Abstract
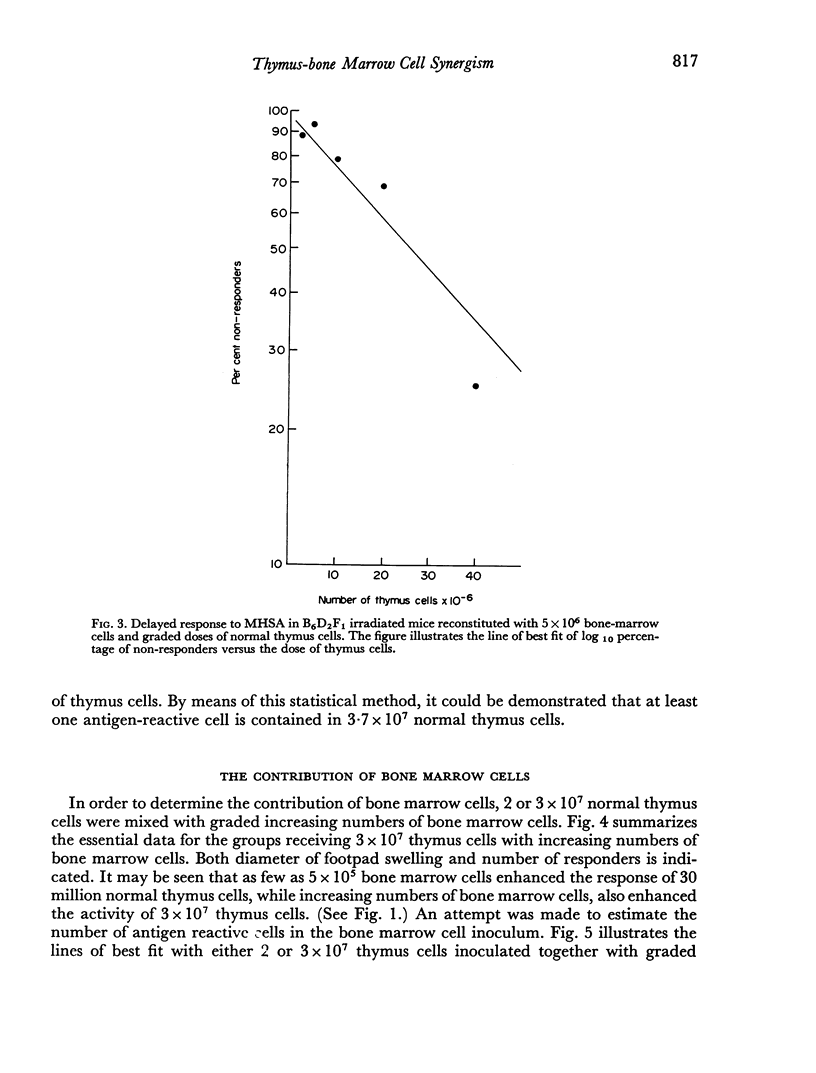
The contribution of syngeneic bone marrow and thymus cell populations obtained from normal and cortisone-treated donor mice for reconstitution of delayed hypersensitivity to methylated human serum albumin (MHSA) was studied in lethally irradiated recipients employing a limiting dilution assay. Normal thymus cells contained at least one antigen-reactive cell (or cell unit) per 3.7 × 107 cells, while cortisone-resistant cell populations contained one cell per 6.7 × 105 cells. Thus, cells immunocompetent to MHSA are not destroyed by steroid. Bone marrow cell populations contribute effector cells, and as few as 2 × 106 cells are capable of fully restoring delayed response when combined with optimum numbers of thymus cells. Large numbers of thymus cells alone will restore immune responsiveness, indicating that such populations do contain some effector cells, either as contaminating blood borne cells, or as glandular cells derived possibly from sinusoids. Similarly, bone marrow cells in larger numbers restore responsiveness, implying contamination with thymus-derived cells.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Cantor H., Asofsky R. Synergy among lymphoid cells mediating the graft-versus-host response. 3. Evidence for interaction between two types of thymus-derived cells. J Exp Med. 1972 Apr 1;135(4):764–779. doi: 10.1084/jem.135.4.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Asofsky R. Synergy among lymphoid cells mediating the graft-versus-host response. II. Synergy in graft-versus-host reactions produced by Balb-c lymphoid cells of differing anatomic origin. J Exp Med. 1970 Feb;131(2):235–246. doi: 10.1084/jem.131.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Fschbach M., Claman H. N. Hydrocortisne resistance of graft vs host activity in mouse thymus, spleen and bone marrow. J Immunol. 1970 Nov;105(5):1146–1150. [PubMed] [Google Scholar]
- Crowle A. J., Patrucco A. Preferential development by mice of delayed hypersensitivity to purified basic proteins. J Allergy. 1968 Sep;42(3):140–156. doi: 10.1016/0021-8707(68)90087-7. [DOI] [PubMed] [Google Scholar]
- Eidinger D., Ackerman A. A cellular deficit in the reconstitutive capacity of immune populations of lymphoid cells demonstrable in studies of delayed hypersensitivity in mice. Evidence for thymus-bone marrow cell synergism. J Exp Med. 1971 May 1;133(5):1061–1073. doi: 10.1084/jem.133.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidinger D., Pross H. Studies of antibody formation in vitro and in lethally irradiated reconstituted mice. Evidence for an inhibitory function of thymus-derived cells. Scand J Immunol. 1972;1(3):193–203. doi: 10.1111/j.1365-3083.1972.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Feldman J. D., Unanue E. R. Role of macrophages in delayed hypersensitivity. II. Effects of anti-macrophage antibody. Cell Immunol. 1971 Jun;2(3):275–282. doi: 10.1016/0008-8749(71)90047-5. [DOI] [PubMed] [Google Scholar]
