T containing 5% milk powder) followed by a peroxidase-conjugated goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA, U.S.A.; 1:6000 dilution for 1 h in the same buffer). Blots were developed with enhanced chemiluminescence (ECL®) reagents according to the manufacturer's instructions (Amersham Biosciences, Orsay, France).
MTP activity assay
MTP activity was measured using an MTP assay kit (Roar Biomedical, New York, NY, U.S.A.) as previously described [26] except that the assay was performed with 400 μg of protein at 37 °C and that fluorescence was measured (485 nm excitation wavelength and 538 nm emission wavelength) at 37 °C every 5 min for 3 h using the Fluostar Ascent FL (Labsystems S.A., Paris, France). Results were expressed in arbitrary units calculated as fluorescence intensity transferred per min per μg of protein.
Lipoprotein analysis
The lipoproteins secreted by Caco-2 cells in the basolateral media were fractionated according to their density by sequential ultracentrifugation and analysed for ApoB and lipids as previously described [14].
Lipid analysis
Lipids from cell culture media (1 ml supplemented with 10 μl of 10× lysis buffer), sonicated cell lysates (0.1 ml, made up to 1 ml with serum-free medium), and cytosolic, microsomal and lipoprotein fractions were extracted with chloroform/methanol (2:1, v/v). Lipid classes were separated by TLC using hexane/diethyl ether/acetic acid (80:20:2, by vol.) as the mobile phase, as described previously [14]. Lipids were visualized with I2 vapour. The incorporation of [1-14C]OA into lipids was measured by liquid-scintillation counting of excised radioactive bands revealed by autoradiography of the TLC plates. The amount of [1-14C]OA incorporated into DGEs (diacylglyceryl ethers) compared with that incorporated into DGE and TG gave an indication of the contribution of the MGAT (MG acyltransferase) pathway to total glycerolipid synthesis by Caco-2 cells.
For quantification of the TG mass, the TG bands were excised and extracted with chloroform/methanol. After solvent evaporation, TGs were quantified using the PAP150 TG kit (Biomérieux, Marcy l'Etoile, France) according to the manufacturer's instructions with the following modifications: the dried TGs were sonicated in 0.5 ml of the buffer provided before the addition of 0.5 ml of the 2-fold concentrated kit reagent, and the absorbance was measured after an incubation of 2 h 30 min at 37 °C.
RNA extraction and real-time PCR analysis
Total RNA was prepared using the TRI Reagent kit according to the manufacturer's instructions (Euromedex, Souffelweyersheim, France). cDNA was synthesized from 2 μg of total RNA in a 40 μl solution containing 1 mM dithiothreitol, 40 units of RNaseOut™, 0.5 mM dNTP, 400 units of MMLV (Moloney-murine-leukaemia virus) superscript reverse transcriptase and 10 μM random hexamers [pd(N)6]. mRNA was quantified using Light-Cycler apparatus according to the manufacturer's instructions (Roche Diagnostics, Meylan, France). PCR was done with a 1:1000 final dilution of the cDNA preparation in the SYBR Green I master mix with 0.4 μM specific primers except for MGAT2 primers (0.6 μM). The sequences of the primers and the PCR conditions are detailed elsewhere for L19, glucose-6-phosphatase and L-pyruvate kinase [27], for GLUT2 [28] and for MTP [26]. The sequences of the primers used for MGAT2 were GACCCCTCTCGGAACTACATTG (forward primer) and CGGAACCACAAGGTCAGCAT (reverse primer), and PCR conditions were as follows: one denaturation step (8 min, 95 °C) followed by 40 cycles of 10 s at 95 °C, 10 s at 58 °C, and 10 s at 72 °C. Amplified DNA from serial dilutions of Caco-2 cDNA was used to make standard curves and acquire quantitative data with the Light-Cycler software. Quantification of L19 mRNA coding for a ribosomal protein was used as a control for RNA extraction and reverse-transcription experiments. Results were expressed as the ratio between the levels of the mRNA of interest and of L19 mRNA.
Confocal fluorescence microscopy
Cells were washed twice with PBS containing 1 mM CaCl2 and 0.5 mM MgCl2, and fixed for 30 min with 4% (w/v) paraformaldehyde in PBS. After washing in PBS, cells were stained for neutral lipids by incubation for 10 min with BODIPY 493/503 (10 μg/ml; Invitrogen). Cells were then mounted in Fluoprep (BioMérieux) and examined by confocal fluorescence microscopy (LSM 510 microscope; Carl Zeiss, Jena, Germany).
Electron microscopy
Cells were processed for electron microscopy and stained for neutral lipids using the imidazole-buffered osmium tetroxide procedure as previously described [14]. For glycogen staining, ultrathin sections were stained for 30 min with 1% periodic acid in distilled water, for 4 h with 0.2% thiosemicarbazide in 20% (v/v) acetic acid, and finally incubated for 30 min in 1% silver proteinate in darkness [29]. Grids were examined with a Jeol 100 CX-II electron microscope.
Statistical analysis
Results were expressed as means±S.E.M. and statistically significant differences were identified by the Student's t test for paired data, carried out with GraphPad Prism software.
RESULTS
Effects of adaptation to a differential glucose supply on the content, synthesis and secretion of lipids by Caco-2 cells
Analyses were performed on cells cultured in a medium containing 25 mM glucose in both compartments (25 mM/25 mM glucose) until confluence to allow optimal cell growth, and then either maintained in the same medium or switched to a low-glucose-containing medium (0 mM/5 mM glucose) for 2 weeks. We had previously determined that a 2-week culture post-confluence was optimal for secretion of TRL in the 25 mM/25 mM glucose conditions [14]. When observed by electron microscopy, Caco-2 cells frequently have an electron-lucent material with an irregular outline at the apical pole [14,30]. This material was shown to be glycogen after staining by the periodic acid/thiosemicarbazide/silver proteinate procedure (Figure 1A, left panel). In the 0 mM/5 mM glucose culture condition, Caco-2 cells contained much less glycogen (Figure 1A, right panel), indicating that Caco-2 cells adapted to the glucose concentration of the culture medium. We also analysed the expression of GLUT2, glucose-6-phosphatase and L-pyruvate kinase genes, which are known to depend both on the differentiation state of the cells [31,32] and on variations of the consumption and intracellular flux of glucose [27,28]. Results reported in Figure 1(B) show that GLUT2, glucose-6-phosphatase and L-pyruvate kinase mRNA levels increased after confluence, as already reported [31], and that these levels were similar in both glucose conditions.
Figure 1. Glycogen distribution and mRNA levels of glucose-responsive genes in Caco-2 cells.
Cells were cultured in 25 mM/25 mM or in 0 mM/5 mM glucose for 2 weeks post-confluence. (A) Glycogen, stained black, is indicated by arrows. MV, microvilli; N, nucleus; Gly, glycogen. Scale bar, 500 nm. (B) GLUT2, glucose-6-phosphatase (G6Pase) and L-pyruvate kinase (L-PK) mRNA levels in cells cultured as indicated. Results shown are the means±S.E.M. for three experiments performed in triplicate. The asterisk (*) indicates significant differences (P<0.05) from the values for Caco-2 cells cultured in the 25 mM/25 mM glucose condition for 2 days post-confluence.
