Abstract
In vitro studies demonstrate that the hydroxyl radical converts L-phenylalanine into m-tyrosine, an unnatural isomer of L-tyrosine. Quantification of m-tyrosine has been widely used as an index of oxidative damage in tissue proteins. However, the possibility that m-tyrosine might be generated oxidatively from free L-phenylalanine that could subsequently be incorporated into proteins as an L-tyrosine analogue has received little attention. In the present study, we demonstrate that free m-tyrosine is toxic to cultured CHO (Chinese-hamster ovary) cells. We readily detected radiolabelled material in proteins isolated from CHO cells that had been incubated with m-[14C]tyrosine, suggesting that the oxygenated amino acid was taken up and incorporated into cellular proteins. m-Tyrosine was detected by co-elution with authentic material on HPLC and by tandem mass spectrometric analysis in acid hydrolysates of proteins isolated from CHO cells exposed to m-tyrosine, indicating that free m-tyrosine was incorporated intact rather than being metabolized to other products that were subsequently incorporated into proteins. Incorporation of m-tyrosine into cellular proteins was sensitive to inhibition by cycloheximide, suggesting that protein synthesis was involved. Protein synthesis using a cell-free transcription/translation system showed that m-tyrosine was incorporated into proteins in vitro by a mechanism that may involve L-phenylalanine-tRNA synthetase. Collectively, these observations indicate that m-tyrosine is toxic to cells by a pathway that may involve incorporation of the oxidized amino acid into proteins. Thus misincorporation of free oxidized amino acids during protein synthesis may represent an alternative mechanism for oxidative stress and tissue injury during aging and disease.
Keywords: amino acid misincorporation, hydroxyl radical, oxidative stress, protein synthesis, tRNA synthetase, m-tyrosine
Abbreviations: CHO, Chinese-hamster ovary; LDH, lactate dehydrogenase; MS/MS, tandem MS; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium; TCA, trichloroacetic acid; TFA, trifluoroacetic acid
INTRODUCTION
A wealth of indirect evidence implicates oxidative damage of cellular constituents in the pathogenesis of diseases and aging [1–5]. One important target is proteins, which are of central importance as enzymes and structural elements in cells. However, the mechanisms for oxidative damage of proteins in vivo are poorly understood because the oxidative intermediates are short-lived and are difficult to detect directly.
One widely studied oxidant is the hydroxyl radical and species of similar reactivity that are generated by metal-catalysed oxidation systems, glucose autoxidation and mitochondrial aerobic metabolism [1–5]. Hydroxyl radical converts L-phenylalanine into m-tyrosine and o-tyrosine [6], isomers of the natural amino acid L-tyrosine. m-Tyrosine is also produced by peroxynitrite in vitro [7]. Because m-tyrosine is stable to acid hydrolysis and is thought to be absent from normal proteins, it has served as a useful marker for oxidative damage. Thus elevated levels of m-tyrosine have been detected in aging lens proteins of humans [8], atherosclerotic tissue of diabetic non-human primates [9], mitochondrial proteins of exercised animals [10], blood of animals subjected to cardiac ischaemia–reperfusion injury [11] and retinal tissue of diabetic rats [12]. Model system studies indicate that when proteins are oxidized by peroxynitrite, the major product is 3-nitrotyrosine, although low levels of o-tyrosine and m-tyrosine are also detectable [13,14]. In contrast, the myeloperoxidase–H2O2–chloride system fails to generate o-tyrosine or m-tyrosine [13,14]. Collectively, these observations indicate that the detection of elevated levels of o-tyrosine and m-tyrosine implicates a hydroxyl radical-like species in tissue damage.
Oxidized amino acids are generated in proteins that are exposed to reactive intermediates in vitro [6,9,15], and most studies of these markers in vivo have assumed that oxidized proteins are generated post-translationally. In contrast, the possibility that oxidized amino acids might be incorporated into proteins during synthesis has received little attention. It is well established that structural analogues of L-tyrosine, such as 3-iodotyrosine, 3-fluorotyrosine and 3,4-dihydroxy-L-phenylalanine, are incorporated by post-transcriptional mechanisms into α-tubulin [16]. The mechanism appears to involve tubulin L-tyrosine ligase, an enzyme that seems to have promiscuous substrate specificity [17,18]. 3-Nitrotyrosine generated by reactive nitrogen species can subsequently be incorporated into tubulin by this mechanism [17]. 3,4-Dihydroxyphenylalanine is incorporated into proteins of a macrophage cell line by a pathway that is sensitive to inhibition by cycloheximide [19]. Proteins containing this oxygenated amino acid were proposed to undergo degradation by the proteasomal and lysosomal pathways [19]. It has not yet been established whether similar mechanisms might contribute to oxidative stress and protein oxidation in vivo.
In the present study, we demonstrate that m-tyrosine is cytotoxic and show that this oxygenated amino acid is incorporated into proteins both in a cell-free translation system and by CHO (Chinese-hamster ovary) cells. Based on these observations, we propose that m-tyrosine might be misincorporated into tissue proteins during translation and that this mechanism could contribute to the toxicity of the oxidized amino acid.
EXPERIMENTAL
Materials
L-[14C]Phenylalanine (specific activity 520 mCi/mmol, 0.5 mCi/ml) was provided by American Radiolabeled Chemicals. CHO cells were purchased from the A.T.C.C. Organic solvents were HPLC grade. Unless indicated otherwise, all other reagents were obtained from Sigma–Aldrich.
Synthesis of 14C-labelled L-tyrosine and m-tyrosine
L-[14C]Phenylalanine was dried under vacuum, dissolved in water and irradiated in a 137Cs irradiator for 30 min at a rate of 3317 rad/min. L-[14C]Tyrosine and m-[14C]tyrosine were isolated from the reaction mixture by HPLC using a reverse-phase column (Beckman Ultrasphere, 5 μm, 4.6 mm×25 cm) as described below. Identity of the amino acids was confirmed by co-elution with authentic standards on HPLC and by GLC/MS analysis [14].
Cell culture
CHO cells were cultured in 10-cm-diameter dishes (Corning) at 1×106 cells/well and maintained in medium A [1:1 mixture (v/v) of Ham's F-12 medium and Dulbecco's modified Eagle's medium supplemented with 10% (v/v) foetal bovine serum, 100 units/ml penicillin, 0.1 mg/ml streptomycin, 2 mM L-glutamine, and 1 mM sodium pyruvate] at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
Cycloheximide treatment
Cycloheximide was added from a 100 mg/ml stock solution dissolved in DMSO to a final concentration of 100 μg/ml in medium A.
