Abstract
PTX (Paclitaxel®) is an antimitotic agent used in the treatment of a number of major solid tumours, particularly in breast and ovarian cancer. This study was undertaken to gain insight into the molecular alterations producing PTX resistance in ovarian cancer. PTX treatment is able to induce apoptosis in the human ovarian carcinoma cell line, CABA I. PTX-induced apoptosis in CABA I cells was accompanied by an increase in the cellular Cer (ceramide) levels and a decrease in the sphingomyelin levels, due to the activation of sphingomyelinases. The inhibition of acid sphingomyelinase decreased PTX-induced apoptosis. Under the same experimental conditions, PTX had no effect on Cer and sphingomyelin levels in the stable PTX-resistant ovarian carcinoma cell line, CABA-PTX.
The acquisition of the PTX-resistant phenotype is accompanied by unique alterations in the complex sphingolipid pattern found on lipid extraction. In the drug-resistant cell line, the levels of sphingomyelin and neutral glycosphingolipids were unchanged compared with the drug-sensitive cell line. The ganglioside pattern in CABA I cells is more complex compared with that of CABA-PTX cells. Specifically, we found that the total ganglioside content in CABA-PTX cells was approximately half of that in CABA I cells, and GM3 ganglioside content was remarkably higher in the drug-resistant cell line.
Taken together our findings indicate that: i) Cer generated by acid sphingomyelinase is involved in PTX-induced apoptosis in ovarian carcinoma cells, and PTX-resistant cells are characterized by their lack of increased Cer upon drug treatment, ii) PTX resistance might be correlated with an alteration in metabolic Cer patterns specifically affecting cellular ganglioside composition.
Keywords: ceramide, drug resistance, ganglioside, ovarian cancer, PTX (Paclitaxel®), sphingolipids
Abbreviations: Cer, ceramide; C6-Cer, N-hexanoyl-sphingosine; C16-Cer, N-palmitoyl-sphingosine; FB1, fumonisin B1; FCS, foetal calf serum; GCS, GlcCer-synthase; GD3S, GD3-synthase; GM3S, GM3-synthase; IMP, imipramine hydrochloride; ManA, manumycin A; MDR, multidrug resistance; MRP, MDR-associated protein; P-gp, P-glycoprotein; PTX, Paclitaxel®; SM, sphingomyelin; SMase, sphingomyelinase; XTT/PMS, tetrazolium/phenazine methosulphate
INTRODUCTION
PTX (Paclitaxel®) is an antineoplastic agent used for the treatment of a variety of solid tumours, including ovarian carcinoma [1]. Despite a significant initial response rate to first-line therapy (surgery plus platinum and taxanes-based chemotherapy), many ovarian cancer patients relapse and less than 15% are long-term survivors [2]. Resistance to PTX has been observed in tumours, representing a major impediment to the successful use of this agent in cancer treatment, and it was found to be associated with the common phenomenon of MDR (multidrug resistance) [3]. In order to investigate mechanisms of PTX-multifactorial acquired resistance in ovarian cancer, we derived a new stable PTX-resistant human ovarian carcinoma cell line, CABA-PTX [4], from the parental cell line CABA I [5]. Remarkably, PTX-resistance in these cells is not related to an overexpression of P-gp (P-glycoprotein) and MRP (MDR-associated protein) 1 and 2, usually regarded as hallmarks of the MDR phenotype.
Since apoptosis often represents the final common event in chemotherapy-induced cell death, insight into the mechanisms of drug resistance can be gained from a better understanding of the pathways involved in drug-induced apoptosis in tumour cells. Indeed, defects in the apoptotic pathway have been observed to confer insensitivity to the cytotoxic effects of chemotherapy and may therefore represent an important mechanism for the development of chemoresistance [6].
Cer (ceramide, N-acyl-sphingosine), the central molecule of sphingolipid metabolism, has been recognized as a cellular second-messenger involved in triggering apoptotic/necrotic events in many normal and cancer cells [7]. In particular, Cer seems to be the mediator of apoptosis induced by a variety of anti-tumour therapeutics in drug-sensitive tumour cell lines, including human ovarian carcinoma cells [8]. Several findings suggest that alterations in Cer metabolism could be at least in part responsible for the acquisition of an MDR phenotype in cancer cells [9]. Some drug-resistant tumour cell lines reportedly escape Cer-mediated cell death by converting Cer into GlcCer [β-Glc-(1→1)-Cer]. Indeed, high levels of GlcCer and of GCS (GlcCer-synthase) were found in drug-resistant cell lines [10–12] and in specimens from cancer patients unresponsive to chemotherapy [13]. Based on these observations, it has been proposed that the balance between Cer production and its scavenging by glycosylation might represent the critical point in regulating the sensitivity of tumour cells to chemoterapeutic agents.
On the other hand, Cer can be converted into a variety of complex metabolites and several other steps in the pathway of sphingolipid metabolism are altered in drug resistant tumour cells. In MDR-2780AD human ovarian carcinoma cells, not only GlcCer, but all direct biosynthetic products of Cer were present at higher levels than in drug-sensitive parental cells, whereas the levels of complex sphingolipids downstream of GlcCer, were decreased [12]. In human neuroblastoma cell lines, differential P-gp and MRP1 expression and activity are accompanied by differential sphingolipid metabolism [14]. In fenretinide-resistant A2780 cells, GM3S {GM3 [II3Neu5AcLacCer, α-Neu5Ac-(2→3)-β-Gal-(1→4)-β-Glc-(1→1)-Cer]-synthase} is markedly up-regulated, leading to ganglioside levels that are 6-fold higher than in sensitive cells [8].
In the present study we report that: i) PTX-induced apoptosis is correlated with Cer generation, by SM (sphingomyelin) hydrolysis through acid SMase (sphingomyelinase); ii) PTX-resistance is associated with the lack of a drug-induced increase in Cer levels, that is not due to enhanced Cer glycosylation and iii) a large alteration in the sphingolipid accumulation pattern is a hallmark of the acquisition of resistance.
MATERIALS AND METHODS
Cell lines
CABA I human ovarian carcinoma cells [5] are an adherent cell line. Cells were grown as monolayers in RPMI 1640 medium with 5% FCS (foetal calf serum) (Euroclone) culture medium, 2 mM glutamine, 100 units/ml penicillin and streptomycin. Resistant CABA-PTX cell lines [4], were grown in the same conditions and in the continuous presence of PTX at a concentration of 500 ng/ml (0.585 μM; Sigma–Aldrich, Milano, Italy). All experiments were conducted using cells in the logarithmic phase of cell proliferation.