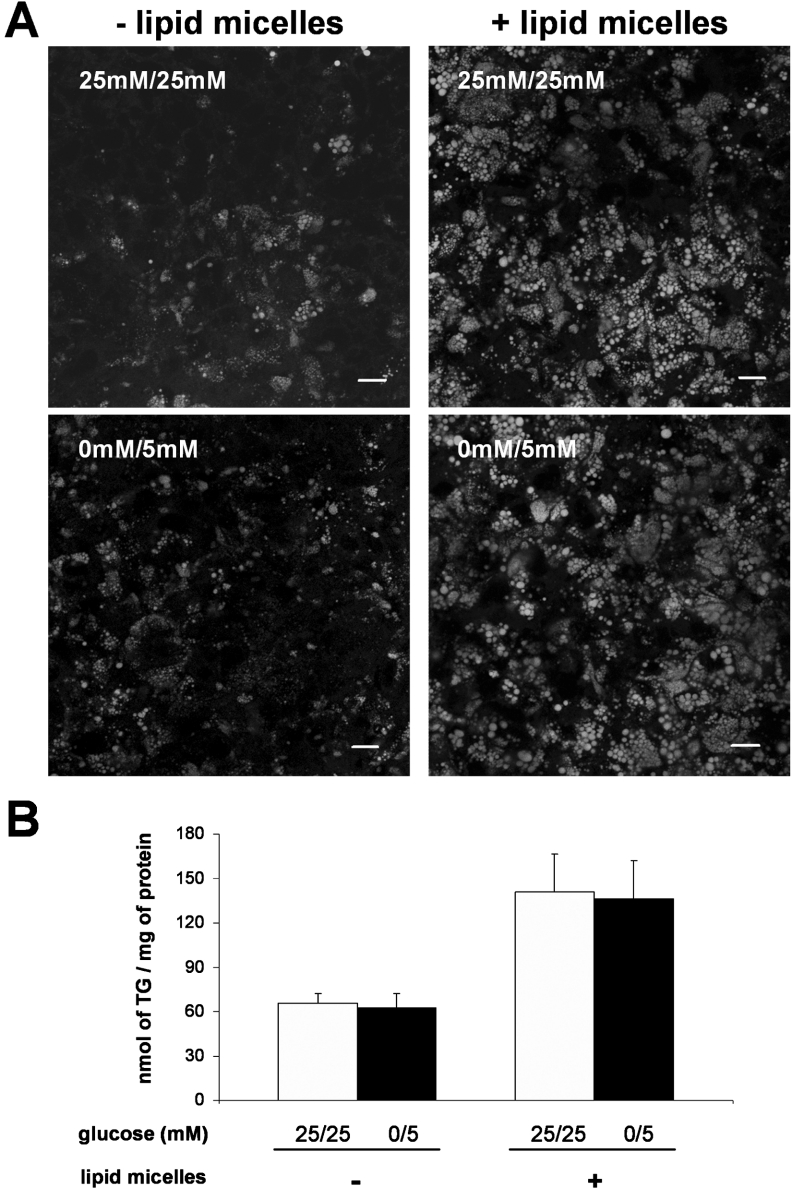
As glucose can be used for fatty acid synthesis [1] and as some Caco-2 cells cultured in 25 mM/25 mM glucose for 2 weeks post-confluence contain cytosolic lipid droplets localized at the basal pole of the cell [14], we compared the TG content of the cells cultured in both glucose conditions. Although a heterogeneous neutral lipid staining was observed from cell to cell (Figure 2A, left panel), the overall TG content of cells was similar in both conditions (Figure 2B).
Figure 2. TG content of Caco-2 cells cultured in 25 mM/25 mM or 0 mM/5 mM glucose for 2 weeks post-confluence.
On the last day of culture, cells were supplied for 24 h with (right panels) or without (left panels) lipid micelles containing 2-MO. (A) Cells were stained for neutral lipids with BODIPY 493/503 and examined by confocal microscopy. The pictures shown are from the basal pole of the cells where cytosolic lipid droplets localize. Scale bar, 10 μm. (B) TG content in cell lysates. After extraction and separation by TLC, the TG spot was quantified using the PAP150 TG kit. Results shown, expressed as nmol of TG per mg of cell protein, are the means±S.E.M. for five independent experiments.
After a 24 h apical supply of complex lipid micelles, each cell contained large cytosolic lipid droplets and the cellular TG content reached a similar value whether cells were cultured in 25 mM/25 mM or 0 mM/5 mM glucose condition (Figures 2A, right panel, and 2B).
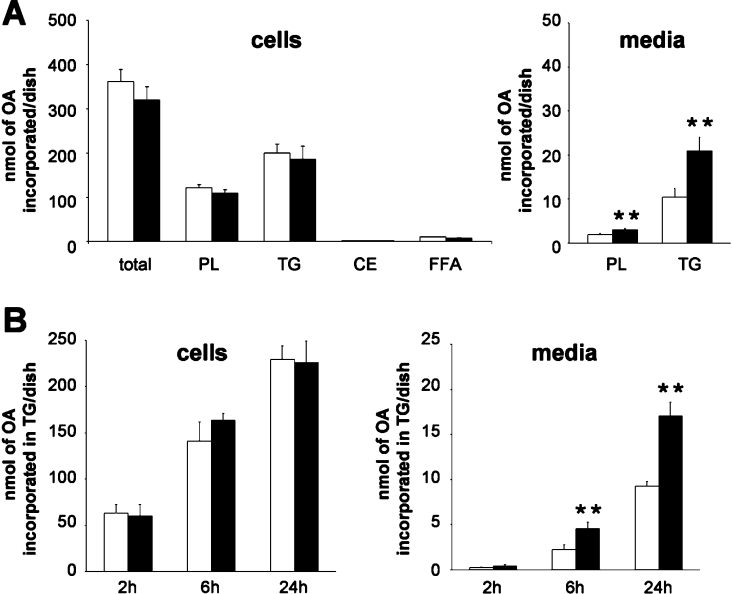
Although no difference in cellular TG accumulation was observed, a possible effect on lipid synthesis and secretion was assessed in cells incubated with complex micelles containing [1-14C]OA for 24 h. Lipids extracted from cell lysates and media were separated into classes by TLC and the radioactivity in the resultant spots was counted. As shown in Figure 3(A), the total amount of OA incorporated into cells and the cellular distribution of labelled OA in different lipid classes were similar in both conditions. However, in the 0 mM/5 mM glucose condition, we observed a significant increase in the secretion of phospholipid and TG [Figure 3A, 1.5±0.1-fold (P<0.01) for phospholipid and 2.1±0.2-fold (P<0.01) for TG]. This increase was detectable as early as 6 h after micelle supply (Figure 3B, media) and was not associated with an increased TG synthesis rate (Figure 3B, cells).
Figure 3. Synthesis and secretion of lipids by Caco-2 cells cultured in 25 mM/25 mM (white bars) or 0 mM/5 mM (black bars) glucose for 2 weeks post-confluence.
On the last day of culture, cells were incubated with complex micelles containing 2-MO and [1-14C]OA for 24 h (A) or for the stated time (B). Lipid classes extracted from cells and basolateral media were separated by TLC and the radioactivity in resultant spots was counted. Results shown, expressed as nmol of OA incorporated per dish, are the means±S.E.M. for five independent experiments. The double asterisk (**) indicates value significantly different (P<0.01) from that for Caco-2 cells cultured in 25 mM/25 mM glucose condition. PL, phospholipid; CE, cholesteryl ester; FFA, ‘free fatty acid’ (non-esterified fatty acid).