Colony formation assay for cytotoxicity
Exponentially growing CHO cells were detached from tissue culture wells using trypsin/EDTA and were collected by centrifugation at 500 g for 5 min at 4 °C. The resulting cell pellet was resuspended in medium A, and the number of cells was established using an aliquot of medium and a haemocytometer. Cells (100–2000) were plated into tissue culture dishes (60 mm diameter; Corning) and were incubated for 4 h to allow them to attach to the surface. They were then exposed to the indicated final concentrations of amino acids in medium A and incubated for 7–10 days. The resulting cell colonies were stained with Methylene Blue and counted. The colony efficiency was calculated as: (colonies counted/cells plated)×100. A cell-survival curve was constructed by plotting the surviving fractions (number of colonies counted/number of cells seeded)×(colony efficiency of the control cells) from cells exposed to various concentrations of L-tyrosine and m-tyrosine.
MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2- (4-sulphophenyl)-2H-tetrazolium] assay for cytotoxicity
The viability of cells was assessed by monitoring the reduction of MTS (Promega). For cytotoxicity assays, CHO cells were plated on 10-cm-diameter dishes as described above and were allowed to adhere for 2 h. The medium was then changed to medium A supplemented with the indicated final concentration of L-tyrosine or m-tyrosine. After 24 h of incubation, the medium was removed, and the cells were washed once with PBS. MTS was added to each well following the manufacturer's instructions. Following 2 h of incubation at 37 °C, 200 μl aliquots of medium from each well of cells were transferred to a 96-well microtitre plate. The absorbance of the medium at 490 nm was determined using a microplate reader.
LDH (lactate dehydrogenase) assay for cytotoxicity
Media were collected and were centrifuged at 1500 g for 15 min, then LDH activity in the supernatants was determined following the addition of NADH (125 μM final concentration) and sodium pyruvate (100 μM final concentration) by monitoring changes in the absorbance of the reaction mixture at 340 nm [20]. The initial rate of reaction was directly proportional to the amount of medium incorporated into the reaction mixture.
Cellular incorporation of 14C-labelled amino acids into proteins
CHO cells (1×106) were plated in 10-cm-diameter dishes and were cultured in 10 ml of medium A as described above. Following 24 h of incubation, the medium was replaced with fresh medium A supplemented with 50 μM 14C-labelled L-tyrosine or m-tyrosine. The cells were then incubated for the indicated period of time, washed once with PBS, detached with 1 ml of trypsin/EDTA, and harvested by centrifugation at 500 g for 5 min after adding 10 ml of medium. Proteins were isolated from the cells by precipitation using TCA (trichloroacetic acid). TCA [0.5 ml of a 20% (w/v) solution] was added to each cell pellet, the cells were resuspended by vortex-mixing, and the cellular lysate was incubated on ice for 30 min. Proteins were collected by centrifuging the lysate at 4000 g for 4 min at 4 °C. The protein pellet was washed twice with ice-cold PBS, and the incorporation of radiolabelled amino acids into proteins was monitored as described below.
To control for non-specific incorporation of radiolabelled material into cellular proteins, CHO cells were incubated under similar conditions, except that medium A included non-radiolabelled L-tyrosine, L-phenylalanine or m-tyrosine. After 24 h of incubation, the same amount of the radiolabelled compound (L-tyrosine or m-tyrosine) was added to the medium, and the cells were immediately harvested as described above.
Incorporation of radiolabelled amino acid into proteins was monitored by solubilizing TCA-precipitated proteins with 0.5 ml of 2% (v/v) Triton X-100. Protein concentrations were determined using the DC Protein Assay (Bio-Rad). An aliquot (100 μl) of the solubilized protein was mixed with 2 ml of scintillation mixture (Optiflor; Packard Instrument Company), and radiolabel was quantified using a liquid-scintillation counter (LS 3081; Beckman Corp.). For HPLC analysis, TCA-precipitated proteins were suspended in 0.5 ml of 6 M HCl (Sequenal Grade; Pierce Chemical) containing 1% (w/v) phenol and were hydrolysed at 110 °C for 24 h [14]. Amino acids were isolated from the hydrolysate by solid-phase extraction on a C18 column (3 ml; Supelclean SPE, Supelco). The columns were conditioned before use by washing with 2 ml of methanol and 6 ml of 0.1% (v/v) TFA (trifluoroacetic acid). The volume of the amino acid hydrolysate was adjusted to 2 ml with 0.1% TFA, passed over the column, and the column was washed with 2 ml of 0.1% TFA. Amino acids were eluted with 2 ml of 25% methanol, and the eluent was dried under nitrogen. Isolated amino acids were dissolved in water and subjected to HPLC analysis.
HPLC analysis of radiolabelled amino acids
Amino acid analysis was performed using Beckman System Gold 125 HPLC apparatus equipped with a Beckman Ultrasphere ODS reverse-phase column (5 μm resin, 4.6 mm×25 cm) coupled to a Waters 484 Tunable Absorbance Detector. Amino acids were separated at a flow rate of 1 ml/min using a linear gradient of 100% solvent A [methanol/TFA/water, 5:1:94, by vol. (pH adjusted to 2.5 with NaOH)] that was changed to 100% solvent B (methanol/TFA/water, 20:1:79, by vol.) over 20 min with monitoring of absorbance at 274 nm.
Detection and quantification of m-tyrosine by HPLC-MS analysis
CHO cells were plated and incubated for 24 h as described above. At the end of the incubation, proteins were isolated using TCA precipitation and washed twice as described above. The protein pellet was hydrolysed with 0.5 ml of 6 M HCl containing 1% (w/v) phenol at 110 °C for 24 h and was centrifuged at 14000 g for 10 min. The hydrolysate was then subjected to HPLC-MS analysis [21]. Briefly, 50 μl of supernatant was injected on to a reverse-phase HPLC column (Varian Chromospheres 5 C18, 25 cm×4 mm). Amino acids were separated at a flow rate of 0.8 ml/min using a linear gradient produced using solvent A (10 mM ammonium acetate, adjusted to pH 4.5 with ethanoic acid) containing 1% (v/v) methanol that was changed to solvent B containing 10% methanol over 30 min. Amino acids were monitored using an ion-trap mass spectrometer (Finnigan LCQ Delta; Thermoquest) equipped with an atmospheric pressure chemical ionization source. L-Phenylalanine and m-tyrosine were monitored by selected reaction monitoring. Protonated precursor and product ions were detected as ions of m/z 166 and 120 for L-phenylalanine and m/z 182 and 136 for m-tyrosine respectively. Product ions were derived by loss of HCOOH [(46 a.m.u. (atomic mass units)] from protonated precursor ions.
