Abstract
The capacity of CBA mouse B lymphocytes to bind polyvalent rabbit anti-mouse immunoglobulin antibody, even when this reagent is present in low concentration, was used as an index of a lymphocyte's B cell status. Various mouse tissues were held for 30 minutes at 0° with 0.2 μ/ml of 125I-labelled anti-Ig, and smear preparations of washed cells were examined, following radioautography, for their content of B cells. The survey covered two areas of B cell differentiation, namely the spontaneous emergence of B cells in the mouse foetus, and the development of B cells in lethally irradiated mice that had received early foetal liver as a source of haematogenous stem cells.
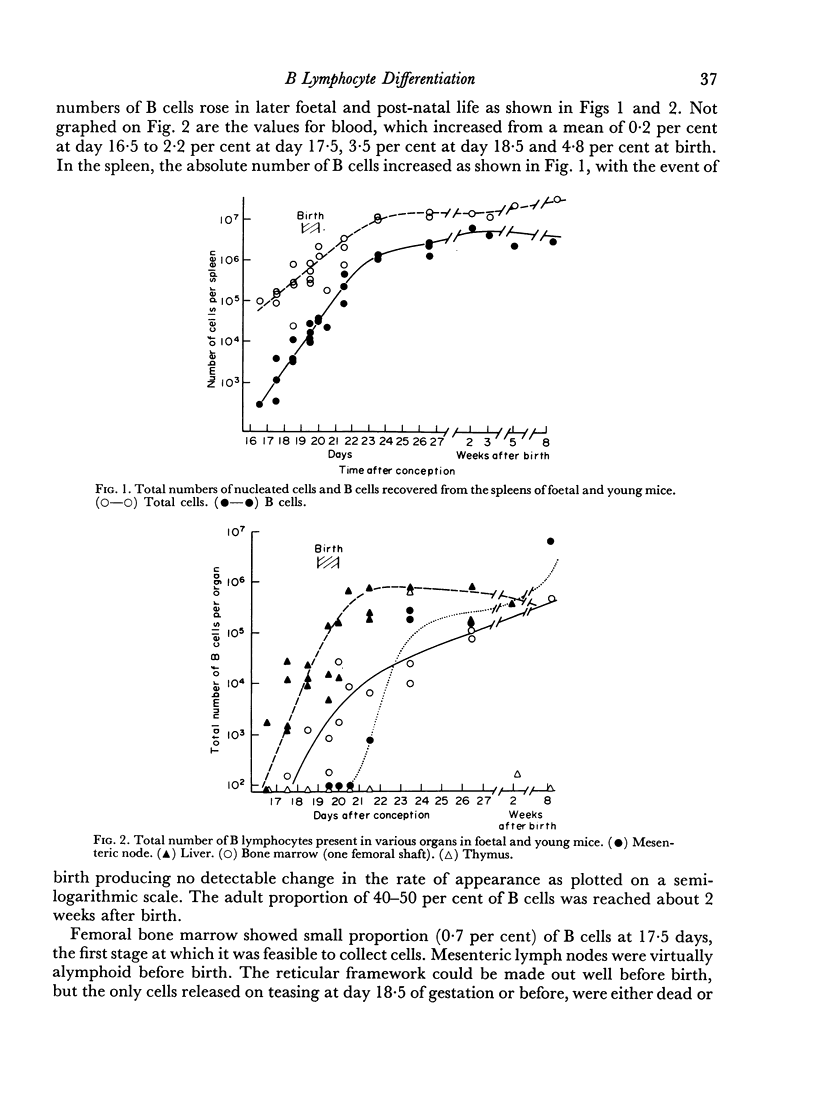
The foetal survey suggested a multifocal origin of B cells, commencing 3 days before birth, in all major sites of erythromyelopoiesis, namely liver, spleen and bone marrow. Lymph nodes showed B cells later than did these organs, and thymus contained virtually no B cells at any stage. A rapid influx of B cells into lymph nodes took place shortly after birth. The CBA mouse is born with somewhat over 105 B lymphocytes in toto, of which the majority are in liver, spleen and blood.