Overall, the 2-fold increase in the secretion of labelled TG in the 0 mM/5 mM glucose condition is unlikely to result from a higher TG specific radioactivity obtained by dilution with smaller pre-existing unlabelled intracellular TG pools, since cell TG content was similar in both conditions (Figure 2B). Therefore it is likely that the increased secretion of labelled TG mirrors a genuine increase in the amount of TG secreted.
Effects of adaptation to a differential glucose supply on the distribution of lipids and ApoB in lipoprotein classes secreted by Caco-2 cells
An increased secretion of lipids can result from the secretion of an increased number and/or of an increased mean size of secreted lipoproteins. The number of lipoprotein particles can be estimated by quantifying ApoB, since ApoB, in contrast with other apolipoproteins, is non-exchangeable and since TRLs contain only one molecule of ApoB per particle [33]. We isolated the lipoproteins secreted by Caco-2 cells adapted to the 0 mM/5 mM or the 25 mM/25 mM glucose condition, and analysed their lipid and ApoB48 and ApoB100 content. As shown in Table 1, in the 0 mM/5 mM glucose condition, total ApoB100 and ApoB48 content of secreted lipoproteins tended to increase (1.4±0.2- and 1.3±0.1-fold respectively), and the total content in TG increased up to 2.1±0.2-fold. A greater increase of TG than of ApoB was also observed for each lipoprotein fraction analysed. Overall, these results indicate that the increased TG secretion by cells adapted to low glucose is mainly due to an increased size of the lipoproteins secreted and, to a smaller extent, to an increase of the number of secreted particles.
Table 1. Quantification of TGs, ApoB100 and ApoB48 in lipoprotein fractions.
Caco-2 cells were grown in medium containing 25 mM glucose in both compartments until confluence and maintained for 2 weeks more in the same condition (25 mM/25 mM) or switched to media containing 0 and 5 mM glucose in the apical and basal compartment respectively (0 mM/5 mM). On the last day of culture, cells were incubated for 24 h in the presence of complex micelles containing either [1-14C]OA (1 μCi/ml), for TG analysis, or [35S]methionine/cysteine (100 μCi/ml), for ApoB analysis. Basolateral media were fractionated by sequential ultracentrifugation and lipoprotein fractions were recovered. For TG analysis, lipids were extracted from lipoprotein fractions, separated by TLC and radioactive spots were counted. For ApoB analysis, lipoprotein fractions were analysed by SDS/6% PAGE and placed against phosphor screens for quantification of the individual bands corresponding to ApoB100 and ApoB48. The total secretion of TG, ApoB100 and ApoB48 by cells cultured in 25 mM/25 mM glucose was arbitrarily set to 100. Results shown are the means±S.E.M. for three independent experiments and are expressed in terms of arbitrary units. d, density; VLDL, very-low-density lipoprotein. *Indicates significant differences (P<0.05) from the values for Caco-2 cells cultured in 25 mM/25 mM glucose condition.
| d<1.006 | |||||
|---|---|---|---|---|---|
| Total | Chylomicron | VLDL | 1.006<d<1.063 | 1.063<d<1.21 | |
| TG | |||||
| 25 mM/25 mM | 100 | 2.6±1 | 30.7±3 | 61.6±4 | 5±1 |
| 0 mM/5 mM | 206.4±19* | 9.6±4 | 74.4±15* | 114.7±6* | 7.8±1 |
| Fold increase | 2.1±0.2 | 3.3±0.7 | 2.5±0.7 | 1.9±0.1 | 1.6±0.1 |
| ApoB100 | |||||
| 25 mM/25 mM | 100 | 1.9±0.8 | 31.5±10 | 62±12 | 4.6±1.6 |
| 0 mM/5 mM | 144±17 | 3.1±1 | 53.3±15 | 83±5 | 4.7±1.7 |
| Fold increase | 1.4±0.2 | 1.8±0.3 | 1.8±0.2 | 1.5±0.4 | 1.1±0.2 |
| ApoB48 | |||||
| 25 mM/25 mM | 100 | 0.8±0.3 | 13±4.2 | 51.3±11 | 34.9±6.8 |
| 0 mM/5 mM | 129±12 | 2.2±1.3 | 20.8±5.3 | 64.2±11 | 41.6±5.9 |
| Fold increase | 1.3±0.1 | 2.5±0.6 | 1.7±0.2 | 1.3±0.2 | 1.3±0.4 |
Effect of adaptation to a differential glucose supply on TG availability for lipoprotein assembly and secretion
Due to a low MGAT activity, Caco-2 cells are thought to synthesize TG through the GPAT (glycerolphosphate acyltransferase) pathway [34], in contrast with enterocytes in vivo that make use of the MGAT pathway from MG and fatty acids brought as post-digestive micelles [7,8]. We wondered whether long-term adaptation to low glucose condition led Caco-2 cells to develop MGAT activity to overcome a reduced availability of glycerol-3-phosphate. Furthermore, it is unknown whether the pathway used for TG synthesis contributes to their availability for lipoprotein assembly. To discriminate between the glycerol-3-phosphate pathway and the 2-MG pathway, we measured TG synthesis using BA, a stable ether analogue of 2-MG; the resulting TG analogue, a DGE, migrates at a higher Rf (retention factor) than TG [22]. After incubating Caco-2 cells for 24 h with micelles containing [1-14C]OA and BA as a 2-MG backbone donor, a band corresponding to DGE was visualized on TLC plates, confirming the expression of a MGAT activity (Figure 4A). As shown in Figure 4(B), although DGE synthesis was similar in both conditions, DGE secretion increased almost 2-fold in the 0 mM/5 mM glucose condition. Nevertheless, the MGAT pathway contributed to TG synthesis to a minor extent (2.9±0.5%, Figure 4C) that was similar in both culture conditions. These results were further confirmed by measuring the level of MGAT2 mRNA by real-time PCR (Figure 4D). Although the MGAT2 mRNA level increased with time post-confluence in culture, it remained far below the level observed in jejunum and was not modified by adaptation to differential glucose supply. Additionally, incubation with complex micelles had no effect on MGAT2 mRNA level. Overall, our results indicate that the MGAT pathway was not stimulated in low-glucose culture conditions and did not account for the increased TG secretion observed.
Figure 4. MGAT activity and expression.
(A) TLC of lipids synthesized by Caco-2 cells incubated for 24 h with lipid micelles containing [1-14C]OA (lane 1) plus 2-MO (lane 2) or BA (lane 3). Cellular lipids were extracted and separated by TLC. An autoradiograph of a representative TLC is shown. Shark liver oil (lane 4) was run in parallel and stained for lipids with I2 vapour. PL, phospholipid; CE, cholesteryl ester. (B, C) Synthesis and secretion of DGE by Caco-2 cells cultured for 2 weeks post-confluence in 25 mM/25 mM (white bars) or 0 mM/5 mM (black bars) glucose. On the last day of culture, cells were incubated with complex micelles containing [1-14C]OA and BA. Lipids extracted from cells and basolateral media were separated by TLC and the amount of radioactivity in spots was measured. Results are expressed as nmol of OA incorporated in DGE per dish (B) or as the percentage of c.p.m. incorporated in DGE compared with the total c.p.m. incorporated in glycerolipids (DGE+TG) (C). Results shown are the means±S.E.M. for five independent experiments. The double asterisk (**) indicates value significantly different (P<0.01) from the value for Caco-2 cells cultured in 25 mM/25 mM glucose condition. (D) MGAT2 mRNA levels in Caco-2 cells cultured and incubated with or without complex micelles containing BA during the last 24 h of culture as indicated. The value for MGAT2 mRNA in jejunum is included for comparison. Results shown are the means±S.E.M. for three experiments performed in triplicate. The asterisk (*) indicates value significantly different (P<0.05) from the value for Caco-2 cells cultured in 25 mM/25 mM glucose condition for 2 days post-confluence; † indicates value significantly different (P<0.001) from the value for Caco-2 cells.