Lipids and radioactive lipids
Sphingolipids to be used as standards were extracted from rat brain, purified and characterized; gangliosides were extracted from bovine brain and purified by partitioning. Sphingosine [(2S,3R,4E)-2-amino-1,3-dihydroxy-octadecene] was prepared from cerebroside. [1-3H]sphingosine {(2S,3R,4E)-2-amino-1,3-dihydroxy-[1-3H]octadecene; radiochemical purity over 98%; specific radioactivity 2.1 Ci/mmol} was prepared by specific chemical oxidation of the primary hydroxyl group of sphingosine followed by reduction with sodium boro[3H]hydride (Amersham Biosciences; specific radioactivity 12.0 Ci/mmol). Labelled C16-Cer {[1-3H(sphingosine)]N-palmitoylsphingosine; radiochemical purity over 99%; specific radioactivity 2.1 Ci/mmol} was prepared by N-acylation of [1-3H]sphingosine using hexadecanoic anhydride [8]. SM tritium-labelled at position 3 of sphingosine, ([3-3H]sphingosine-SM; radiochemical purity 98%, specific radioactivity 0.37 Ci/mmol) was prepared by the dichlorodicyanobenzoquinone/sodium borohydride method [8], and the natural erythro-diastereoisomer was purified by reverse phase HPLC as described [15]. [3H]lipids used as chromatographic standards were prepared from [1-3H]sphingosine-fed cell cultures as previously described [16].
Flow cytometric analysis
In all experiments, CABA I and CABA-PTX cells were kept subconfluent to avoid contact inhibition. The cells were treated with PTX alone (0.3–2 μg/ml) or in combination with the different agents tested, such as 50 μM FB1 (fumonisin B1), 20 μM monensin, 50 μM IMP (imipramine hydrochloride), 50 μg/ml D609, 20 μM GW4869, 1 μM ManA (manumycin A) and 10 mM GSH (all from Sigma–Aldrich, Milano, Italy).
Adherent cells were trypsinized, pooled with the culture supernatant containing the apoptotic cells that were detached from the dish, and centrifuged. Cells (1×106) were washed in PBS and fixed by the addition of 1 ml of 70% ethanol. After 30 min, the cells were pelleted by centrifugation (720 g; 5 min), and resuspended in 1 ml of DNA staining solution (PBS containing 200 μg/ml RNAase A, 20 μg/ml propidium iodide and 0.1% Triton X-100) and stained by incubation at room temperature for 60 min. All cells were then counted on a FACScan flow cytometer (Becton Dickinson) with an argon laser at 488 nm for excitation and analysed using Cell Quest software (Becton Dickinson).
Growth inhibition assay
Cytotoxicity of C6-Cer (N-hexanoyl-sphingosine) was determined for both cell lines. Cell viability was determined by the XTT/PMS (tetrazolium/phenazine methosulphate) cell-viability dye assay (Sigma–Aldrich, Milano, Italy). Briefly, CABA I and CABA-PTX cells were plated onto flat-bottomed 96-well plates (Nunc) and cultured in RPMI 1640 medium supplemented with 5% FCS for 72 h (CABA-PTX) in the presence or absence of serial Cer dilutions before addition of a 50 μl aliquot of XTT/PMS solution (final concentrations 50 and 0.38 μg/ml respectively) and incubated for 4 h at 37 °C. Plates were read on a microplate ELISA reader at absorbance 450 nm. To exclude any effects from continuous PTX exposure on the assays, CABA-PTX cells were grown in PTX-free medium for 2 days before the assay. C6-Cer was purchased from Sigma and tested at a concentration range of 2–40 μM.
The IC50 value was calculated using a linear regression analysis of the dose response curves and reported as means±S.D. for at least 3 independent experiments.
DNA fragmentation assay
Total DNA was extracted according to the method of Lee and Shacter [17]. Briefly, after treatment cells were harvested, washed with PBS and lysed with lysis buffer [10 mM Tris/HCl (pH 7.8), 10 mM EDTA, 0.5% Triton X-100 and 200 μg/ml proteinase K]. After DNA was precipitated with isopropanol, it was then resuspended in a Tris/EDTA solution. Samples were resolved by electrophoresis on a 1.5% agarose gel and visualized by ethidium bromide staining and UV transillumination.
[3H]Sphingosine metabolic radiolabelling
After seeding (24 h), cells were incubated in the presence of 3×10−8 M [3-3H]sphingosine {(2S,3R,4E)-2-amino-1,3-dihydroxy-[3-3H]octadecene}, (5 ml/dish; labelled sphingosine, specific radioactivity 23.00 Ci/mmol, was from Pelkin Elmer, Boston, MA, U.S.A.) in culture medium for 2 h (pulse), followed by a 48 h chase in medium without radioactive sphingosine. Under these conditions all sphingolipids (including Cer, SM, neutral glycolipids and gangliosides) were metabolically radiolabelled [8,16].
Lipid extraction and determination
In the experiments described above, at the end of the treatment period, cells adherent to the dishes were harvested in ice-cold water (2 ml) by scraping with a rubber policeman. Cells floating in the culture medium were collected by centrifugation. Adherent and floating cells were analysed to determine lipid-associated radioactivity content. Samples were freeze-dried and lipids were extracted and subjected to a two-phase partitioning as previously described [8], resulting in the separation of an aqueous phase containing gangliosides and an organic phase containing all other lipids. Aliquots of total lipid extracts, aqueous and organic phases, were analysed by HPTLC as described below followed by radioactivity imaging for quantification of radioactivity. Identity of radioactive lipids separated by HPTLC (using HPTLC Silica Gel 60 plates from Merck, Germany) was assessed by comigration with standard lipids and confirmed as previously described [18,19].
Enzymatic assay
For acid and neutral SMase assays, cells cultured as described above were harvested using a plastic scraper and washed twice with PBS. Cells were lysed in 0.2% (v/v) Triton-X 100/H2O for 10 min at 4 °C and then sonicated 3 times for 10 s. For the ceramidase assay, cells cultured as described above were harvested using a plastic scraper and washed twice with PBS. Cells were resuspended in 20 mM Tris/HCl (pH 7.4), 20 mg of cell-protein/ml, with protease inhibitors [2 mM 4-(2-aminoethyl)-benzenesulphonyl fluoride, 0.0016 mM aprotinin, 0.044 mM leupeptin, 0.08 mM bestatin, 0.03 mM pepstatin A and 0.028 mM E-64] (Sigma) and homogenized with a Dounce homogenizer (10 strokes, tight). The activity of ceramidases, and acid and neutral SMases was assayed as previously described [8,20,21].
Radioactive lipids were detected and quantified by radioactivity imaging performed with a Beta-Imager 2000 (Biospace, Paris, France) using an acquisition time of approx. 70 h. The radioactivity associated with individual lipids was determined with specific β-Vision software provided by Biospace.
Real-time PCR analysis
Real-time PCR was performed with cDNA synthesized from 5 μg of RNA using the ImProm-II™ Reverse Transcription System kit (Promega) according to the manufacturer's instructions. RNA was isolated using the SV Total RNA Isolation System (Promega). Independent measurements (three per sample) were performed using 2 μl of cDNA as the sample. For PCR reactions, light cycler Fast Start DNA Master SYBR® Green (Roche) was used.