- Grant C. K., Currie G. A., Alexander P. Thymocytes from mice immunized against an allograft render bone-marrow cells specifically cytotoxic. J Exp Med. 1972 Jan;135(1):150–164. doi: 10.1084/jem.135.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday W. J., Webb M. Delayed hypersensitivity to chemically induced tumors in mice and correlation with an in vitro test. J Natl Cancer Inst. 1969 Jul;43(1):141–150. [PubMed] [Google Scholar]
- Hilgard H. R. Synergism of thymus and bone marrow in the production of gra a5hilgard HR: Synergism of thymus and bone marrow in the production of graft-versus-host splenomegaly in x-irradiated hosts. J Exp Med. 1970 Aug 1;132(2):317–328. doi: 10.1084/jem.132.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiki A. T., Parish C. R. Cleavage of bacterial flagellin with proteolytic enzymes. II. Induction of enhanced delayed-type hypersensitivity to flagellin by peptic fragments of the protein. Cell Immunol. 1972 Jul;4(3):264–278. doi: 10.1016/0008-8749(72)90030-5. [DOI] [PubMed] [Google Scholar]
- Kennedy J. C., Till J. E., Siminovitch L., McCulloch E. A. The proliferative capacity of antigen-sensitive precursors of hemolytic plaque-forming cells. J Immunol. 1966 Jun;96(6):973–980. [PubMed] [Google Scholar]
- Lev W. R., Robbins J. H. Antigen-induced blastogenesis: the human cell determining the specificity of response in vitro. J Immunol. 1970 May;104(5):1295–1299. [PubMed] [Google Scholar]
- Lonai P., Feldman M. Cooperation of lymphoid cells in an in vitro graft reaction system. The role of the thymus cell. Transplantation. 1970 Nov;10(5):372–381. doi: 10.1097/00007890-197011000-00003. [DOI] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Bone marrow as source of cells in reactions of cellular hypersensitivity. II. Identification of allogeneic or hybrid cells by immunofluorescence in passively transferred tuberculin reactions. J Exp Med. 1968 Dec 1;128(6):1437–1447. doi: 10.1084/jem.128.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Delayed hypersensitivity: bone marrow as the source of cells in delayed skin reactions. Science. 1967 Jul 21;157(3786):322–323. doi: 10.1126/science.157.3786.322. [DOI] [PubMed] [Google Scholar]
- Parish C. R., Liew F. Y. Immune response to chemically modified flagellin. 3. Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J Exp Med. 1972 Feb 1;135(2):298–311. doi: 10.1084/jem.135.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A., Collins F. M. The restorative effect of peritoneal macrophages on delayed hypersensitivity following ionizing radiation. Cell Immunol. 1971 Dec;2(6):552–566. doi: 10.1016/0008-8749(71)90004-9. [DOI] [PubMed] [Google Scholar]
- WEISSMANN G., THOMAS L. Studies on lysosomes. II. The effect of cortisone on the release of acid hydrolases from a large granule fraction of rabbit liver induced by an excess of vitamin A. J Clin Invest. 1963 May;42:661–669. doi: 10.1172/JCI104757. [DOI] [PMC free article] [PubMed] [Google Scholar]