In vitro transcription and translation
Incorporation of m-tyrosine into luciferase during in vitro coupled transcription/translation was studied using a cell-free reticulate lysate preparation (TNT SP6 System; Promega) following the manufacturer's instructions. Unless indicated otherwise, all 20 of the common amino acids required for protein synthesis were included in the reaction mixture at 1 mM.
SDS/PAGE and autoradiography of radiolabelled proteins
14C-Labelled luciferase prepared using the in vitro transcription/translation system was subjected to SDS/PAGE with a Bio-Rad Mini-PROTEAN II cell. Samples were applied to gels after dilution (1:1, v/v) with loading buffer [50 μl of 2-mercaptoethanol (Fisher) and 950 μl of Laemmli sample buffer (Bio-Rad) 1:19 (v/v)]. Running buffer was 25 mM Tris/HCl, 192 mM glycine and 0.1% (w/v) SDS (pH 8.3). Following electrophoresis, proteins were fixed by placing the gel in a 10% (v/v) ethanoic acid and 30% (v/v) methanol mixture for 1 h. Gels were treated with EN3HANCE (NEN) for 1 h and then with ice-cold water for 30 min and were dried under vacuum at 60 °C. Luciferase was visualized by exposing the gel to X-ray film (X-OMAT AR; Kodak) at −80 °C for 2 weeks.
Statistical analysis
Student's t test was used to examine the significance of the difference between groups. P<0.05 was accepted as significant.
RESULTS
The free amino acid m-tyrosine is cytotoxic to cultured cells
To determine whether free oxidized amino acids can be cytotoxic, we incubated CHO cells with increasing concentrations of m-tyrosine or its isomer L-tyrosine (which contains the hydroxy group in the para position on the phenolic ring) for 24 h in medium containing 40 μM L-tyrosine, 30 μM L-phenylalanine and 10% foetal bovine serum. We monitored cellular injury using three different assays: colony formation, MTS reduction and LDH release.
Incubation of CHO cells with m-tyrosine inhibited colony formation in a concentration-dependent manner. Survival and proliferation of the cells was inhibited approx. 60% by 0.25 mM m-tyrosine (Figure 1). In contrast, 1 mM L-tyrosine had little effect on the fraction of CHO cells that survived. Since colony formation assesses the ability of individual cells to proliferate, it is a sensitive measure of cell viability.
Figure 1. Colony formation of CHO cells incubated with m-tyrosine.
Exponentially growing CHO cells were plated on to tissue culture dishes and incubated for 4 h to allow them to attach to the surface. The cells were then exposed to the indicated final concentrations of amino acid in medium A [a 1:1 (v/v) mixture of Ham's F-12 medium and Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum, penicillin, streptomycin, 2 mM L-glutamine and 1 mM sodium pyruvate] and incubated for 7–10 days. The resulting cell colonies were stained with Methylene Blue and counted. Survival curves were constructed by plotting the surviving fractions of cells (number of colonies counted/number of cells seeded)×(colony efficiency of the control cells). Results are means±S.D. for three independent experiments.
CHO cells incubated for 24 h with increasing concentrations of m-tyrosine in medium containing 10% foetal bovine serum and physiological concentrations of amino acids progressively lost their ability to reduce MTS (results not shown). Reduction of MTS was inhibited approx. 50% by 0.5 mM m-tyrosine; 3 mM m-tyrosine produced >90% inhibition. In contrast, L-tyrosine had little effect at any concentration investigated. These observations suggest that relatively high concentrations of m-tyrosine were cytotoxic to CHO cells as assessed by the MTS assay.
Alterations in membrane integrity cause the cytosolic enzyme LDH to leak into the medium around the cells. LDH release therefore indicates significant damage to cells. Release of LDH into the medium increased markedly when CHO cells were exposed to a high concentration (5 mM) of m-tyrosine for 24 or 48 h (results not shown). As in the cytotoxicity assay, the same concentration of L-tyrosine had little effect as monitored by this method. Collectively, these observations indicate that m-tyrosine is toxic to CHO cells. The differences in m-tyrosine concentrations required to inflict cell injury in the various assays probably reflects the fact that these assays monitor different aspects of cellular function and viability.
Radiolabelled material derived from m-tyrosine is incorporated into cellular proteins
One potential mechanism for m-tyrosine cytotoxicity might involve incorporation of the oxidized amino acid into proteins in place of L-tyrosine. To explore this hypothesis, we monitored the incorporation of radiolabelled L-tyrosine and m-tyrosine into proteins isolated from CHO cells. The cells were incubated for various times in medium that contained 10% foetal bovine serum and physiological concentrations of amino acids and was supplemented with L-[14C]tyrosine or m-[14C]tyrosine. At the end of the incubation, we lysed the cells and precipitated their proteins with TCA. Following extensive washing to remove non-covalently associated radiolabel, the amount of [14C]amino acid incorporated into cellular proteins was quantified by liquid-scintillation counting.