Primers employed for PCR amplifications are as follows: GCS, forward TTCGTCCTCTTCTTGGTGCT, reverse AGACACCTGGGAGCTTGCTA (115 bp); GM3S forward CCCTGAACCAGTTCGATGTT, reverse CATTGCTTGAAGCCAGTTGA (197 bp); GD3S {GD3 [II3(Neu5Ac)2LacCer, α-Neu5Ac-(2→8)-α-Neu5Ac-(2→3)-β-Gal-(1→4)-β-Glc-(1→1)-Cer]-synthase}, forward TACATCTTCCCCGTCTACCG, reverse CCCCATAGGGGAATTCATTT (178 bp); GAPDH (glyceraldehyde-3-phosphate dehydrogenase) forward GGCCTCCAAGGAGTAAGACC, reverse AGGGGTCTACATGGCAACTG (147 bp). The comparative ΔCT method was used to calculate the expression levels of RNA transcripts. The quantified individual RNA expression levels were normalized to the respective GAPDH expression levels. Because we measured the relative RNA expression levels, the CABA I expression level was set at 1.
RESULTS
PTX-induced apoptosis in CABA I and CABA-PTX cells
Previously we described marked differences in PTX sensitivity for CABA I (IC50, 800 ng/ml PTX) and CABA-PTX cells (IC50, 256000 ng/ml PTX) [4]. In the present study, we have investigated whether the cytotoxic effect of PTX in these cells might be due to the induction of apoptosis.
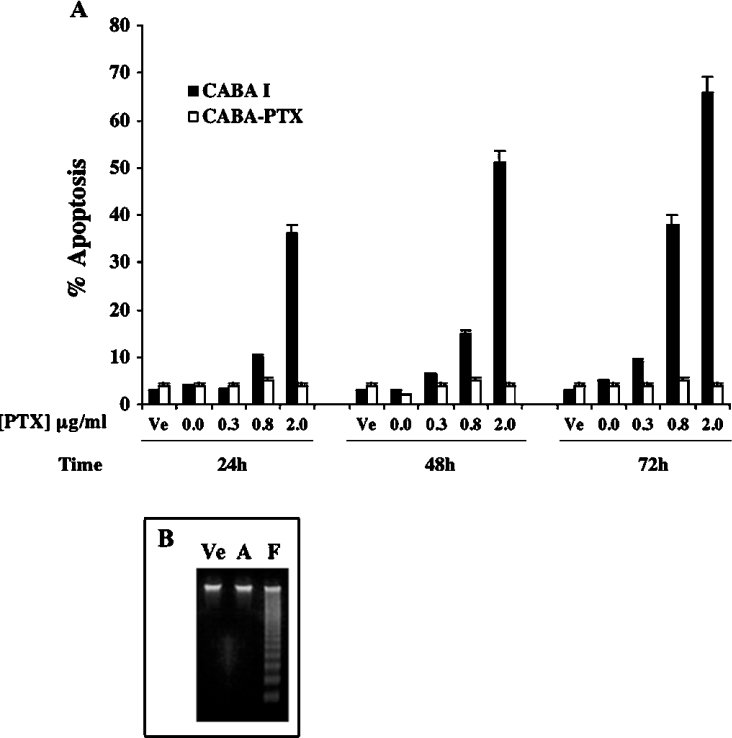
Both cell lines were treated with PTX at different concentrations (0.3, 0.8 and 2 μg/ml) and for different times (24, 48 and 72 h). Flow cytometric analysis of cells floating in the culture medium indicated that exposure to PTX induced apoptosis in a dose- and time-dependent manner in CABA I cells. Apoptosis was maximal at 72 h, with 2 μg/ml PTX (66% of cells were apoptotic); no evidence of apoptosis was observed in untreated cells and in adherent treated cells (Figure 1A). According to these results, gel electrophoresis of DNA extracted from cells floating in the culture medium following 72 h of treatment with 2 μg/ml PTX, showed low molecular mass DNA fragments typical of apoptotic cells (Figure 1B, lane F); no DNA fragmentation was observed in adherent treated-cells (lane A) or in control cells (lane Ve). The same results were obtained at different times and/or concentrations of PTX (results not shown). Under the same experimental conditions, CABA-PTX cells did not undergo apoptosis (Figure 1A).
Figure 1. PTX-induced apoptosis in detached CABA I cells and CABA-PTX cells.
(A) PTX-sensitive CABA I cells (black bars) and resistant CABA-PTX (white bars) cells were treated with PTX 0.3, 0.8 and 2 μg/ml, or vehicle control (Ve), for 24, 48 and 72 h (106 cells/10 cm dish). Apoptotic nuclear fragmentation was evaluated by propidium iodide staining and FACScan analysis. Data are means±S.D. for 3 independent experiments. (B) Agarose gel analysis of DNA fragmentantion induced by PTX. After 72 h of treatment with 2 μg/ml PTX only floating cells (F) show DNA fragmentation compared with adherent cells (A) under the same conditions, or cells treated with vehicle alone (Ve).
Effect of PTX on Cer and SM levels in CABA I and CABA-PTX cells
Cer is a mediator of apoptosis induced by anti-tumour drugs in a number of cell models. To assess whether PTX treatment might affect Cer production, we performed metabolic steady-state radiolabelling with [3-3H]sphingosine in CABA I and CABA-PTX cells. After labelling, cells were treated with 2 μg/ml PTX for 72 h.
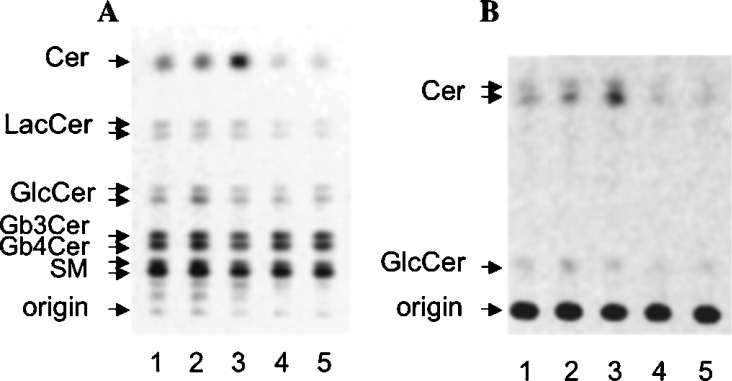
Cell lipids were extracted from control and treated CABA-PTX cells, from control CABA I cells and from adherent and floating PTX-treated CABA I cells. The total lipid extracts were subjected to a two-phase partitioning to allow the separation of an organic phase containing Cer, SM and neutral glycosphingolipids, and of an aqueous phase containing gangliosides. Organic phase lipids were separated by HPTLC and quantitatively analysed by digital autoradiography. The patterns of radioactive lipids present in the organic phase obtained from the lipid extract from CABA I and CABA-PTX cells is presented in Figure 2(A). To allow a better resolution of Cer, the organic phase lipids were separated by HPTLC using a solvent system that left all of the more hydrophilic lipids at the origin (Figure 2B). The radioactivity associated with each lipid species was quantitatively determined and is reported in Table 1. Treatment of CABA I cells with PTX increased the Cer levels in floating cells compared with untreated cells or adherent cells. Cer levels in floating PTX-treated cells were approx. 2-fold higher than in untreated cells. In floating PTX-treated CABA I cells, SM levels were decreased in parallel compared with untreated or adherent cells. In the case of CABA-PTX cells, the Cer level was significantly lower than in CABA I cells and was very similar to treated and control cells. PTX treatment in these cells also left SM levels unchanged.
Figure 2. Cer detection: CABA I (lanes 1–3) and CABA-PTX (lanes 4–5) cells were treated with vehicle (lanes 1 and 4) or 2 μg/ml PTX for 72 h (lanes 2, 3 and 5).