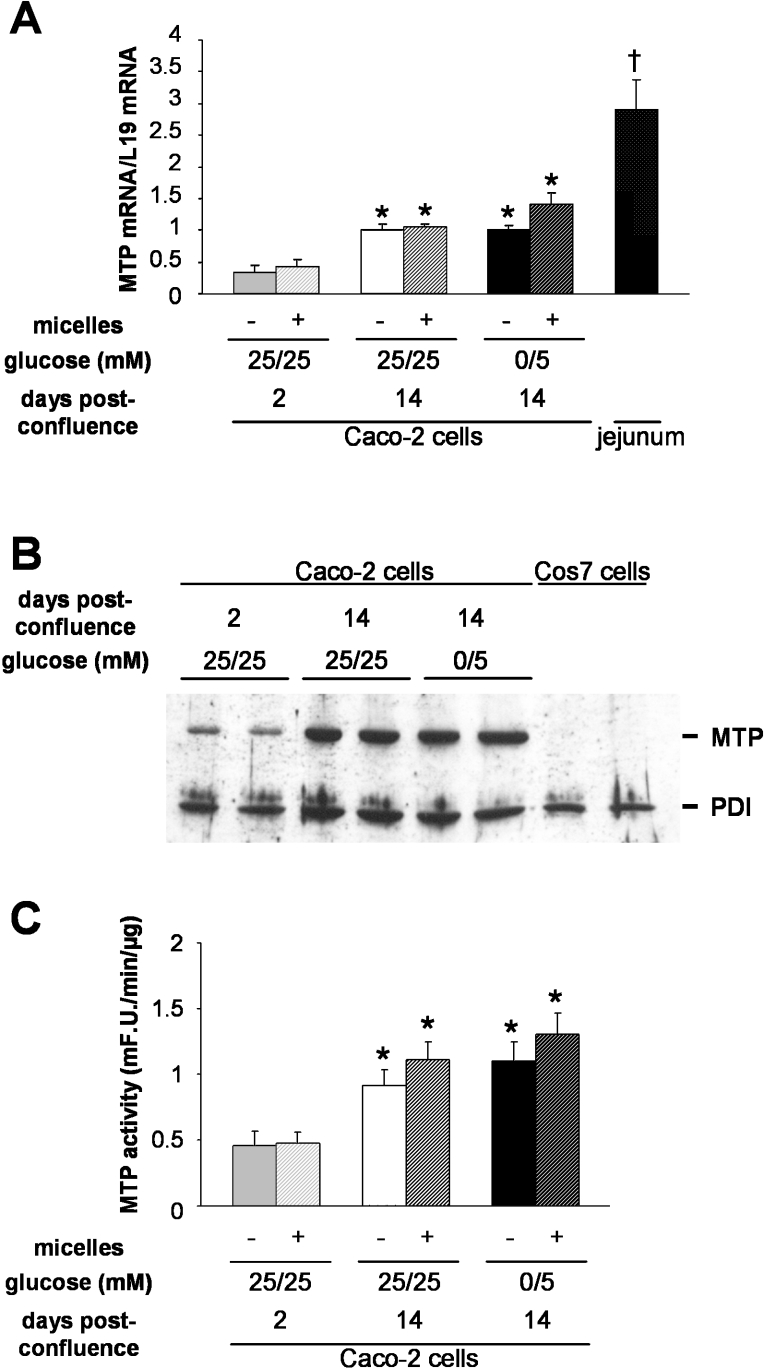
MTP is considered to play an important role in the transfer of TG to nascent ApoB, in the formation and stabilization of lipid droplets in the lumen of the ER and in lipoprotein assembly [35–37]. We thus examined the expression and activity of MTP in cells adapted to each glucose condition (Figure 5). The MTP mRNA level was similar in cells cultured for 2 weeks post-confluence in 25 mM/25 mM or 0 mM/5 mM glucose condition, although it was almost 3-fold higher than at 2 days post-confluence (Figure 5A) when cells were not yet able to secrete TG in response to lipid supply [14]. Additionally, incubation with complex micelles for the last 24 h of culture did not modify the MTP mRNA level (Figure 5A). Accordingly, while MTP protein expression (Figure 5B) and MTP activity (Figure 5C) significantly increased between day 2 and 14 post-confluence, they were similar in both glucose conditions and were not modified by a micelle supply during the last 24 h of culture.
Figure 5. MTP expression and activity.
Caco-2 cells were cultured and incubated with or without complex micelles during the last 24 h of culture as indicated. (A) MTP mRNA levels. Quantification of MTP mRNA level in jejunum is included for comparison. Results shown are the means±S.E.M. for three experiments performed in triplicate. The asterisk (*) indicates value significantly different (P<0.05) from that for Caco-2 cells cultured in 25 mM/25 mM glucose for 2 days post-confluence; † indicates value significantly different (P<0.001) from that for Caco-2 cells. (B) Immunoblotting of MTP expressed by Caco-2 cells cultured in the conditions indicated. Cos7 cell lysates are included as a negative control for MTP expression. A representative experiment from duplicate dishes is shown. (C): MTP activity in Caco-2 cell lysates cultured in the conditions indicated. Results shown, expressed as m-fluorescence units·min−1·(μg of protein)−1, are the means±S.E.M. for three independent experiments done in triplicate. The asterisk (*) indicates values that are significantly different (P<0.05) from that of Caco-2 cells cultured in 25 mM/25 mM glucose condition for 2 days post-confluence. mF.U., m-fluorescence units.
Effect of adaptation to a differential glucose supply on the intracellular distribution of TGs in Caco-2 cells
To identify further the cellular events responsible for the increased TG secretion in low-glucose-adapted Caco-2 cells, we studied the intracellular distribution of TGs. In Caco-2 cells, TGs accumulate in the cytosol as lipid droplets and in the lumen of the secretory compartment [14], where they are assembled into lipoproteins. In order to compare the capacity of cells cultured in both conditions to direct lipid droplets towards the ER lumen, cells were incubated with lipid micelles for 2 h, then stained for lipids, and examined by electron microscopy (Figure 6A). Cells adapted to low glucose exhibited the morphology of fully differentiated enterocytes and had more lipid droplets within the ER and the Golgi apparatus than cells maintained in 25 mM glucose. This increase was further demonstrated by quantification of the amount of TG in microsomes and ER lumen in both conditions, after a 3 h incubation of Caco-2 cells with lipid micelles containing labelled OA. Intact microsomes contained more TG (2.4±0.3-fold) when isolated from cells adapted to the 0 mM/5 mM glucose condition than from cells maintained in the 25 mM/25 mM glucose condition, while the phospholipid content was similar (Figures 6B and 6C). The cytosolic/microsomal TG levels were reduced in the low glucose condition (104±8.6 and 38±16.1 for the 25 mM/25 mM and 0 mM/5 mM glucose condition respectively), while the cytosolic/microsomal phospholipid levels remained similar (0.52±0.17 and 0.55±0.1 respectively). These results, combined with those showing that the total cellular TG content was similar in both glucose conditions (Figures 2 and 3), suggest a redistribution of TG from the cytosol to microsomes. Furthermore, the amount of luminal TG increased more (3.5±0.4-fold) than the membrane-associated TG (2.1±0.2-fold). Thus results suggest that Caco-2 cells that have been adapted for 2 weeks to 0 mM/5 mM glucose condition are able to direct more TG to the ER lumen where it is available for TRL assembly and secretion.