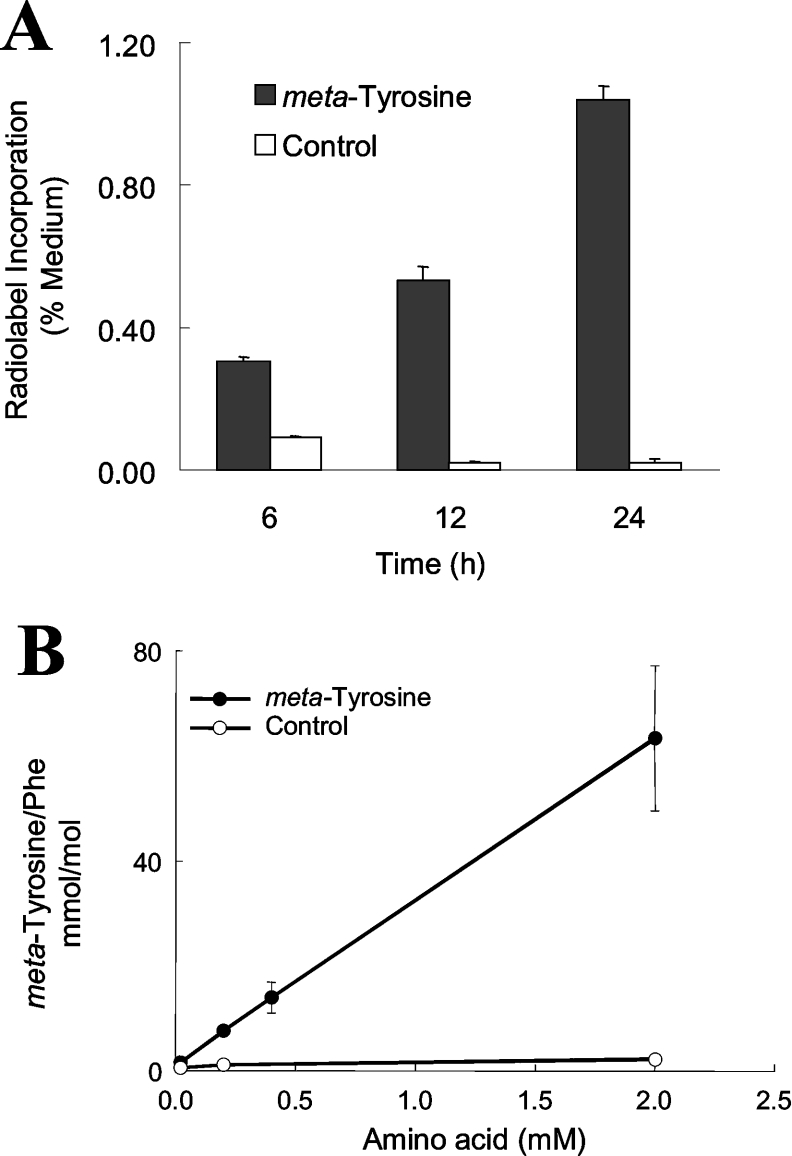
m-[14C]Tyrosine was incorporated into cellular proteins in a time-dependent manner (Figure 2A). After 24 h incubation, 1% of the radiolabelled m-tyrosine included in the medium was found in isolated cellular proteins. In contrast, radiolabelled L-tyrosine was incorporated into cellular proteins at a 20-fold higher level (1.04% m-tyrosine and 21.5% L-tyrosine) when the cells were incubated under identical conditions. Non-specific binding was not likely to have accounted for the radiolabelled m-tyrosine detected in isolated cellular proteins because, when the same concentration of radiolabelled m-tyrosine was added to the cells immediately before they were harvested, the isolated proteins contained a much smaller amount of radiolabel (Figure 2A; open bars). Similar levels of radiolabelled L-tyrosine and m-tyrosine were found in isolated proteins of control cells (L-[14C]tyrosine, 0.13±0.03%; m-[14C] tyrosine, 0.02±0.01%) under these conditions. Moreover, the amount of radiolabel detected in isolated proteins of control cells under these conditions did not increase with the length of time the cells were cultured (Figure 2A). These findings suggest strongly that the radiolabel detected in proteins isolated from CHO cells incubated with m-[14C]tyrosine had been covalently incorporated into proteins.
Figure 2. m-[14C]Tyrosine was incorporated into CHO cell proteins in a concentration-dependent manner.
(A) Incorporation of radiolabel into proteins isolated from CHO cells incubated with m-[14C]tyrosine. Cells were cultured for 6, 12 or 24 h in medium A supplemented with m-[14C]tyrosine (closed bars). At the end of the incubation, the cells were harvested and lysed, and their proteins were isolated by acid precipitation. After the proteins were washed extensively, the amount of radiolabel they had incorporated was quantified by liquid-scintillation counting. Control cells were incubated under similar conditions, except that radioactive amino acid was added to the medium immediately before the cells were harvested (open bars). Results are means±S.D. for three independent experiments. (B) MS/MS quantification of m-tyrosine and L-phenylalanine in proteins from CHO cells that had been incubated with increasing concentrations of m-tyrosine. CHO cells were incubated with the indicated final concentration of m-tyrosine or L-tyrosine for 24 h in medium A. At the end of the incubation, cellular proteins were isolated by acid precipitation, washed extensively and hydrolysed with acid. Levels of m-tyrosine in acid hydrolysates were quantified by HPLC and MS/MS analysis with selected reaction monitoring. The content of m-tyrosine is normalized to that of its precursor amino acid, L-phenylalanine. Results are means±S.D. for three independent analyses.
Free m-tyrosine is incorporated intact into cellular proteins
Free amino acids and oxidized amino acids are metabolized to a wide range of compounds (including other amino acids) that might subsequently be incorporated into proteins [22]. To determine whether m-[14C]tyrosine or a compound derived from the radiolabelled amino acid became incorporated into proteins, we subjected the radiolabelled material isolated from CHO cells to acid hydrolysis and reverse-phase HPLC analysis (Figure 3). Previous studies have shown that this chromatography system completely separates a wide range of L-tyrosine and L-phenylalanine oxidation products [13,23]. More than 85% of the radioactivity in the protein pellet (n=4) isolated from CHO cells that had been incubated with m-[14C]tyrosine co-migrated with authentic m-tyrosine. Similarly, essentially all of the radioactivity in the protein pellet isolated from CHO cells that had been incubated with L-[14C]tyrosine co-migrated with authentic L-tyrosine (results not shown). These results suggest that most of the radiolabelled material that was incubated with the cells and recovered from isolated protein was m-[14C]tyrosine under our analytical conditions. The remaining radiolabelled material probably represented metabolites derived from m-[14C]tyrosine.
Figure 3. HPLC analysis of radiolabelled material isolated from tissue proteins of CHO cells incubated with m-[14C]tyrosine.
Cells were cultured for 24 h in medium A supplemented with m-[14C]tyrosine. At the end of the incubation, cellular proteins were isolated by acid precipitation, washed extensively and hydrolysed with acid. The hydrolysate was supplemented with authentic m-tyrosine, o-tyrosine, L-tyrosine or L-phenylalanine and was then analysed by HPLC using a C18 reverse-phase column. Elution of radiolabelled material and aromatic amino acids was monitored by liquid-scintillation counting and absorbance at 274 nm respectively.