In the case of PTX-treated CABA I cells, both adherent (lane 2) and floating (lane 3) cells were collected separately and analysed for their lipid content. Radioactive lipids were detected by digital autoradiography (250 d.p.m. applied on a 3 mm line; acquisition time, 70 h). (A) Solvent system, chloroform/methanol/water (55:20:3, by vol.); (B) solvent system, hexane/chloroform/acetone/acetic acid (10:35:10:1, by vol.). The position of pure standard lipids is indicated on the left of each panel. Experiments were performed in triplicate.
Table 1. Incorporation of radioactivity into different sphingolipids extracted from untreated or PTX-treated CABA I, and CABA-PTX cells.
Data are expressed as mean nCi/mg of protein±S.D. and as a percentage of total sphingolipids.
| Glycosphingolipids | Gangliosides | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell line | Total sphingolipids | SM nCi/mg | % | Cer nCi/mg | % | Total nCi/mg | % | GlcCer nCi/mg | % | LacCer nCi/mg | % | (Hex)nCer nCi/mg | % | Total nCi/mg | % | GM3 nCi/mg | % | Other nCi/mg | % |
| CABA I | 493.26±37.5 | 138.19±10.6 | 28 | 70.79±6.4 | 14.3 | 222.39±17.4 | 45.1 | 38.15±2.1 | 7.7 | 33.06±2.2 | 6.7 | 81.81±7.3 | 16.6 | 69.38±5.8 | 14.06 | 15.89±0.9 | 3.2 | 53.48±4.9 | 10.8 |
| CABA I+PTX | |||||||||||||||||||
| Adherent cells | 450.76±36.2 | 121.37±11.2 | 26.9 | 72.66±6.6 | 16.1 | 208.44±17.9 | 46.2 | 34.17±2.6 | 7.6 | 42.81±3.8 | 9.5 | 73.44±6.5 | 16.3 | 58.02±5.0 | 12.9 | 12.88±1.1 | 2.9 | 45.14±3.9 | 10.8 |
| Floating cells | 434.99±33.7 | 97.08±8.4 | 22.3 | 121.74±10.9 | 28 | 170.28±12.2 | 39.1 | 33.37±1.9 | 7.7 | 26.17±1.9 | 6.0 | 54.99±4.4 | 12.6 | 55.74±4.0 | 12.8 | 18.95±1.3 | 4.4 | 36.79±2.7 | 8.5 |
| CABA I-PTX | 397.02±29.1 | 113.01±9.1 | 28.5 | 49.81±3.2 | 12.5 | 153.51±12.8 | 38.7 | 24.54±1.7 | 6.2 | 30.11±2.1 | 7.6 | 73.61±6.9 | 18.5 | 25.25±2.1 | 6.3 | 14.01±1.0 | 3.5 | 11.24±1.1 | 2.8 |
| CABA I-PTX+PTX | 382.00±28.7 | 127.04±10.3 | 33.2 | 42.46±2.4 | 11.1 | 149.18±11.1 | 39 | 20.17±1.4 | 5.3 | 23.00±1.1 | 6 | 77.85±6.8 | 20.4 | 28.16±1.8 | 7.4 | 16.81±1.2 | 4.4 | 11.35±0.6 | 3 |
In order to further evaluate the extent of Cer involvement on the effect of PTX on both CABA cell lines, we examined the cellular response to the addition of exogenous C6-Cer. The cytotoxic effect of C6-Cer at different concentrations for 48 h of exposure was evaluated, and the IC50 for C6-Cer in CABA I cells was 20 μM and 35 μM in CABA-PTX resistant cells respectively.
Metabolic mechanisms of PTX-induced Cer production
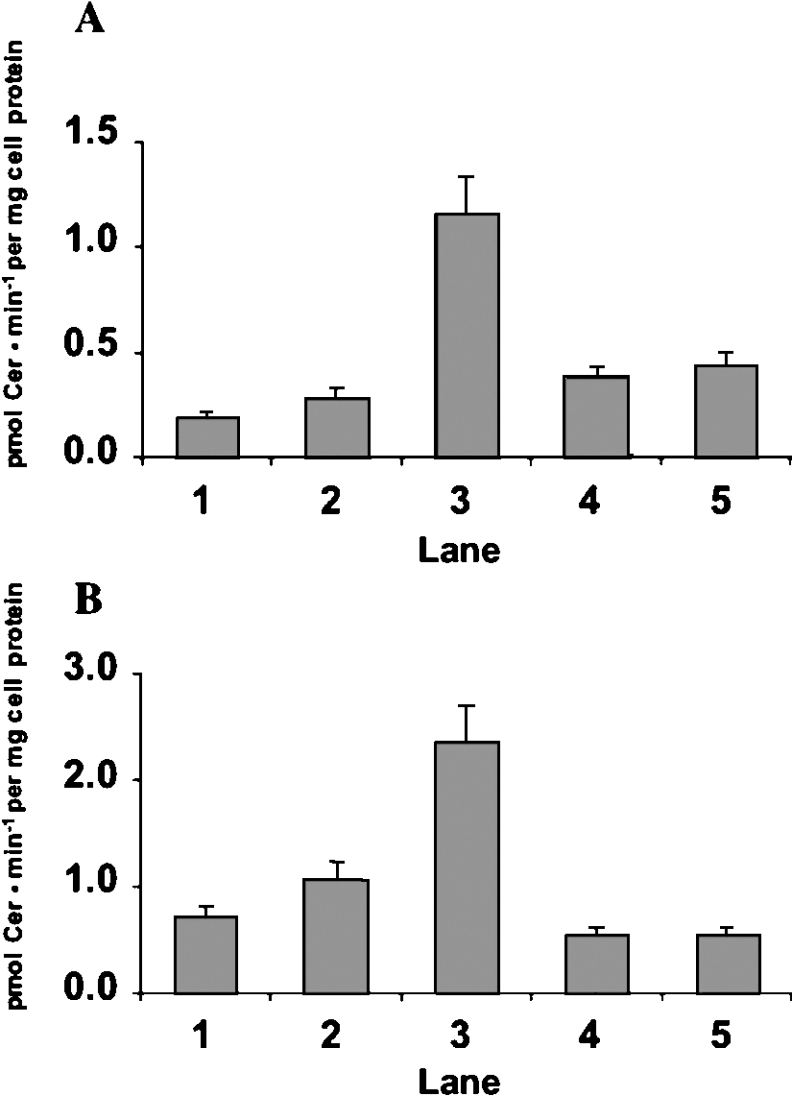
PTX treatment decreases SM levels in CABA I cells (Table 1), suggesting that PTX-induced Cer production is due to SM hydrolysis. Thus we measured the activity of acid SMase and neutral SMase in CABA I and CABA-PTX cells treated or untreated with PTX. As shown in Figure 3, treatment of CABA I cells with PTX produced a 6-fold and 3-fold increase in the in vitro activity of acid SMase (A) and neutral SMase (B) respectively in floating cells compared with control-untreated cells or adherent cells. In the case of CABA-PTX cells, the in vitro activity of acid SMase and neutral SMase was not affected by PTX treatment (Figure 3, lane 5).