Figure 6. TG content in the secretory compartment of Caco-2 cells cultured in 25 mM/25 mM or 0 mM/5 mM glucose for 2 weeks after confluence.
(A) Electron micrographs showing the presence of lipid droplets in the secretory compartment. Cells were incubated for 2 h with complex lipid micelles. Arrows show lipid droplets within the ER and the Golgi apparatus (G). MV, microvilli; N, nucleus. Scale bar, 500 nm. (B) TLC of lipids present in whole microsomes, and in the membranes and the lumen of microsomes. Cells were incubated for 3 h with lipid micelles containing [1-14C]OA and BA. An autoradiography of a representative TLC is shown. (C) Quantification of the labelled TG (upper panel) and phospholipid (PL; lower panel) present in the microsomal fractions after separation by TLC. The fractions were isolated from cells cultured in 25 mM/25 mM (white bars) or 0 mM/5 mM (black bars) glucose. Results are expressed as c.p.m. per μg of protein in each fraction. Results shown are the means±S.E.M. for three independent experiments. The asterisk (*) indicates a significant difference (P<0.05) from the value for Caco-2 cells cultured in 25 mM/25 mM glucose condition. Note: triglycerides=TGs.
Effect of verapamil on intracellular distribution of TG in Caco-2 cells adapted to a differential glucose supply
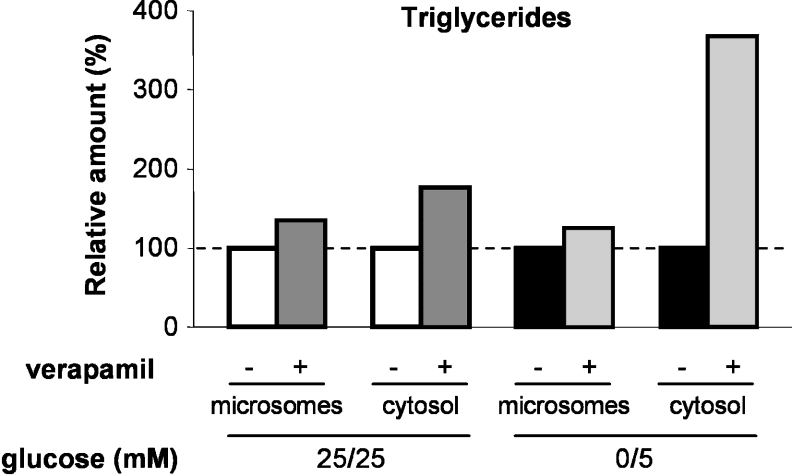
As reported by Higashi et al. [38] in a hepatoma cell line, TG transfer into the microsomal fraction can be inhibited by verapamil without affecting MTP activity, suggesting a verapamil-sensitive mechanism for TG transfer across ER membranes. Since MTP was not involved in the increase of the microsomal lipid content induced by low glucose, we tested whether this increase could be inhibited by verapamil. For that means, cells cultured in the 25 mM/25 mM or 0 mM/5 mM glucose condition for 2 weeks post-confluence were treated for 3 h in the presence of lipid micelles with or without 25 μM verapamil, a concentration that we found to cause a 50% reduction of TG secretion after a 24 h treatment, as already reported in Caco-2 cells by Field et al. [39]. In both glucose conditions, verapamil treatment resulted in a slight increase of the TG content in microsomes and a strong increase of TG content in the cytosolic fraction (Figure 7). The TG increase in the cytosolic fraction was clearly higher for cells cultured in the low glucose condition as compared with cells cultured in 25 mM/25 mM glucose. Overall, verapamil treatment reduced the relative amounts of TG present in the ER, particularly in cells cultured in the low glucose condition.
Figure 7. Effect of verapamil on microsomal and cytosolic TG content.
Caco-2 cells were cultured in 25 mM/25 mM (white bars) or 0 mM/5 mM (black bars) glucose for 2 weeks post-confluence, and incubated for 3 h with lipid micelles supplemented with [1-14C]OA and with or without verapamil as indicated. Labelled TG present in each fraction resulting from subcellular fractionation was quantified after separation by TLC. Results are expressed as relative amounts of radioactive TG in the fractions from the verapamil-treated cells in comparison with those of untreated cells. Results shown are the means for two independent experiments. Note: triglycerides=TGs.
DISCUSSION
In the present paper, we show that TG secretion is modulated when Caco-2 enterocytes adapt to a differential glucose supply. Adaptation of Caco-2 cells to low glucose (0 and 5 mM glucose in the apical and basal compartments respectively) for 2 weeks post-confluence resulted in the doubling of TG secretion induced by the supply of complex lipid micelles compared with cells cultured in 25 mM glucose at both poles. Our results show that the increased TG secretion by cells adapted to low glucose is mainly due to an increased size of the lipoproteins secreted and, to a smaller extent, to an increase in the number of secreted particles. We further demonstrate that this increased availability of TG for assembly and secretion in TG-enriched lipoproteins is not due to an increase in TG synthesis nor to an increase in MTP activity. Rather, we show that the partitioning of TG between cytosolic droplets and the ER lumen differs according to differential glucose supply, and that this could account for the greater TG secretion in the low glucose condition. This mechanism differs from that reported for decreases in TG secretion induced by taxifolin [40] and niacin [41] in hepatic cells (HepG2), consisting of a parallel decrease in lipid synthesis and secretion. It rather resembles the verapamil-sensitive mechanism proposed by Higashi et al. [38] to regulate TG transfer across ER membrane. Our results are in accordance with their results which showed, in a human hepatoma cell line, that verapamil inhibited the transfer of TG across ER membranes, without affecting MTP activity. Moreover, we report that verapamil treatment strongly reduced the relative amount of TG present in the ER in cells cultured in low glucose condition.
It has been shown in many cell types, including muscle cells, pancreatic β cells, hepatocytes [42] and intestinal epithelial cells [43], that the balance between fatty acid esterification and β-oxidation can be controlled by glucose. In the presence of high concentrations of glucose and fatty acids, fatty acids are directed towards esterification rather than β-oxidation. However, this is unlikely to have occurred in our experiments since fatty acid esterification remained constant in both conditions. Although we observed the accumulation of less glycogen in Caco-2 cells adapted to low glucose, the increased TRL secretion, a high energy-consuming process, and the fully differentiated enterocyte cell morphology demonstrate that the energy supply was sufficient to ensure the differentiation process in cells adapted to low glucose after confluence. This is consistent with the already reported sharp decrease in glucose consumption in Caco-2 cells at confluence [31,44] and with the results reported here, which show that the mRNA levels of three glucose-responsive genes reached similar values in both glucose supply conditions. Overall, our results suggest that culture for 2 weeks post-confluence in 0 mM/5 mM glucose favours the absorptive phenotype of Caco-2 enterocytes, and provides a more suitable environment for physiological studies.