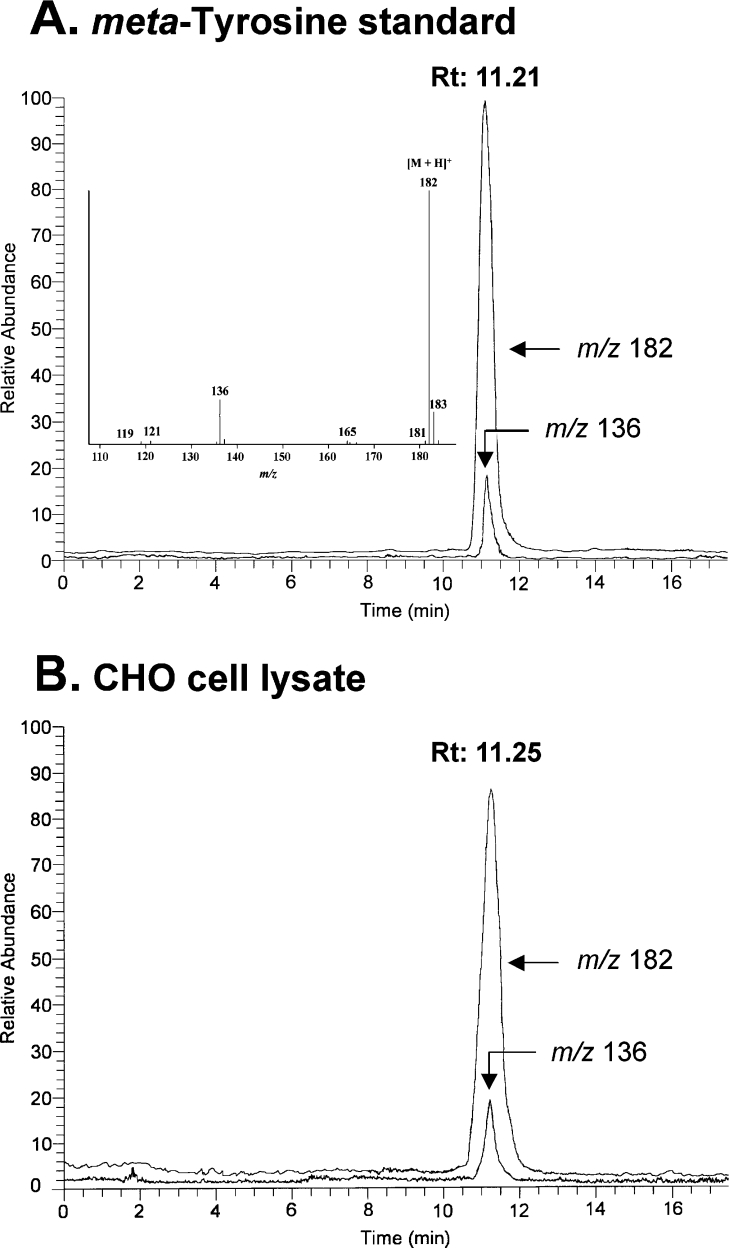
To confirm that proteins isolated from CHO cells incubated with m-tyrosine contain the unmodified amino acid, we first used MS to establish the mass spectrum of authentic m-tyrosine [21]. We then used MS/MS (tandem MS analysis) to analyse acid hydrolysates of proteins isolated from CHO cells. Amino acids were separated by HPLC and were subjected to atmospheric pressure ionization followed by analysis with an ion-trap instrument. MS analysis of authentic m-tyrosine (Figure 4A, inset) revealed a major ion at m/z 182, consistent with the anticipated m/z of the protonated molecular ion [M+H]+. Low-energy collisionally activated MS/MS analysis of m-tyrosine revealed that the [M+H]+ ion (m/z 182) decomposed into major ions at m/z 136 [M+H−HCOOH]+, m/z 165 [M+H−NH3]+, m/z 121 [M+H−NH2COOH]+ and m/z 119 [M+H−HCOOH−NH3]+.
Figure 4. Detection of m-tyrosine in CHO cell proteins by positive-ion atmospheric pressure chemical ionization MS analysis.
(A) HPLC-MS analysis of authentic m-tyrosine subjected to HPLC on a reverse-phase column. Ions derived from the protonated molecular ion [M+H]+ and its major product ion [M−HCOOH+H]+ were monitored in the ion chromatogram. Note that the two ions co-elute and have the same relative abundance as in the full scan mass spectrum. The inset shows the mass spectrum of m-tyrosine (retention time 11.2 min). (B) HPLC-MS analysis of acid hydrolysates of CHO proteins subjected to HPLC on a reverse-phase column. Proteins were isolated from CHO cells incubated with medium A as described in Figure 2. Note that the relative abundance of the ions (m/z 182 and m/z 136) and retention times (Rt; 11.21 and 11.25 min) of authentic m-tyrosine and the material in hydrolysates of CHO proteins are virtually identical.
To quantify cellular m-tyrosine content, we used MS/MS to analyse proteins isolated from cells that had been incubated with m-tyrosine [21]. Collisionally activated MS analysis of acid hydrolysates of CHO cell proteins revealed ions of m/z 182 and m/z 136 that co-eluted on HPLC (Figure 4B). The retention times and relative abundance of the ions were virtually identical with those of authentic m-tyrosine (compare Figures 4A and 4B). These results indicate that m-tyrosine was present in proteins of CHO cells.
To confirm that free m-tyrosine was incorporated as the intact amino acid into cellular proteins, we incubated CHO cells with increasing concentrations of m-tyrosine for 24 h, isolated cellular proteins and quantified the levels of protein-bound m-tyrosine by MS/MS. We used the same approach to quantify L-phenylalanine, the precursor of m-tyrosine. For L-phenylalanine, we monitored the precursor and product ions of m/z 166 and m/z 120 (results not shown).
Proteins were isolated from lysed cells by TCA precipitation, washed extensively and subjected to acid hydrolysis. Amino acids were separated by HPLC on a reverse-phase column and were detected using an ion-trap mass spectrometer as described above. m-Tyrosine was present at 0.6 mmol/mol of L-phenylalanine in proteins isolated from cells that had been incubated in medium A that was not supplemented with m-tyrosine. The protein content of m-tyrosine in cells incubated with the oxidized amino increased significantly (2 mmol/mol of L-phenylalanine; P<0.05) at the lowest concentration of m-tyrosine investigated (20 μM). The amount of m-tyrosine incorporated into cellular proteins increased linearly with the amount of m-tyrosine available in the medium (Figure 2B). These observations indicate that free m-tyrosine is taken up by CHO cells and incorporated intact into proteins. They also suggest that cells will incorporate m-tyrosine into their proteins even when physiological concentrations of the 20 common amino acids are present in extracellular fluid and m-tyrosine is available only at a low concentration.