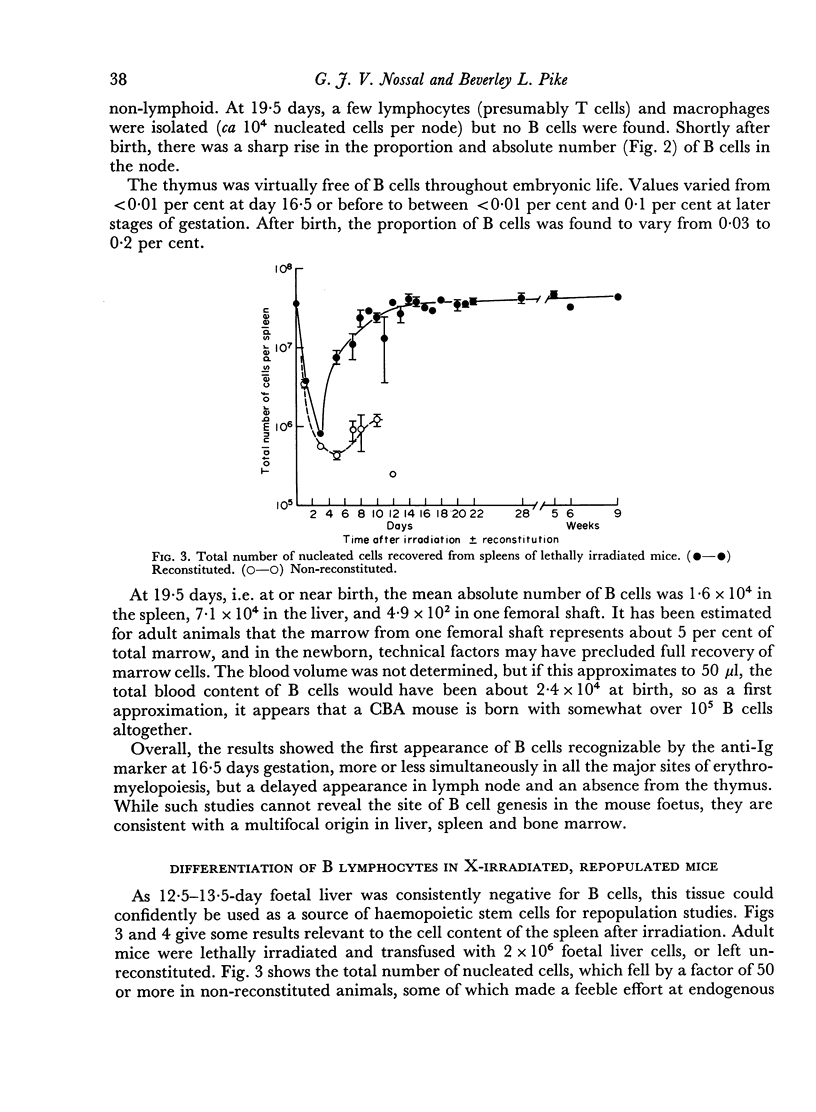
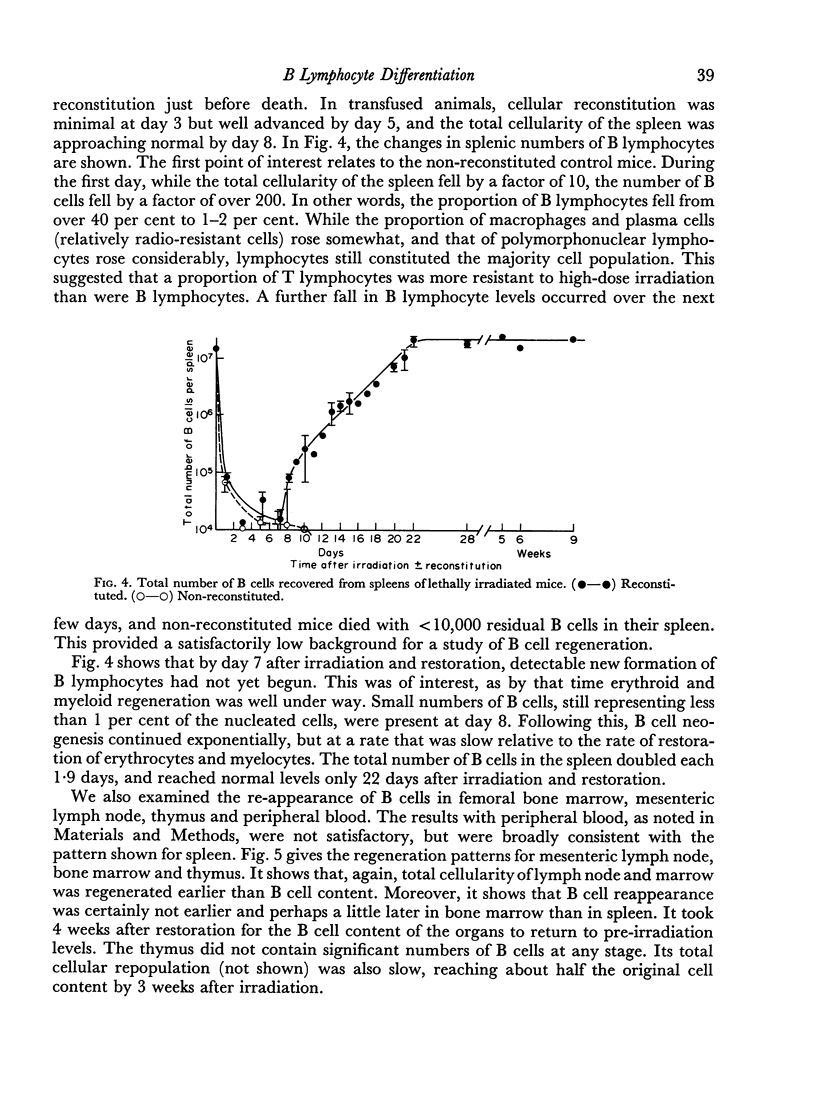
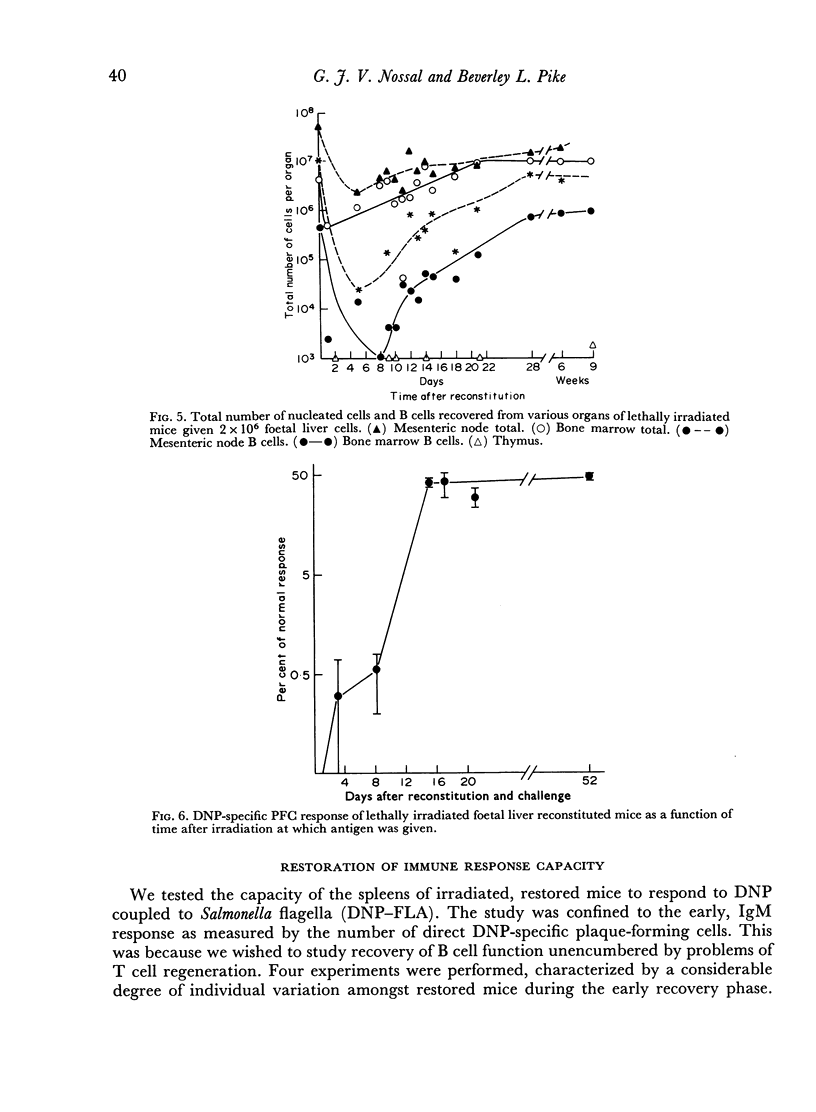
The irradiation-recovery study showed: (1) that B cells are, statistically, more sensitive to high dose irradiation than T cells; (2) that a B cell-free stem cell source can repopulate the B cell pool; (3) that repopulation is surprisingly slow; and (4) that numerical and functional recovery of B cells parallel each other to a reasonable degree.