Our results indicate that the targeting of de novo synthesized TG towards the ER lumen may represent a key step in chylomicron secretion. In vivo, a transient accumulation of cytosolic lipid droplets is observed in enterocytes during the post-prandial period [15–18] and may result from a transitory overflow in the capacity for chylomicron assembly.
The molecular mechanisms governing TG partitioning between the cytosol and the ER lumen, and the formation of lipid droplets in the lumen of the ER, are still poorly understood. It has been known for a long time that the enzymes necessary for lipid synthesis are intrinsic membrane proteins present in the ER membrane (for reviews, see [5,6]). However, although a critical issue, the fact of whether the catalytic sites of the enzymes face the cytosol or the lumen of the ER remains elusive. In hepatocytes, several lines of evidence suggested that TG synthesis occurs on both sides of the ER membrane [45,46]. TGs are not permeant whereas diacylglycerol, an intermediary product of TG synthesis, is able to rapidly migrate across a membrane bilayer [47]. Based on the inverted topologies of the two forms of acyl-CoA:cholesterol acyltransferase at the ER membrane [48], it has been proposed that luminal forms of DGAT (diacylglycerol acyltransferase) [49] could be specifically involved in the synthesis of TG for lipoprotein assembly in the ER lumen. However, it is as yet unknown whether DGAT1 is involved in TG partitioning in the cell [50,51].
An alternative model proposes that synthesized TG would first accumulate between the two phospholipid leaflets of the ER membrane [19,20,52]. After reaching a critical size, the lipid droplet would bud off the cytosolic or the luminal side of the ER membrane. The proteins that regulate the inward and outward budding of the growing lipid droplet remain to be determined.
In any case, MTP is required for TG assembly into TRL in the lumen of the secretory pathway [35–37]. In a fructose-fed Syrian golden hamster model, the overproduction of intestinal lipoproteins was found to correlate with an increase in intestinal MTP protein and activity [21]. Accordingly, in Caco-2 cells, the inhibition of MTP activity results in a reduction in lipid and TRL secretion [53,54], but it is unknown whether increased MTP expression results in an enhanced production of TRL in Caco-2 cells, as reported for hepatic cells [55]. Such a mechanism cannot account for the increased TRL secretion we observed, since we found similar levels of MTP mRNA, protein and activity in our two culture conditions. Consequently, MTP appears not to be rate-limiting for the formation of the TG droplet inside the ER and for the assembly and secretion of TRL in our conditions. Therefore the differential TRL production we observed most probably depends on factors acting upstream of MTP in this pathway, at the stage of TG partitioning between the cytosol and ER lumen as discussed above.
The physiological significance of the presence of two pathways for TG synthesis in the intestine is not known. Does the balance between these two pathways determine the targeting of the newly synthesized TG towards cytosolic storage or lipoprotein secretion? In Caco-2 cells cultured in 25 mM glucose, the MGAT activity is low [34]. We wondered whether a lower glucose supply might decrease the production of glycerol-3-phosphate, the precursor of the GPAT pathway, and consequently turn on the MGAT pathway for TG synthesis, which should decrease cell dependency on glucose for TG synthesis. Although we showed that the MGAT2 mRNA level increased with time post-confluence, as does the TG secretion capacity [14], it did not vary significantly between the two glucose culture conditions; neither did the contribution of the MGAT pathway to TG synthesis, as measured using the 2-MG analogue BA, vary.
Interestingly, the increased secretion of TRL in cells adapted to low glucose was only correlated with an increase in the ER luminal TG content, as observed by electron microscopy and confirmed by TG quantification in the lumen of microsomes. Neither the TG synthesis rate nor ApoB production nor MTP transfer activity was responsible for this different TRL secretion by Caco-2 cells adapted to differential glucose supply. Therefore the target of this modulation appears to be the TG compartmentalization between the cytoplasm and the ER lumen. Candidate protein(s) could therefore be proteins involved (i) in TG synthesis, possibly acting as a partitioning orientator, such as DGAT(s) [51,56], MGAT(s), TG hydrolase [52,57], (ii) in membrane budding, such as flippases [58], and/or (iii) in vesicular trafficking, such as rab proteins that have been found associated with cytosolic lipid droplets [59]. Furthermore, the activity of the candidate protein may be sensitive to verapamil [38].
In conclusion, we have shown that Caco-2 cells modulate the TG partitioning between the cytosol and the ER lumen, depending on the nutrient supply. During the post-prandial period, the transient cytosolic lipid droplet accumulation that can be observed in enterocytes may represent a way for enterocytes to regulate TRL secretion as a function of time after a meal, and thus be relevant to post-prandial hypertriglyceridaemia, a risk factor in the development of atherosclerosis [60]. Our model, in which TG partitioning is modulated by a differential glucose supply, will help in identifying the proteins involved, and in making progress in the understanding of the mechanisms controlling the balance between TRL assembly and cytosolic lipid storage.
Acknowledgments
Electron and confocal microscopy were done using the facilities of IFR 58 (Centre de Recherches Biomédicales des Cordeliers, 15 rue de l'Ecole de Médecine, Paris, France). We thank Christophe Klein (IFR 58, Paris, France) for his kind assistance with confocal microscopy, and Véronique Carrière, Armelle Leturque (UMR 505, Paris, France) and Rachel Carol (Ecole Pratique des Hautes Etudes, Paris, France) for helpful suggestions and critical reading of this paper. T. P. is a recipient of a MENRT (Ministère de l'Education Nationale, de la Recherche, et de la Technologie) fellowship and J. B. is a recipient of an ANRS (Agence Nationale de Recherches sur le Sida, Paris, France) fellowship. This work was supported by ATC (Action Thématique Concertée) Nutrition INSERM grant number ASE02129DSA and ANRS.