Cycloheximide inhibits the incorporation of m-tyrosine into cellular proteins
To determine whether synthesis of new proteins was required for the incorporation of m-tyrosine into cellular proteins, we incubated CHO cells for 24 h in medium A alone, medium A supplemented with 100 μg/ml cycloheximide, medium A supplemented with 1 mM m-tyrosine, or medium A supplemented with m-tyrosine and 100 μg/ml cycloheximide. CHO cells incubated with 1 mM m-tyrosine exhibited a 4.6-fold increase in the amount of m-tyrosine that was incorporated into cellular proteins, as monitored using isotope-dilution GC/MS analysis of an acid hydrolysate of cellular proteins [12]. Incorporation of m-tyrosine into cellular proteins was inhibited 88% by cycloheximide. In contrast, cycloheximide had little effect on the levels of protein-bound m-tyrosine in CHO cells incubated in medium A alone. These observations strongly suggest that the pathway for the incorporation of m-tyrosine into cellular proteins requires protein synthesis.
m-Tyrosine is incorporated into proteins in vitro by a coupled transcription/translation system
Aminoacyl-tRNA synthetase enzymes attach cognate amino acids to the corresponding tRNAs, which can subsequently be incorporated into proteins [24]. To explore the potential role of this mechanism in the incorporation of m-tyrosine into cellular proteins, we monitored the incorporation of radiolabelled L-tyrosine and m-tyrosine into proteins expressed by an in vitro coupled transcription/translation system. This luciferase expression system was composed of reticulate lysates supplemented with all 20 common amino acids (1 mM) required for protein synthesis. 14C-Labelled amino acids (L-tyrosine, L-phenylalanine or m-tyrosine) were included in the incubation system, both in the presence of the complete amino acid mixture and in the absence of unlabelled L-tyrosine or L-phenylalanine. Incorporation of radiolabelled amino acids into transcribed and translated proteins was monitored by SDS/PAGE and fluorography.
We were unable to detect incorporation of radiolabel into proteins when m-[14C]tyrosine was added to reticulocyte lysates, and the complete amino acid mixture was available. However, when L-phenylalanine was omitted from the incubation, a band of radiolabelled material that co-migrated with authentic luciferase was apparent on SDS/PAGE and fluorography. In contrast, no labelled protein was found when L-tyrosine was omitted from the incubation (Figure 5). These observations indicate that m-tyrosine can be incorporated into proteins during transcription and translation by a reticulocyte lysate system and suggest that one potential mechanism involves the binding of m-tyrosine to the aminoacyl-tRNA synthetase for L-phenylalanine.
Figure 5. Incorporation of m-[14C]tyrosine into proteins by a cell-free transcription/translation system.

Incorporation of m-[14C]tyrosine into luciferase protein was monitored using a cell-free reticulate lysate preparation containing the 20 common amino acids required for protein synthesis and either L-[14C]tyrosine or m-[14C]tyrosine. Radiolabelled protein was detected by SDS/PAGE and autoradiography. Lane A, amino acid mixture+L-[14C]tyrosine; lane B, amino acid mixture lacking L-tyrosine+m-[14C]tyrosine; lane C, amino acid mixture lacking L-phenylalanine+m-[14C]tyrosine. LUC, luciferase. Molecular-mass sizes are indicated in kDa.
Phenylalanine reverses the cytotoxicity of m-tyrosine
To test the proposal that m-tyrosine might bind to aminoacyl-tRNA synthetase for L-phenylalanine in cells, we incubated CHO cells with 0.2 mM m-tyrosine alone or with 0.2 mM m-tyrosine and either L-phenylalanine or L-tyrosine for 10 days in medium containing 10% foetal bovine serum. We then monitored cellular injury as assessed by colony formation.
Incubation of CHO cells with 0.2 mM m-tyrosine inhibited colony formation by approx. 30% (Figure 6). L-Tyrosine (0.2 or 1 mM) had little effect on the fraction of CHO cells that survived. In contrast, L-phenylalanine at 0.2 mM partially protected and at 1 mM almost completely preserved the ability of the cells to undergo proliferation. These observations are consistent with the proposal that m-tyrosine mediates its cytotoxic effects via a pathway that requires protein synthesis and that is mediated in part by L-phenylalanine-tRNA synthetase.
Figure 6. Effects of L-phenylalanine and L-tyrosine on m-tyrosine-induced cytotoxicity.
CHO cells were plated on to tissue culture dishes and were incubated for 4 h to allow them to attach to the surface. The cells were then exposed for 7–10 days to the indicated final concentrations of 0.2 mM m-tyrosine alone or 0.2 mM m-tyrosine with either 0.2 mM or 1 mM L-phenylalanine or L-tyrosine in medium A containing 10% foetal bovine serum. The resulting cell colonies were stained with Methylene Blue and were counted. Survival curves were constructed by plotting the surviving fractions of cells. Results are means±S.D. for three independent experiments.
DISCUSSION
Elevated levels of oxidatively modified proteins have been detected in diseased and aging tissues [3,9,25], implicating protein oxidation in the pathogenesis of disorders ranging from atherosclerosis to ischaemia–reperfusion injury and perhaps the aging process itself. Oxidized amino acid residues are generated in proteins that are exposed to reactive intermediates in vitro, and most studies of these markers of protein damage in vivo have assumed that oxidized proteins are generated post-translationally. In contrast, the possibility that oxidized amino acids might be incorporated into proteins during synthesis has received little attention.
In the present study, we found that m-tyrosine, a stable product of the oxidation of L-phenylalanine by the hydroxyl radical, was toxic to CHO cells as assessed by three different assays. We hypothesized that one potential cytotoxicity mechanism could be the incorporation of this unnatural isomer of L-tyrosine into proteins. Indeed, we found that m-tyrosine was incorporated into proteins of CHO cells as assessed by the uptake of radiolabelled m-tyrosine. Incorporation of m-tyrosine into cellular proteins was sensitive to inhibition by cycloheximide, suggesting that protein synthesis was involved. Rodgers et al. [19] used a similar approach to demonstrate that another oxygenated amino acid, 3,4-dihydroxyphenylalanine, was incorporated into proteins of a macrophage cell line. Importantly, these workers demonstrated that the degradation rates of proteins containing 3,4-dihydroxyphenylalanine differed from those of control proteins, suggesting that the metabolism and perhaps function of proteins might be altered by incorporation of oxidized amino acids.
In the present study, two lines of evidence indicated that intact m-[14C]tyrosine, rather than one of its metabolites, was incorporated into cellular proteins. First, the radiolabelled material isolated from acid hydrolysates of cellular proteins co-migrated with authentic m-tyrosine on HPLC analysis. Secondly, MS/MS analysis readily detected m-tyrosine in hydrolysed proteins isolated from CHO cells that had been incubated with the oxidized amino acid. When increasing concentrations of m-tyrosine were provided, the concentration of m-tyrosine detected in cellular proteins increased in a linear manner. These observations suggest that even cells exposed to physiological concentrations of the 20 common amino acids and low concentrations of m-tyrosine will take up the oxidized amino acid and incorporate it into proteins.
We also detected m-tyrosine in proteins isolated from cells that were not exposed to m-tyrosine in the medium. This finding suggests that cells might routinely produce a low level of free m-tyrosine and incorporate it into proteins. Consistent with this hypothesis, cultured neuronal, adrenal and liver cells readily convert L-phenylalanine into m-tyrosine and o-tyrosine by a metabolic pathway that has been proposed to require L-tyrosine hydroxylase [26,27]. Moreover, free m-tyrosine has been detected in blood and urine of animals and humans [28,29], suggesting that one pathway for producing this ‘abnormal’ amino acid involves enzymatic oxygenation of the free amino acid by metabolic pathways. Collectively, these observations suggest that detection of elevated levels of m-tyrosine in tissue proteins might reflect biosynthetic incorporation of the oxygenated amino acid rather than direct oxidative damage of proteins.