Figure 3. SMase activities in CABA I and CABA-PTX cells. CABA I (lanes 1–3) and CABA-PTX (lanes 4–5) cells were treated with vehicle (lanes 1 and 4) or 2 μg/ml PTX for 24 h (lanes 2, 3 and 5).
In the case of PTX-treated CABA I cells, both adherent (lane 2) and floating (lane 3) cells were collected separately and assayed. Acid (A) and neutral (B) SMase activities were assayed in cell homogenates as described in the Materials and methods section. Negative controls were heat-inactivated cell homogenates. The reaction mixture was analysed by HPTLC. Data are expressed as Cer pmoles formed/min per mg of cell protein and are the means±S.D. for 3 independent experiments.
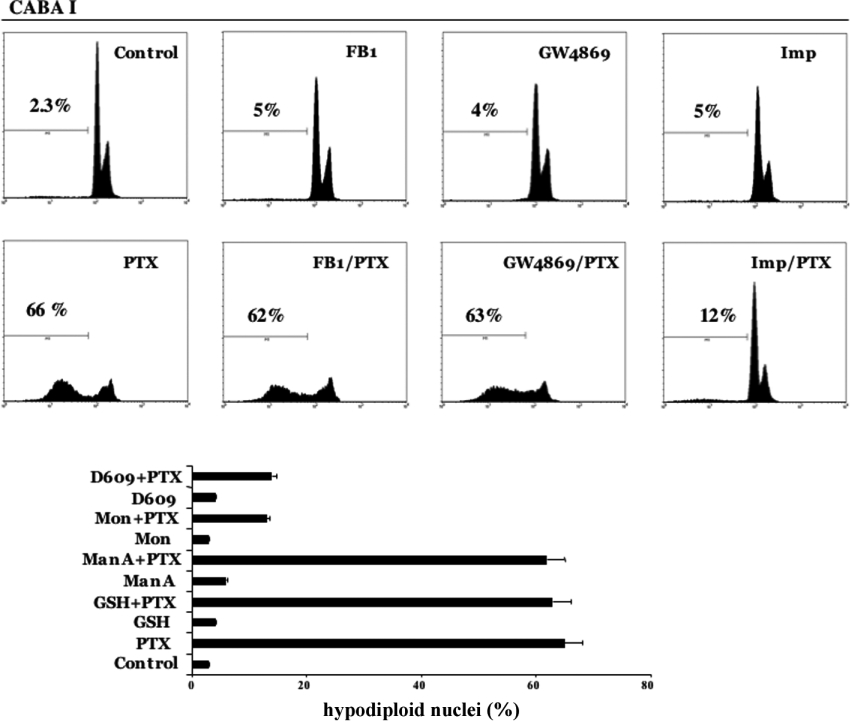
We investigated the effects on PTX-induced apoptosis by the use of specific inhibitors of SMases. When cells were exposed to 2 μg/ml PTX after a pre-treatment of 30 min with 10 mM GSH, 1 μM ManA [22] or 20 μM GW4869 [23], all of which are inhibitors of neutral SMases, no effect was observed on PTX-induced apoptosis (Figure 4).
Figure 4. Effect of inhibitors in PTX-induced apoptosis in CABA I cells.
Parental cells were treated with PTX (2 μg/ml) for 72 h in the presence or absence of several inhibitors. Data obtained for FB1 (50 μM), GW4869 (20 μM) and IMP (50 μM) are shown as flow cytometric plots, and data from GSH (10 mM), ManA (1 μM), monensin (20 μM) and D609 (50 μg/ml) are shown as histograms. The percentage of hypodiploid nuclei are reported for each condition. The results are the means±S.D. for 3 independent experiments. Mon, monensin.
When CABA I cells were incubated for 30 min before PTX treatment with 20 μM monensin, 50 μg/ml D609 [24] or 50 μM IMP [25], all inhibitors of acid SMase, the apoptotic effect of PTX was drastically decreased (Figure 4), suggesting that Cer involved in PTX-induced apoptosis in CABA I cells is generated by acid SMase. The involvement of the de novo pathway was excluded by pretreatment with 50 μM FB1, an inhibitor of Cer synthase. No anti-apoptotic effect was observed after PTX treatment (Figure 4).
Effect of PTX on sphingolipid composition in CABA I and CABA-PTX cells
To evaluate whether the acquisition of PTX resistance might be linked to an altered sphingolipid metabolism, we analysed the levels of all radioactive sphingolipids present in CABA I and CABA-PTX cells after metabolic labelling with radioactive sphingosine. The patterns of organic phase sphingolipids after HPTLC are shown in Figure 2(A). The levels of all neutral glycosphingolipids {including GlcCer, LacCer [β-Gal-(1→4)-β-Glc-(1→1)-Cer] and globosides} were similar in CABA I and in CABA-PTX cells, and they were not significantly altered by PTX treatment (Table 1). The only exception was presented by globosides, which were decreased in CABA I cells that were detached after PTX treatment.
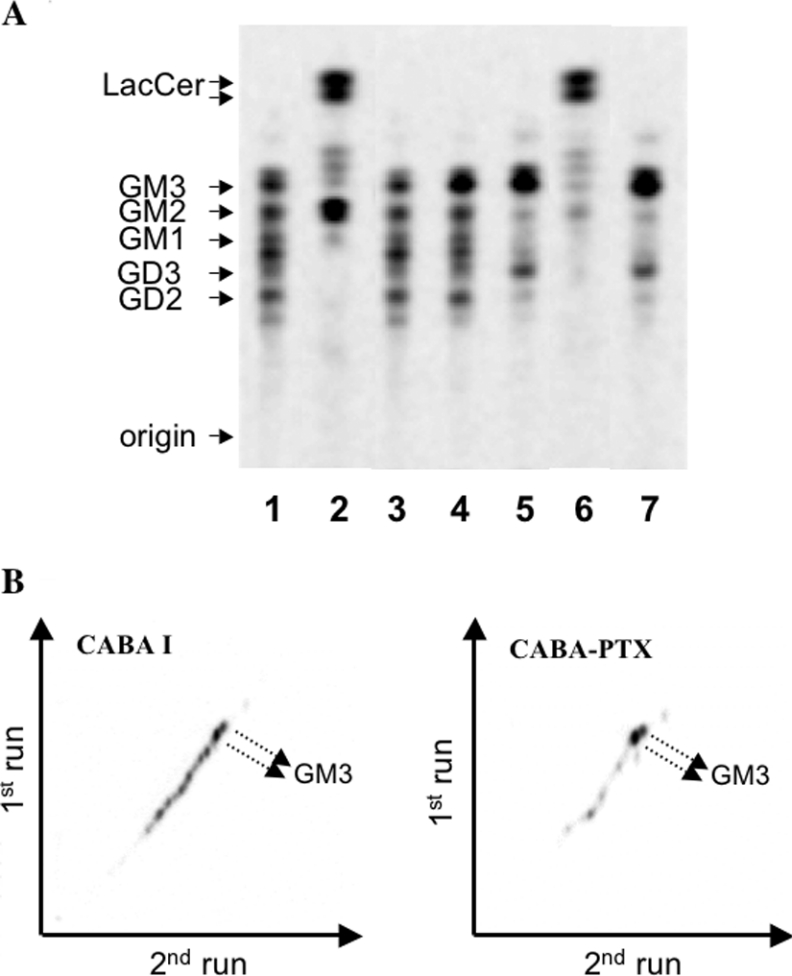
The amount of radioactivity associated with the aqueous phase (gangliosides) was much higher in CABA I than in CABA-PTX cells, independently of PTX treatment (Table 1). The ganglioside patterns present in the aqueous phase from both cell lines are shown in Figure 5(A). In CABA I cells, at least 5 major radioactive bands co-migrating with standards GM3, GM2, GM1, GD3 and GD2 were evident (Figure 5A, lane 1). In striking contrast, the ganglioside pattern in CABA-PTX cells was much simpler (Figure 5A, lane 5), with 2 main bands co-migrating with standards GM3 and GD3. Bacterial sialidase treatment of the aqueous phase from CABA-PTX and CABA I cells led to LacCer as the only hydrolysis product (Figure 5A, lanes 2 and 6); moreover CABA I cells showed the presence of GM2, probably formed after hydrolysis of GD2. From these results we can conclude that the doublet band co-migrating with the GM3 standard that is detectable in both cell lines is indeed authentic GM3, and that the lower band in CABA-PTX cells is authentic GD3. GM3 represented about 22% of total gangliosides in adherent untreated and PTX-treated CABA I cells. PTX treatment induced a slight increase in the levels of GM3 in floating treated-CABA I cells (Figure 5A, lane 4) compared with adherent untreated or treated cells. In PTX floating treated-CABA I cells GM3 increased to 34% of total gangliosides. In CABA-PTX cells, independent of PTX treatment, GM3 was by far the major cell ganglioside (approx. 56% of total gangliosides). On the other hand, the amount of radioactivity associated with gangliosides other than GM3 (mainly represented by GD3) in CABA-PTX cells was approx. 4-fold lower than in CABA I cells. Further characterization of the ganglioside mixtures from CABA I and CABA-PTX cells was accomplished by two-dimensional thin layer chromatography. Remarkably, no alkali-labile gangliosides were present in the aqueous phase obtained from CABA I and CABA-PTX cells (Figure 5B).
Figure 5. Ganglioside patterns of CABA I and CABA-PTX cells.