References
- 1.Schutz Y. Dietary fat, lipogenesis and energy balance. Physiol. Behav. 2004;83:557–564. doi: 10.1016/j.physbeh.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Tso P. Intestinal lipid absorption. In: Johnson L. R., editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 1867–1907. [Google Scholar]
- 3.Towle H. C., Kaytor E. N., Shih H. M. Regulation of the expression of lipogenic enzyme genes by carbohydrate. Annu. Rev. Nutr. 1997;17:405–433. doi: 10.1146/annurev.nutr.17.1.405. [DOI] [PubMed] [Google Scholar]
- 4.Foufelle F., Ferre P. New perspectives in the regulation of hepatic glycolytic and lipogenic genes by insulin and glucose: a role for the transcription factor sterol regulatory element binding protein-1c. Biochem. J. 2002;366:377–391. doi: 10.1042/BJ20020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehner R., Kuksis A. Biosynthesis of triacylglycerols. Prog. Lipid Res. 1996;35:169–201. doi: 10.1016/0163-7827(96)00005-7. [DOI] [PubMed] [Google Scholar]
- 6.Coleman R. A., Lee D. P. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 2004;43:134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 7.Kayden H. J., Senior J. R., Mattson F. H. The monoglyceride pathway of fat absorption in man. J. Clin. Invest. 1967;46:1695–1703. doi: 10.1172/JCI105660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breckenridge W. C., Kuksis A. Triacylglycerol biosynthesis in everted sacs of rat intestinal mucosa. Can. J. Biochem. 1975;53:1184–1195. doi: 10.1139/o75-163. [DOI] [PubMed] [Google Scholar]
- 9.Vance J. E. Assembly and secretion of lipoproteins. In: Vance D. E., Vance J. E., editors. Biochemistry of Lipids, Lipoproteins and Membranes. 4th edn. Amsterdam: Elsevier; 2002. pp. 505–526. [Google Scholar]
- 10.Olofsson S. O., Stillemark-Billton P., Asp L. Intracellular assembly of VLDL: two major steps in separate cell compartments. Trends Cardiovasc. Med. 2000;10:338–345. doi: 10.1016/s1050-1738(01)00071-8. [DOI] [PubMed] [Google Scholar]
- 11.Shelness G. S., Sellers J. A. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 2001;12:151–157. doi: 10.1097/00041433-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Fisher E. A., Ginsberg H. N. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 13.Morel E., Demignot S., Chateau D., Chambaz J., Rousset M., Delers F. Lipid-dependent bidirectional traffic of apolipoprotein B in polarized enterocytes. Mol. Biol. Cell. 2004;15:132–141. doi: 10.1091/mbc.E03-04-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chateau D., Pauquai T., Delers F., Rousset M., Chambaz J., Demignot S. Lipid micelles stimulate the secretion of triglyceride-enriched apolipoprotein B48-containing lipoproteins by Caco-2 cells. J. Cell. Physiol. 2005;202:767–776. doi: 10.1002/jcp.20173. [DOI] [PubMed] [Google Scholar]
- 15.Sabesin S. M., Frase S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J. Lipid Res. 1977;18:496–511. [PubMed] [Google Scholar]
- 16.Buschmann R. J., Manke D. J. Morphometric analysis of the membranes and organelles of small intestinal enterocytes. II. Lipid-fed hamster. J. Ultrastruct. Res. 1981;76:15–26. doi: 10.1016/s0022-5320(81)80047-0. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton R. L., Wong J. S., Cham C. M., Nielsen L. B., Young S. G. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J. Lipid Res. 1998;39:1543–1557. [PubMed] [Google Scholar]
- 18.Cartwright I. J., Plonne D., Higgins J. A. Intracellular events in the assembly of chylomicrons in rabbit enterocytes. J. Lipid Res. 2000;41:1728–1739. [PubMed] [Google Scholar]
- 19.Murphy D. J., Vance J. Mechanisms of lipid-body formation. Trends Biochem. Sci. 1999;24:109–115. doi: 10.1016/s0968-0004(98)01349-8. [DOI] [PubMed] [Google Scholar]
- 20.van Meer G. Caveolin, cholesterol, and lipid droplets? J. Cell Biol. 2001;152:F29–F34. doi: 10.1083/jcb.152.5.f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haidari M., Leung N., Mahbub F., Uffelman K. D., Kohen-Avramoglu R., Lewis G. F., Adeli K. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J. Biol. Chem. 2002;277:31646–31655. doi: 10.1074/jbc.M200544200. [DOI] [PubMed] [Google Scholar]
- 22.Malins D. C., Wekell J. C., Houle C. R. Composition of the diacyl glyceryl ethers and triglycerides of the flesh and liver of the dogfish (Squalus acanthias) J. Lipid Res. 1965;79:100–105. [PubMed] [Google Scholar]
- 23.Hallgren B., Larsson S. The glycerylethers in the liver oils of elasmobranch fish. J. Lipid Res. 1962;3:31–38. [Google Scholar]
- 24.Kulinski A., Rustaeus S., Vance J. E. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J. Biol. Chem. 2002;277:31516–31525. doi: 10.1074/jbc.M202015200. [DOI] [PubMed] [Google Scholar]
- 25.Yu W., Cassara J., Weller P. F. Phosphatidylinositide 3-kinase localizes to cytoplasmic lipid bodies in human polymorphonuclear leukocytes and other myeloid-derived cells. Blood. 2000;95:1078–1085. [PubMed] [Google Scholar]
- 26.Vidal R., Hernandez-Vallejo S., Pauquai T., Texier O., Rousset M., Chambaz J., Demignot S., Lacorte J. M. Apple procyanidins decrease cholesterol esterification and lipoprotein secretion in Caco-2/TC7 enterocytes. J. Lipid Res. 2005;46:258–268. doi: 10.1194/jlr.M400209-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Carriere V., Le Gall M., Gouyon-Saumande F., Schmoll D., Brot-Laroche E., Chauffeton V., Chambaz J., Rousset M. Intestinal glucose-dependent expression of glucose-6-phosphatase: involvement of the aryl receptor nuclear translocator transcription factor. J. Biol. Chem. 2005;280:20094–20101. doi: 10.1074/jbc.M502192200. [DOI] [PubMed] [Google Scholar]
- 28.Gouyon F., Caillaud L., Carriere V., Klein C., Dalet V., Citadelle D., Kellett G. L., Thorens B., Leturque A., Brot-Laroche E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J. Physiol. (Cambridge, U.K.) 2003;552:823–832. doi: 10.1113/jphysiol.2003.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiery J. P. Mise en évidence des polysaccharides sur coupes fines en microscopie électronique. J. Microsc. 1967;6:987–1018. [Google Scholar]
- 30.Rousset M. The human colon carcinoma cell lines HT-29 and Caco-2: two in vitro models for the study of intestinal differentiation. Biochimie. 1986;68:1035–1040. doi: 10.1016/s0300-9084(86)80177-8. [DOI] [PubMed] [Google Scholar]
- 31.Mahraoui L., Rodolosse A., Barbat A., Dussaulx E., Zweibaum A., Rousset M., Brot-Laroche E. Presence and differential expression of SGLT1, GLUT1, GLUT2, GLUT3 and GLUT5 hexose-transporter mRNAs in Caco-2 cell clones in relation to cell growth and glucose consumption. Biochem. J. 1994;298:629–633. doi: 10.1042/bj2980629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maulen N. P., Henriquez E. A., Kempe S., Carcamo J. G., Schmid-Kotsas A., Bachem M., Grunert A., Bustamante M. E., Nualart F., Vera J. C. Up-regulation and polarized expression of the sodium-ascorbic acid transporter SVCT1 in post-confluent differentiated CaCo-2 cells. J. Biol. Chem. 2003;278:9035–9041. doi: 10.1074/jbc.M205119200. [DOI] [PubMed] [Google Scholar]