Free m-tyrosine might be incorporated into cellular proteins during or after protein synthesis. One potential mechanism involves aminoacyl-tRNA synthetase, a family of enzymes that attach cognate amino acids to their corresponding tRNAs. It is well established that amino acid analogues that are structurally similar to their natural counterparts are both aminoacylated and incorporated into proteins [30]. Therefore structural similarities among m-tyrosine, L-phenylalanine and L-tyrosine might increase mischarging of tRNA and consequent misincorporation of m-tyrosine into proteins. We used a cell-free in vitro translation system to begin to explore this possibility. We found that m-tyrosine was incorporated into luciferase protein during in vitro translation of the luciferase gene. However, this misincorporation occurred only in the absence of L-phenylalanine and not in the presence of a complete amino acid mixture or an amino acid mixture lacking L-tyrosine. Moreover, the cytotoxicity of m-tyrosine to CHO cells was blocked when the medium contained 1 mM L-phenylalanine, but not when the medium contained 1 mM L-tyrosine. These results suggest that L-phenylalanine-tRNA synthetase recognizes m-tyrosine and that this mechanism might contribute in part to the misincorporation of m-tyrosine into cellular proteins.
It is important to note that we observed a much higher level of incorporation of m-tyrosine into proteins when we incubated the oxygenated amino acid with CHO cells than when we used the in vitro transcription/translation system. Also, the CHO cells were incubated with m-tyrosine in medium that contained both L-tyrosine and L-phenylalanine, indicating that the unnatural amino acid can be incorporated into proteins even when the parent amino acid is available at physiological levels. These cells were thus likely to have normal cellular pools of L-tyrosine and L-phenylalanine, suggesting that the in vitro transcription/translation system was not accurately mimicking the cellular milieu. One factor that might contribute to this apparent discrepancy is premature termination of protein synthesis in the in vitro system when high levels of m-tyrosine are included in the reaction mixture. In future studies, it will be important to use intact cells and animals to investigate further the pathways that can misincorporate oxygenated amino acids into proteins.
A key issue is whether the relatively low levels of misincorporation of m-tyrosine into cellular proteins that we observed in cultured cells is likely to be physiologically relevant. It is of interest that relatively low concentrations of m-tyrosine (0.2 mM) inhibited the proliferation of CHO cells in the colony formation assay. This observation suggests that m-tyrosine-induced alterations in protein catalytic function or structure could be cytotoxic. It is also possible that even a low level of misincorporation of amino acids into an enzyme such as DNA polymerase could lead to errors in DNA replication and long-term consequences such as impaired cellular viability or even mutagenesis.
Our observations have implications for using m-tyrosine as an indicator of protein oxidation in vivo. Measurement of amino acid oxidation products in proteins has been widely employed as a non-invasive way to monitor contributions of oxidative stress to disease and the efficacy of antioxidant therapy [10,31]. An important question regarding the validity of using such amino acid oxidation products as biomarkers in vivo is the fate of the biomarker itself in the body. Our findings suggest that the steady-state level of m-tyrosine in a tissue protein reflects the initial level of misincorporation of the amino acid as well as post-translational oxidation and the subsequent rate of protein degradation. It is also important to note that oxidized amino acids can be metabolized to other products. For example, when high doses of m-tyrosine were given to four healthy subjects, the major metabolic product was m-hydroxyphenylacetic acid, which was excreted in urine [32]. It will clearly be important to investigate the metabolic fate of oxidized amino acids in greater detail.
In summary, the present study provides strong evidence that m-tyrosine is cytotoxic and is incorporated into proteins by cultured cells. Misincorporation of m-tyrosine into critical enzymes or structural proteins may contribute to the cytotoxic effects of the oxygenated amino acid. Our findings suggest further that post-translational oxidative modification may not be the only factor that contributes to the level of m-tyrosine in tissue proteins. In future studies, it will be of great interest to determine whether free m-tyrosine is incorporated into proteins of whole animals and to assess the physiological role of this pathway.
Acknowledgments
This research was supported by grants from the National Institutes of Health (DK02456, AG021191, P50 HL073996, P30 DK017047, P30 ES07033 and PO1 HL030086) and from the Donald W. Reynolds Foundation. We thank Dianne Mueller for excellent technical assistance.
References
- 1.Berliner J. A., Heinecke J. W. The role of oxidized lipoproteins in atherogenesis. Free Radical Biol. Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 2.Baynes J. The role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 3.Stadtman E. R. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 4.Chapman M. L., Rubin B. R., Gracy R. W. Increased carbonyl content of proteins in synovial fluid from patients with rheumatoid arthritis. J. Rheumatol. 1989;16:15–19. [PubMed] [Google Scholar]
- 5.Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants and the degenerative diseases of aging. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huggins T. G., Wells-Knecht M. C., Detorie N. A., Baynes J. W., Thorpe S. R. Formation of o-tyrosine and dityrosine in proteins during radiolytic and metal-catalyzed oxidation. J. Biol. Chem. 1993;268:12341–12347. [PubMed] [Google Scholar]
- 7.van der Vliet A., O'Neill C. A., Halliwell B., Cross C. E., Kaur H. Aromatic hydroxylation and nitration of L-phenylalanine and L-tyrosine by peroxynitrite: evidence for hydroxyl radical production from peroxynitrite. FEBS Lett. 1994;339:89–92. doi: 10.1016/0014-5793(94)80391-9. [DOI] [PubMed] [Google Scholar]