(A) CABA I (lanes 1–4) and CABA-PTX (lanes 5–7) cells were treated with vehicle (lanes 1, 2, 5 and 6) or 2 μg/ml PTX for 72 h (lanes 3, 4 and 7). In the case of PTX-treated CABA I cells, both adherent (lane 3) and floating (lane 4) cells were separately collected and analysed for their lipid content. For ganglioside characterization, aliquots of the aqueous phase from vehicle-treated CABA I and CABA-PTX cells were treated with Vibrio cholerae sialidase (lanes 2 and 6). The position of pure standard lipids is indicated on the left of the panel. (B) Two-dimensional ganglioside patterns in CABA I and CABA-PTX cells. The position of GM3, the main ganglioside in the mixtures, is indicated by arrows. Radioactive lipids were detected by digital autoradiography (250 d.p.m. applied on a 3 mm line; acquisition time 70 h). Patterns were representative of those obtained from 3 independent experiments.
In order to understand the possible mechanisms responsible for the observed differences in sphingolipid levels in CABA I and CABA-PTX cells, the mRNA expression of the enzymes GCS, GM3S and GD3S was evaluated in drug-sensitive and drug-resistant cells by real-time PCR assay. For all enzymes tested, resistant cells showed mRNA levels similar to those of CABA I cells (results not shown), suggesting that Cer glycosylation is not involved in PTX resistance in CABA cells. Furthermore, since ceramidases have been implied as possible regulators of cellular Cer levels [26], we measured in vitro ceramidase activity at acid, neutral and alkaline pHs in CABA I and CABA-PTX cells using [1-3H(sphingosine)]C16-Cer as substrate, without any significant difference between the sensitive and resistant cells (results not shown).
DISCUSSION
PTX is an anti-neoplastic agent that is clinically available for the treatment of a variety of solid tumours, including ovarian carcinoma [1]. Resistance to PTX has been observed and it represents a major impediment to the successful use of this agent in cancer treatment [27]. However, the exact mechanisms by which drug-induced apoptosis and resistance are accomplished have not been clearly defined.
We have previously described the derivation of a new stable PTX-resistant human ovarian carcinoma cell line, CABA-PTX, from a parental cell line CABA I, that represents a useful in vitro model to investigate the mechanisms of PTX multifactorial acquired resistance in ovarian cancer. Despite the observation that drug resistance in tumour cells is often associated with the overexpression of MDR proteins, in CABA-PTX cells the level of these proteins is very similar to that in parental cells [4].
In the present study, we showed that the cytotoxic effect of PTX in CABA I cells is due to an apoptotic mechanism, and we have identified Cer as the mediator molecule in PTX-induced apoptosis of these cells. This finding is in agreement with the undisputed link between Cer and sensitivity to anti-tumour drugs that is emerging in the literature. Cer generation during anti-tumour drug-induced apoptosis can be accomplished by SM hydrolysis due to the action of acid SMase and/or neutral SMases [28] or by the activation of Cer-synthase [29]. In CABA I cells, cellular Cer elevation upon PTX-induced apoptosis is accompanied by a decrease in the cellular levels of SM. These changes in the Cer and SM content are concomitant with 6-fold and 3-fold increases in the in vitro activity of acid SMase and neutral SMase respectively. Incubation of CABA I cells with FB1 [30] (an inhibitor of Cer-synthase) or with inhibitors of neutral SMases [22,23] did not affect PTX-induced apoptosis. On the other hand, different inhibitors of acid Smase [24,25] were able to prevent the onset of apoptosis by PTX treatment in CABA I cells. Taken together, these data suggest that the generation of Cer that is responsible for PTX-induced apoptosis is due to the activation of SM hydrolysis by acid SMase.
The second piece of evidence from our work is that PTX-resistance is associated with a lack of increased cellular Cer upon drug treatment. In MDR cells, this seems to be due to increased GCS expression and/or activity [11]. In human ovarian carcinoma cells resistant to fenretinide [8], with a resistant phenotype unrelated to MDR proteins, increased expression of GM3S leading to elevated cellular GM3 levels could represent another metabolic mechanism to remove Cer by converting it into glycosylated metabolites.
However, in CABA-PTX cells the expression of GCS and the levels of GlcCer and other neutral sphingolipids were identical to those in CABA I cells. The total ganglioside content was indeed lower in drug-resistant than in drug-sensitive cells, whereas GM3 levels and the expression of GM3S and GD3S were similar in the two cell lines. Thus we can conclude that in these cells, removal of Cer by enhanced glycosylation is not the mechanism underlying the acquisition of drug resistance.