- 33.Jonas A. Lipoprotein structure. In: Vance D. E., Vance J. E., editors. Biochemistry of Lipids, Lipoproteins and Membranes. 4th edn. Amsterdam: Elsevier; 2002. pp. 483–504. [Google Scholar]
- 34.Trotter P. J., Storch J. Fatty acid esterification during differentiation of the human intestinal cell line Caco-2. J. Biol. Chem. 1993;268:10017–10023. [PubMed] [Google Scholar]
- 35.Hussain M. M., Iqbal J., Anwar K., Rava P., Dai K. Microsomal triglyceride transfer protein: a multifunctional protein. Front. Biosci. 2003;8:s500–s506. doi: 10.2741/1071. [DOI] [PubMed] [Google Scholar]
- 36.Gordon D. A., Jamil H. Progress towards understanding the role of microsomal triglyceride transfer protein in apolipoprotein-B lipoprotein assembly. Biochim. Biophys. Acta. 2000;1486:72–83. doi: 10.1016/s1388-1981(00)00049-4. [DOI] [PubMed] [Google Scholar]
- 37.Berriot-Varoqueaux N., Aggerbeck L. P., Samson-Bouma M., Wetterau J. R. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu. Rev. Nutr. 2000;20:663–697. doi: 10.1146/annurev.nutr.20.1.663. [DOI] [PubMed] [Google Scholar]
- 38.Higashi Y., Itabe H., Fukase H., Mori M., Fujimoto Y., Takano T. Transmembrane lipid transfer is crucial for providing neutral lipids during very low density lipoprotein assembly in endoplasmic reticulum. J. Biol. Chem. 2003;278:21450–21458. doi: 10.1074/jbc.M301376200. [DOI] [PubMed] [Google Scholar]
- 39.Field F. J., Born E., Chen H., Murthy S., Mathur S. N. Esterification of plasma membrane cholesterol and triacylglycerol-rich lipoprotein secretion in CaCo-2 cells: possible role of p-glycoprotein. J. Lipid Res. 1995;36:1533–1543. [PubMed] [Google Scholar]
- 40.Casaschi A., Rubio B. K., Maiyoh G. K., Theriault A. G. Inhibitory activity of diacylglycerol acyltransferase (DGAT) and microsomal triglyceride transfer protein (MTP) by the flavonoid, taxifolin, in HepG2 cells: potential role in the regulation of apolipoprotein B secretion. Atherosclerosis. 2004;176:247–253. doi: 10.1016/j.atherosclerosis.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Ganji S. H., Tavintharan S., Zhu D., Xing Y., Kamanna V. S., Kashyap M. L. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J. Lipid Res. 2004;45:1835–1845. doi: 10.1194/jlr.M300403-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Muoio D. M., Seefeld K., Witters L. A., Coleman R. A. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem. J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- 43.Bierbach H., Haag G. F., Holldorf A. W. Uptake and metabolism of long chain fatty acids in isolated chicken intestinal epithelial cells. Digestion. 1979;19:392–403. doi: 10.1159/000198400. [DOI] [PubMed] [Google Scholar]
- 44.Rousset M., Laburthe M., Pinto M., Chevalier G., Rouyer-Fessard C., Dussaulx E., Trugnan G., Boige N., Brun J. L., Zweibaum A. Enterocytic differentiation and glucose utilization in the human colon tumor cell line Caco-2: modulation by forskolin. J. Cell. Physiol. 1985;123:377–385. doi: 10.1002/jcp.1041230313. [DOI] [PubMed] [Google Scholar]
- 45.Owen M. R., Corstorphine C. C., Zammit V. A. Overt and latent activities of diacylglycerol acytransferase in rat liver microsomes: possible roles in very-low-density lipoprotein triacylglycerol secretion. Biochem. J. 1997;323:17–21. doi: 10.1042/bj3230017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abo-Hashema K. A., Cake M. H., Power G. W., Clarke D. Evidence for triacylglycerol synthesis in the lumen of microsomes via a lipolysis-esterification pathway involving carnitine acyltransferases. J. Biol. Chem. 1999;274:35577–35582. doi: 10.1074/jbc.274.50.35577. [DOI] [PubMed] [Google Scholar]
- 47.Allan D., Thomas P., Michell R. H. Rapid transbilayer diffusion of 1,2-diacylglycerol and its relevance to control of membrane curvature. Nature (London) 1978;276:289–290. doi: 10.1038/276289a0. [DOI] [PubMed] [Google Scholar]
- 48.Joyce C. W., Shelness G. S., Davis M. A., Lee R. G., Skinner K., Anderson R. A., Rudel L. L. ACAT1 and ACAT2 membrane topology segregates a serine residue essential for activity to opposite sides of the endoplasmic reticulum membrane. Mol. Biol. Cell. 2000;11:3675–3687. doi: 10.1091/mbc.11.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cases S., Stone S. J., Zhou P., Yen E., Tow B., Lardizabal K. D., Voelker T., Farese R. V., Jr Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 2001;276:38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 50.Liang J. J., Oelkers P., Guo C., Chu P. C., Dixon J. L., Ginsberg H. N., Sturley S. L. Overexpression of human diacylglycerol acyltransferase 1, acyl-CoA:cholesterol acyltransferase 1, or acyl-CoA:cholesterol acyltransferase 2 stimulates secretion of apolipoprotein B-containing lipoproteins in McA-RH7777 cells. J. Biol. Chem. 2004;279:44938–44944. doi: 10.1074/jbc.M408507200. [DOI] [PubMed] [Google Scholar]
- 51.Chen H. C., Farese R. V., Jr Inhibition of triglyceride synthesis as a treatment strategy for obesity: lessons from DGAT1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:482–486. doi: 10.1161/01.ATV.0000151874.81059.ad. [DOI] [PubMed] [Google Scholar]
- 52.Gibbons G. F., Wiggins D., Brown A. M., Hebbachi A. M. Synthesis and function of hepatic very-low-density lipoprotein. Biochem. Soc. Trans. 2004;32:59–64. doi: 10.1042/bst0320059. [DOI] [PubMed] [Google Scholar]
- 53.van Greevenbroek M. M., Robertus-Teunissen M. G., Erkelens D. W., de Bruin T. W. Participation of the microsomal triglyceride transfer protein in lipoprotein assembly in Caco-2 cells: interaction with saturated and unsaturated dietary fatty acids. J. Lipid Res. 1998;39:173–185. [PubMed] [Google Scholar]
- 54.Marcil V., Delvin E., Garofalo C., Levy E. Butyrate impairs lipid transport by inhibiting microsomal triglyceride transfer protein in Caco-2 cells. J. Nutr. 2003;133:2180–2183. doi: 10.1093/jn/133.7.2180. [DOI] [PubMed] [Google Scholar]
- 55.Tietge U. J., Bakillah A., Maugeais C., Tsukamoto K., Hussain M., Rader D. J. Hepatic overexpression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J. Lipid Res. 1999;40:2134–2139. [PubMed] [Google Scholar]
- 56.Meegalla R. L., Billheimer J. T., Cheng D. Concerted elevation of acyl-coenzyme A:diacylglycerol acyltransferase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem. Biophys. Res. Commun. 2002;298:317–323. doi: 10.1016/s0006-291x(02)02466-x. [DOI] [PubMed] [Google Scholar]
- 57.Gilham D., Ho S., Rasouli M., Martres P., Vance D. E., Lehner R. Inhibitors of hepatic microsomal triacylglycerol hydrolase decrease very low density lipoprotein secretion. FASEB J. 2003;17:1685–1687. doi: 10.1096/fj.02-0728fje. [DOI] [PubMed] [Google Scholar]
- 58.Pomorski T., Holthuis J. C., Herrmann A., van Meer G. Tracking down lipid flippases and their biological functions. J. Cell Sci. 2004;117:805–813. doi: 10.1242/jcs.01055. [DOI] [PubMed] [Google Scholar]
- 59.Liu P., Ying Y., Zhao Y., Mundy D. I., Zhu M., Anderson R. G. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 60.Hennig B., Toborek M., McClain C. J. High-energy diets, fatty acids and endothelial cell function: implications for atherosclerosis. J. Am. Coll. Nutr. 2001;20:97–105. doi: 10.1080/07315724.2001.10719021. [DOI] [PubMed] [Google Scholar]