- 8.Wells-Knecht M. C., Huggins T. G., Dyer D. G., Thorpe S. R., Baynes J. W. Oxidized amino acids in lens protein with age: measurement of o-tyrosine and dityrosine in the aging human lens. J. Biol. Chem. 1993;268:12348–12352. [PubMed] [Google Scholar]
- 9.Pennathur S., Wagner J. D., Leeuwenburgh C., Litwak K. N., Heinecke J. W. A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J. Clin. Invest. 2001;107:853–860. doi: 10.1172/JCI11194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leeuwenburgh C., Hansen P., Holloszy J. O., Heinecke J. W. Hydroxyl radical generation during exercise increases mitochondrial protein oxidation and levels of urinary dityrosine. Free Radical Biol. Med. 1999;27:186–192. doi: 10.1016/s0891-5849(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill C. A., Fu L. W., Halliwell B., Longhurst J. C. Hydroxyl radical production during myocardial ischemia and reperfusion in cats. Am. J. Physiol. 1996;271:H660–H667. doi: 10.1152/ajpheart.1996.271.2.H660. [DOI] [PubMed] [Google Scholar]
- 12.Pennathur S., Ido Y., Heller J. I., Byun J., Danda R., Pergola P., Williamson J. R., Heinecke J. W. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J. Biol. Chem. 2005;280:22706–22714. doi: 10.1074/jbc.M500839200. [DOI] [PubMed] [Google Scholar]
- 13.Leeuwenburgh C., Rasmussen J. E., Hsu F. F., Mueller D. M., Pennathur S., Heinecke J. W. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J. Biol. Chem. 1997;272:3520–3526. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 14.Pennathur S., Jackson-Lewis V., Przedborski S., Heinecke J. W. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o,o′-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson's disease. J. Biol. Chem. 1999;274:34621–34628. doi: 10.1074/jbc.274.49.34621. [DOI] [PubMed] [Google Scholar]
- 15.Fu S., Dean R., Southan M., Truscott R. The hydroxyl radical in lens nuclear cataractogenesis. J. Biol. Chem. 1998;273:28603–28609. doi: 10.1074/jbc.273.44.28603. [DOI] [PubMed] [Google Scholar]
- 16.Joniau M., Coudijzer K., De Cuyper M. Reaction of α-tubulin with iodotyrosines catalyzed by tubulin:tyrosine ligase: carboxy-terminal labeling of tubulin with [125I]monoiodotyrosine. Anal. Biochem. 1990;184:325–329. doi: 10.1016/0003-2697(90)90689-7. [DOI] [PubMed] [Google Scholar]
- 17.Eiserich J. P., Estevez A. G., Bamberg T. V., Ye Y. Z., Chumley P. H., Beckman J. S., Freeman B. A. Microtubule dysfunction by post-translational nitrotyrosination of α-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6365–6370. doi: 10.1073/pnas.96.11.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalisz H. M., Erck C., Plessmann U., Wehland J. Incorporation of nitrotyrosine into α-tubulin by recombinant mammalian tubulin-tyrosine ligase. Biochim. Biophys. Acta. 2000;1481:131–138. doi: 10.1016/s0167-4838(00)00110-2. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers K. J., Wang H., Fu S., Dean R. T. Biosynthetic incorporation of oxidized amino acids into proteins ad their cellular proteolysis. Free Radical Biol. Med. 2002;32:766–775. doi: 10.1016/s0891-5849(02)00768-2. [DOI] [PubMed] [Google Scholar]
- 20.Hassoun E. A., Roche V. F., Stohs J. Release of enzymes by ricin from macrophages and Chinese hamster ovary cells in culture. Toxicol. Methods. 1993;3:119–129. [Google Scholar]
- 21.Orhan H., Vermeulen N. P. E., Tump C., Zappey H., Meerman J. H. N. Simultaneous determination of tyrosine and phenylalanine oxidation products and 8-hydroxy-2′-deoxyguanosine in biological samples as non-invasive multi-parameter biomarker for oxidative damage. J. Chromatogr. B. 2004;799:245–254. doi: 10.1016/j.jchromb.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 22.Ohshima H., Friesen M., Brout I., Bartsch H. Nitrotyrosine as a new marker for endogenous nitrosation and nitration of proteins. Food Chem. Toxicol. 1990;28:647–652. doi: 10.1016/0278-6915(90)90173-k. [DOI] [PubMed] [Google Scholar]
- 23.Jacob J. S., Cistola D. P., Hsu F. F., Muzaffar S., Mueller D. M., Hazen S. L., Heinecke J. W. Human phagocytes employ the myeloperoxidase–hydrogen peroxide system to synthesize dityrosine, trityrosine, pulcherosine, and isodityrosine by a tyrosyl radical-dependent pathway. J. Biol. Chem. 1996;271:19950–19956. doi: 10.1074/jbc.271.33.19950. [DOI] [PubMed] [Google Scholar]
- 24.Ibba M., Soll D. Quality control mechanisms during translation. Science. 1999;286:1893–1897. doi: 10.1126/science.286.5446.1893. [DOI] [PubMed] [Google Scholar]
- 25.Uchida K., Toyokuni S., Kishikawa S., Oda H., Hiaia H., Stadtman E. R. Michael addition-type 4-hydroxy-2-nonrenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry. 1994;33:12487–12494. doi: 10.1021/bi00207a016. [DOI] [PubMed] [Google Scholar]
- 26.Ishimitsu S., Fujimoto S., Ohara A. Formation of meta-tyrosine and o-tyrosine from L-phenylalanine by rat brain homogenate. Chem. Pharm. Bull. (Tokyo) 1980;28:1653–1655. doi: 10.1248/cpb.28.1653. [DOI] [PubMed] [Google Scholar]
- 27.Ishimitsu S., Fujimoto S., Ohara A. Formation of meta-tyrosine and o-tyrosine from L-phenylalanine in various tissues of rats. Chem. Pharm. Bull. (Tokyo) 1985;33:3887–3892. doi: 10.1248/cpb.33.3887. [DOI] [PubMed] [Google Scholar]
- 28.Ishimitsu S., Fujimoto S., Ohara A. The determination of m-tyrosine in human plasma by high performance liquid chromatography. Chem. Pharm. Bull. (Tokyo) 1982;30:1889–1891. doi: 10.1248/cpb.30.1889. [DOI] [PubMed] [Google Scholar]
- 29.Ishimitsu S., Fujimoto S., Ohara A. The formation of m-tyrosine and o-tyrosine in rats. Chem. Pharm. Bull. (Tokyo) 1984;32:2439–2441. doi: 10.1248/cpb.32.2439. [DOI] [PubMed] [Google Scholar]
- 30.Ibba M., Hennecke H. Towards engineering proteins by site-directed incorporation in vivo of non-natural amino acids. Bio/Technology. 1994;12:678–682. doi: 10.1038/nbt0794-678. [DOI] [PubMed] [Google Scholar]
- 31.Leeuwenburgh C., Hansen P., Holloszy J. O., Heinecke J. W. Oxidized amino acids in the urine of aging rats: potential markers for assessing oxidative stress in vivo. Am. J. Physiol. 1999;276:R128–R135. doi: 10.1152/ajpregu.1999.276.1.R128. [DOI] [PubMed] [Google Scholar]
- 32.Fell V., Greenway A. M., Hoskins J. A. The metabolism of L-meta-tyrosine in man. Biochem. Med. 1979;22:246–255. doi: 10.1016/0006-2944(79)90010-3. [DOI] [PubMed] [Google Scholar]