On the other hand CABA I and CABA-PTX cells differ greatly in their ganglioside composition. The total ganglioside content is approx. 2.5-fold higher in sensitive than in resistant cells, whereas the GM3 content is almost identical. CABA I and CABA-PTX gangliosides are alkali-stable, excluding the differential expression of alkali-labile O-acetylated gangliosides, a proposed mechanism to regulate apoptosis in tumour cells [31]. Greater changes in the expression patterns of gangliosides were observed in MDR cells, such as 2780AD human ovarian carcinoma cells [12] and neuroblastoma cells [14]. Complex sphingolipids in the cell membrane are organized together with selected proteins into multimolecular assemblies that can be isolated as detergent-insoluble low density material (reviewed in [32]). These membrane structures are regarded as complex organizers of protein–lipid interactions involved in signal transduction. Thus changes in cellular sphingolipid composition could affect several different properties of the tumour cell that require the organization of cell membrane components into detergent-insoluble membrane domains, contributing to the onset of resistant phenotypes. Acid and neutral SMases are activated by PTX in drug-sensitive cells, but are not affected in resistant cells, and our data suggest that the regulation of SMase activity is likely to be responsible for the lack of apoptosis due to raised cellular Cer levels in this resistant phenotype. Both neutral [33] and acid SMases [34] have been reported to be enriched in detergent-insoluble membrane domains, and it has been suggested that these membrane areas could represent the cellular site where the generation of Cer signalling occurs [35]. Interestingly, it has been recently shown [36] that cisplatin induces clustering of CD95 into detergent-insoluble membrane domains in human colon cancer cells, and that acid SMase is required for this event. Therefore we can hypothesize that altered sphingolipid composition associated with drug resistance in tumour cells might be responsible for the negative modulation of SMase(s) activity at the level of detergent-insoluble membrane domains. A greater understanding of the mechanisms responsible for the changes in ganglioside composition in the resistant cell line is needed to define the mode whereby SMases activity is modulated upon the acquisition of the resistant phenotype. A possibility is that the expression levels or activity of key enzymes in glycosphingolipid biosynthesis are differentially modulated in CABA-PTX versus CABA I cells, as observed for GCS in MDR cells [10,11] and for GM3S in fenretinide-resistant ovarian carcinoma cells [8]. Alternatively, we can hypothesize that trafficking of glycolipids along the biosynthetic pathway in the Golgi is altered, as observed in MDR ovarian cancer cells [12].
Acknowledgments
This work was supported by grants from the Italian Cofinanziamento Progetti di Ricerca di Rilevante Interesse Nazionale (COFIN-PRIN) project, and Ministero della Sanità Fondo per gli Investimenti della Ricerca di Base (FIRB) and Associazione Italiana per la Ricerca sul Cancro (AIRC) projects.
References
- 1.Spencer C. M., Faulds D. Paclitaxel. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in the treatment of cancer. Drugs. 1994;48:794–847. doi: 10.2165/00003495-199448050-00009. [DOI] [PubMed] [Google Scholar]
- 2.Colozza M., Mosconi A. M., Gori S., Belanti V., Basurto C., De Angelis V., Giansanti M., Tonato M. Long-term results in patients with advanced epithelial ovarian carcinoma treated with a combination of cisplatin, doxorubicin, and cyclophosphamide. Am. J. Clin. Oncol. 1997;20:522–526. doi: 10.1097/00000421-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y., Ibrado A. M., Reed J. C., Bullock G., Ray S., Tang C., Bhalla K. Co-expression of several molecular mechanisms of multidrug resistance and their significance for paclitaxel cytotoxicity in human AML HL-60 cells. Leukemia. 1997;11:253–257. doi: 10.1038/sj.leu.2400557. [DOI] [PubMed] [Google Scholar]
- 4.Violini S., D'Ascenzo S., Bagnoli M., Millimaggi D., Miotti S., Canevari S., Pavan A., Dolo V. Induction of a multifactorial resistance phenotype by high paclitaxel selective pressure in a human ovarian carcinoma cell line. J. Exp. Clin. Cancer Res. 2004;23:83–91. [PubMed] [Google Scholar]
- 5.Dolo V., Ginestra A., Violini S., Miotti S., Festuccia C., Miceli D., Migliavacca M., Rinaudo C., Romano F. M., Brisdelli F., et al. Ultrastructural and phenotypic characterization of CABA I, a new human ovarian cancer cell line. Oncol. Res. 1997;9:129–138. [PubMed] [Google Scholar]
- 6.Tolomeo M., Simoni D. Drug resistance and apoptosis in cancer treatment: development of new apoptosis-inducing agents active in drug resistant malignancies. Curr. Med. Chem. Anti-Canc. Agents. 2002;2:387–401. doi: 10.2174/1568011024606361. [DOI] [PubMed] [Google Scholar]
- 7.Mimeault M. New advances on structural and biological functions of ceramide in apoptotic/necrotic cell death and cancer. FEBS Lett. 2002;530:9–16. doi: 10.1016/s0014-5793(02)03432-4. [DOI] [PubMed] [Google Scholar]
- 8.Prinetti A., Basso L., Appierto V., Villani M. G., Valsecchi M., Loberto N., Prioni S., Chigorno V., Cavadini E., Formelli F., Sonnino S. Altered sphingolipid metabolism in N-(4-hydroxyphenyl)-retinamide-resistant A2780 human ovarian carcinoma cells. J. Biol. Chem. 2003;278:5574–5583. doi: 10.1074/jbc.M207269200. [DOI] [PubMed] [Google Scholar]
- 9.Cai Z., Bettaieb A., Mahdani N. E., Legres L. G., Stancou R., Masliah J., Chouaib S. Alteration of the sphingomyelin/ceramide pathway is associated with resistance of human breast carcinoma MCF7 cells to tumor necrosis factor-alphamediated cytotoxicity. J. Biol. Chem. 1997;272:6918–6926. doi: 10.1074/jbc.272.11.6918. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y. Y., Han T. Y., Giuliano A. E., Cabot M. C. Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J. Biol. Chem. 1999;274:1140–1146. doi: 10.1074/jbc.274.2.1140. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y. Y., Han T. Y., Giuliano A. E., Cabot M. C. Ceramide glycosylation potentiates cellular multidrug resistance. FASEB J. 2001;15:719–730. doi: 10.1096/fj.00-0223com. [DOI] [PubMed] [Google Scholar]
- 12.Veldman R. J., Klappe K., Hinrichs J., Hummel I., van der Schaaf G., Sietsma H., Kok J. W. Altered sphingolipid metabolism in multidrug-resistant ovarian cancer cells is due to uncoupling of glycolipid biosynthesis in the Golgi apparatus. FASEB J. 2002;16:1111–1113. doi: 10.1096/fj.01-0863fje. [DOI] [PubMed] [Google Scholar]