The significance of the results is discussed from the viewpoints of lymphocyte differentiation and immunological tolerance.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basten A., Miller J. F., Sprent J., Pye J. A receptor for antibody on B lymphocytes. I. Method of detection and functional significance. J Exp Med. 1972 Mar 1;135(3):610–626. doi: 10.1084/jem.135.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahim F., Osmond D. G. Migration of bone marrow lymphocytes demonstrated by selective bone marrow labeling with thymidine-H3. Anat Rec. 1970 Oct;168(2):139–159. doi: 10.1002/ar.1091680202. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A. Immunologic complementation between thymus and marrow cells--a model for the two-cell theory of immunocompetence. Transplant Rev. 1969;1:92–113. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Everett N. B., Tyler R. W. Lymphopoiesis in the thymus and other tissues: functional implications. Int Rev Cytol. 1967;22:205–237. doi: 10.1016/s0074-7696(08)61836-7. [DOI] [PubMed] [Google Scholar]
- Feldmann M. Induction of immunity and tolerance in vitro by hapten protein conjugates. I. The relationship between the degree of hapten conjugation and the immunogenicity of dinitrophenylated polymerized flagellin. J Exp Med. 1972 Apr 1;135(4):735–753. doi: 10.1084/jem.135.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F. Biological effects of anti-immunoglobulins: evidence for immunoglobulin receptors on 'T' and 'B' lymphocytes. Transplant Rev. 1970;5:45–75. doi: 10.1111/j.1600-065x.1970.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Gregory C. J., Lajtha L. G. Recovery of immune responsiveness in lethally-irradiated mice protected with syngeneic marrow cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(2):117–126. doi: 10.1080/09553007014550161. [DOI] [PubMed] [Google Scholar]
- Kincade P. W., Cooper M. D. Development and distribution of immunoglobulin-containing cells in the chicken. An immunofluorescent analysis using purified antibodies to mu, gamma and light chains. J Immunol. 1971 Feb;106(2):371–382. [PubMed] [Google Scholar]
- Lafleur L., Miller R. G., Phillips R. A. A quantitative assay for the progenitors of bone marrow-associated lymphocytes. J Exp Med. 1972 Jun 1;135(6):1363–1374. doi: 10.1084/jem.135.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Owen J. J. Experimental studies on the development of the thymus. J Exp Med. 1967 Oct 1;126(4):715–726. doi: 10.1084/jem.126.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Lewis H. Variation in accessible cell surface immunoglobulin among antibody-firming cells. J Exp Med. 1972 Jun 1;135(6):1416–1422. doi: 10.1084/jem.135.6.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J. J., Raff M. C. Studies on the differentiation of thymus-derived lymphocytes. J Exp Med. 1970 Dec 1;132(6):1216–1232. doi: 10.1084/jem.132.6.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Williams N., Adams P. The separation of different cell classes from lymphoid organs. V. Simple procedures for the removal of cell debris. Damaged cells and erythroid cells from lymphoid cell suspensions. J Immunol Methods. 1972 May;1(3):273–287. doi: 10.1016/0022-1759(72)90005-1. [DOI] [PubMed] [Google Scholar]
- Weissman I. L. Thymus cell migration. J Exp Med. 1967 Aug 1;126(2):291–304. doi: 10.1084/jem.126.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J., Lewis H. Metabolic characteristics of lymphocyte surface immunoglobulins. Eur J Immunol. 1972 Jun;2(3):225–232. doi: 10.1002/eji.1830020306. [DOI] [PubMed] [Google Scholar]