- 13.Olshefski R. S., Ladisch S. Glucosylceramide synthase inhibition enhances vincristine-induced cytotoxicity. Int. J. Cancer. 2001;93:131–181. doi: 10.1002/ijc.1301. [DOI] [PubMed] [Google Scholar]
- 14.Dijkhuis A. J., Douwes J., Kamps W., Sietsma H., Kok J. W. Differential expression of sphingolipids in P-glycoprotein or multidrug resistance-related protein 1 expressing human neuroblastoma cell lines. FEBS Lett. 2003;31:28–32. doi: 10.1016/s0014-5793(03)00721-x. [DOI] [PubMed] [Google Scholar]
- 15.Negroni E., Chigorno V., Tettamanti G., Sonnino S. Evaluation of the efficiency of an assay procedure for gangliosides in human serum. Glycoconjugate J. 1996;13:347–352. doi: 10.1007/BF00731466. [DOI] [PubMed] [Google Scholar]
- 16.Prinetti A., Chigorno V., Tettamanti G., Sonnino S. Sphingolipid-enriched membrane domains from rat cerebellar granule cells differentiated in culture. A compositional study. J. Biol. Chem. 2000;275:11658–11665. doi: 10.1074/jbc.275.16.11658. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y. J., Shacter E. Oxidative stress inhibits apoptosis in human lymphoma cells. J. Biol. Chem. 1999;274:19792–19798. doi: 10.1074/jbc.274.28.19792. [DOI] [PubMed] [Google Scholar]
- 18.Prinetti A., Chigorno V., Prioni S., Loberto N., Marano N., Tettamanti G., Sonnino S. Changes in the lipid turnover, composition, and organization, as sphingolipid-enriched membrane domains, in rat cerebellar granule cells developing in vitro. J. Biol. Chem. 2001;276:21136–21145. doi: 10.1074/jbc.M010666200. [DOI] [PubMed] [Google Scholar]
- 19.Sonnino S., Ghidoni R., Chigorno V., Masserini M., Tettamanti G. Recognition by two-dimensional thin-layer chromatography and densitometric quantification of alkali-labile gangliosides from the brain of different animals. Anal. Biochem. 1983;128:104–114. doi: 10.1016/0003-2697(83)90350-0. [DOI] [PubMed] [Google Scholar]
- 20.Bielawska A., Greenberg M. S., Perry D., Jayadev S., Shayman J. A., McKay C., Hannun Y. A. (1S,2R)-D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol as an inhibitor of ceramidase. J. Biol. Chem. 1996;271:12646–12654. doi: 10.1074/jbc.271.21.12646. [DOI] [PubMed] [Google Scholar]
- 21.Riboni L., Viani P., Bassi R., Stabilini A., Tettamanti G. Biomodulatory role of ceramide in basic fibroblast growth factor-induced proliferation of cerebellar astrocytes in primary culture. Glia. 2000;32:137–145. doi: 10.1002/1098-1136(200011)32:2<137::aid-glia30>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Arenz C., Gartner M., Wascholowski V., Giannis A. Synthesis and biochemical investigation of Scyphostatin analogues as inhibitors of neutral sphingomyelinase. Bioorg. Med. Chem. 2001;9:2901–2904. doi: 10.1016/s0968-0896(01)00165-1. [DOI] [PubMed] [Google Scholar]
- 23.Kolmakova A., Kwiterovich P., Virgil D., Alaupovic P., Knight-Gibson C., Martin S. F., Chatterjee S. Apolipoprotein C-I induces apoptosis in human aortic smooth muscle cells via recruiting neutral sphingomyelinase. Arterioscler. Thromb. Vasc. Biol. 2004;24:264–269. doi: 10.1161/01.ATV.0000112036.72200.ac. [DOI] [PubMed] [Google Scholar]
- 24.Cifone M. G., Migliorati G., Parroni R., Marchetti C., Millimaggi D., Santoni A., Riccardi C. Dexamethasone-induced thymocyte apoptosis: apoptotic signal involves the sequential activation of phosphoinositide-specific phospholipase C, acidic sphingomyelinase, and caspases. Blood. 1999;93:2282–2296. [PubMed] [Google Scholar]
- 25.Osawa Y., Uchinami H., Bielawski J., Schwabe R. F., Hannun Y. A., Brenner D. A. Roles for C16-ceramide and sphingosine-1-phosphate in regulating hepatocyte apoptosis in response to TNF-α. J. Biol. Chem. 2005 doi: 10.1074/jbc.M503002200. in the press. [DOI] [PubMed] [Google Scholar]
- 26.El Bawab S., Mao C., Obeid L. M., Hannun Y. A. Ceramidases in the regulation of ceramide levels and function. Subcell. Biochem. 2002;36:187–205. doi: 10.1007/0-306-47931-1_10. [DOI] [PubMed] [Google Scholar]
- 27.Milas L., Hunter N. R., Kurdoglu B., Mason K. A., Meyn R. E., Stephens L. C., Peters L. J. Kinetics of mitotic arrest and apoptosis in murine mammary and ovarian tumors treated with taxol. Cancer Chemother. Pharmacol. 1995;35:297–303. doi: 10.1007/BF00689448. [DOI] [PubMed] [Google Scholar]
- 28.Hannun Y. A. The sphingomyelin cycle and the second messenger function of ceramide. J. Biol. Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 29.Bose R., Verheij M., Haimovitz-Friedman A., Scotto K., Fuks Z., Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 30.Strle K., Broussard S. R., McCusker R. H., Shen W. H., Johnson R. W., Freund G. G., Dantzer R., Kelley K. W. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology. 2004;145:4592–4602. doi: 10.1210/en.2003-1749. [DOI] [PubMed] [Google Scholar]
- 31.Malisan F., Franchi L., Tommasini B., Ventura N., Condo I., Rippo M. R., Rufini A., Liberati L., Nachtigall C., Kniep B., Testi R. Acetylation suppresses the proapoptotic activity of GD3 ganglioside. J. Exp. Med. 2002;169:1535–1541. doi: 10.1084/jem.20020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hakomori S., Handa K., Iwabuchi K., Yamamura S., Prinetti A. New insights in glycosphingolipid function: “glycosignaling domain,” a cell surface assembly of glycosphingolipids with signal transducer molecules, involved in cell adhesion coupled with signaling. Glycobiology. 1998;8:11–19. doi: 10.1093/oxfordjournals.glycob.a018822. [DOI] [PubMed] [Google Scholar]
- 33.Kilkus J., Goswami R., Testai F. D., Dawson G. Ceramide in rafts (detergent-insoluble fraction) mediates cell death in neurotumor cell lines. J. Neurosci. Res. 2003;72:65–75. doi: 10.1002/jnr.10549. [DOI] [PubMed] [Google Scholar]
- 34.Gulbins E., Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 35.Bezombes C., Laurent G., Jaffrezou J. P. Implication of raft microdomains in drug induced apoptosis. Curr. Med. Chem. Anti-Canc. Agents. 2003;3:263–270. doi: 10.2174/1568011033482413. [DOI] [PubMed] [Google Scholar]
- 36.Lacour S., Hammann A., Grazide S., Lagadic-Gossmann D., Athias A., Sergent O., Laurent G., Gambert P., Solary E., Dimanche-Boitrel M. T. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593–3598. doi: 10.1158/0008-5472.CAN-03-2787. [DOI] [PubMed] [Google Scholar]
- 37.Svennerholm L. Ganglioside designation. Adv. Exp. Biol. Med. 1980;125:11. doi: 10.1007/978-1-4684-7844-0_2. [DOI] [PubMed] [Google Scholar]
- 38.IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. Pure Appl. Chem. 1997;69:2475–2487. [Google Scholar]
- 39.IUPAC-IUBMB Joint Commission on Biochemical Nomenclature. Carbohydr. Res. 1998;312:167–175. [Google Scholar]