Abstract
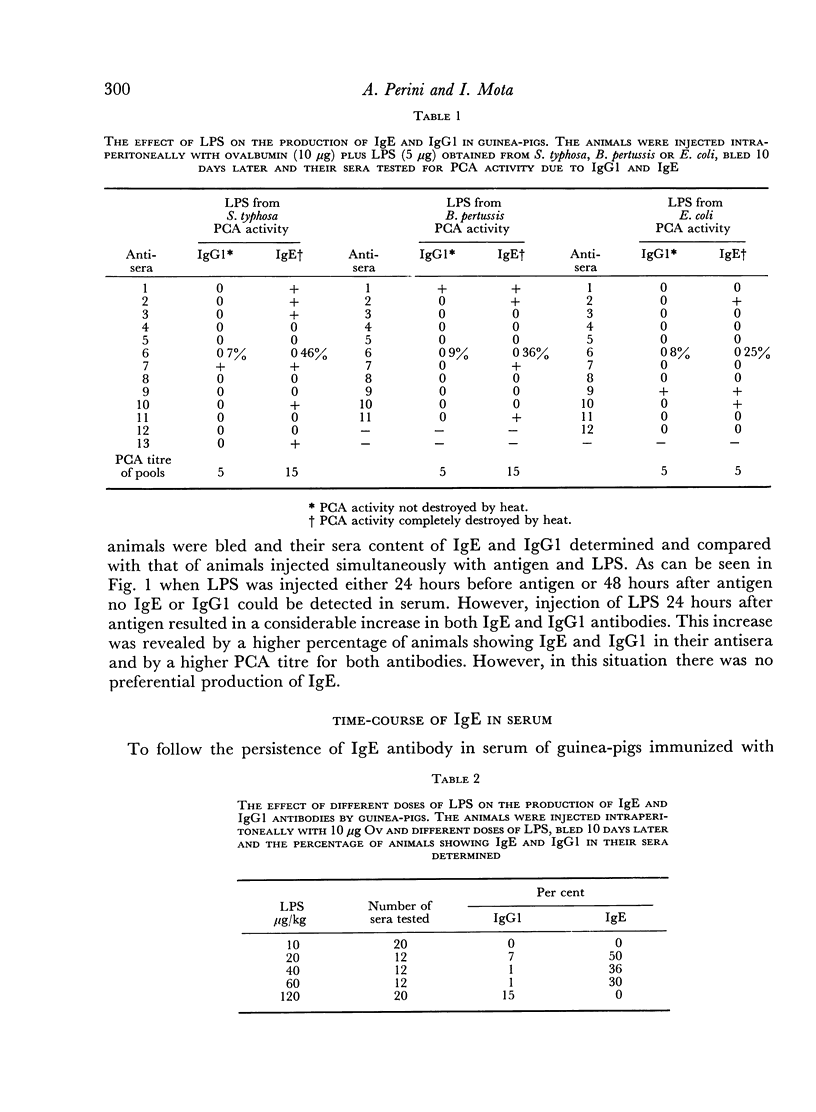
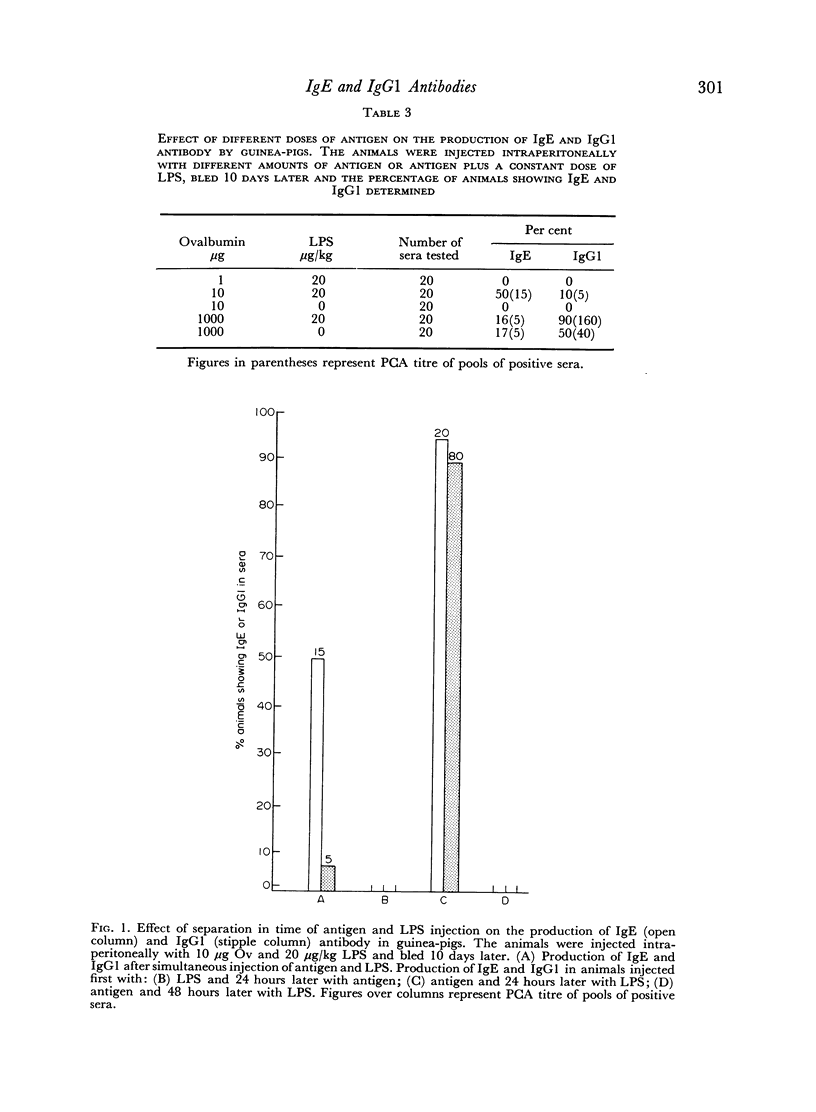
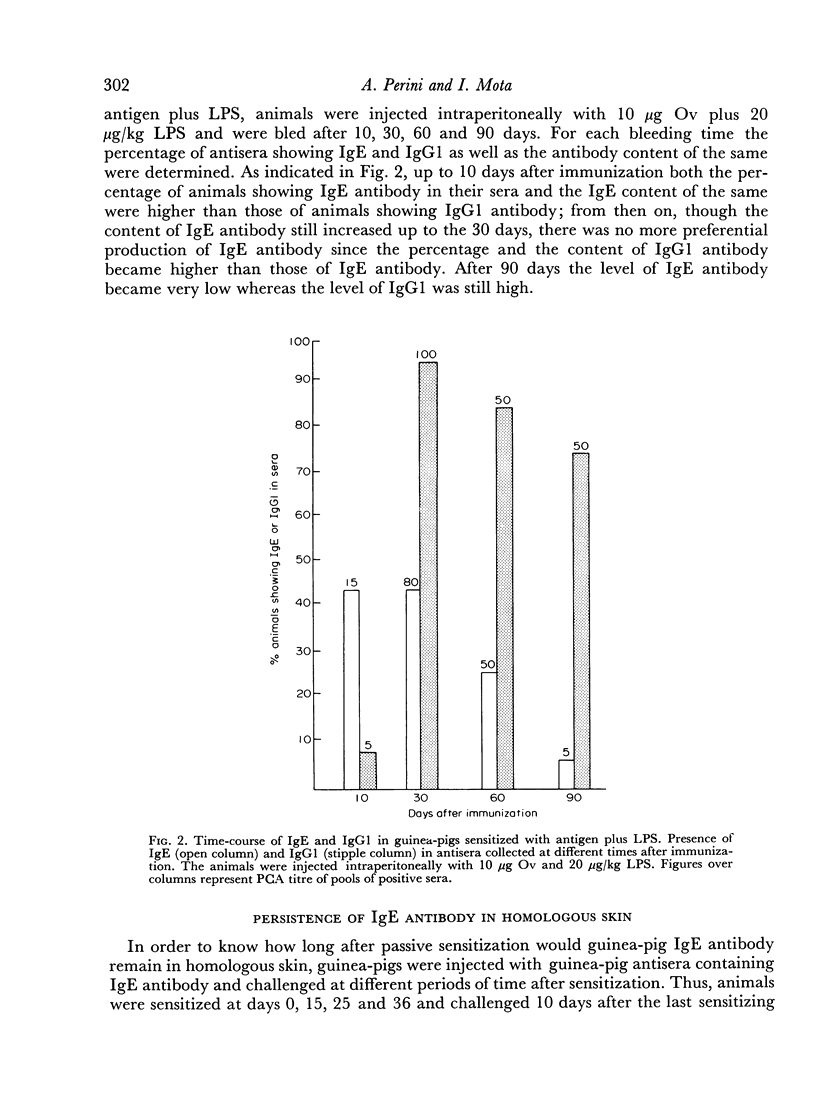
The adjuvant action of lipopolysaccharide (LPS) on the IgE and IgG1 antibody production by guinea-pigs was studied. It was observed that LPS induces an early preferential production of IgE antibody. The optimal dose of LPS to cause this effect was of the order of 20 μg/kg. Low amounts of LPS as well as low amounts of antigen favoured the production of IgE antibody whereas high amounts of LPS or antigen favoured the production of IgG1. Later, in the course of immunization, the IgE antibody content decreased and the IgG1 antibody content increased. Booster injection of antigen resulted in a considerable and simultaneous increase in both IgE and IgG1. The preferential production of IgE was consistently obtained when LPS and antigen were injected simultaneously. Injection of LPS 24 hours after antigen resulted in a higher production of IgE and IgG1 but in the loss of the preferential production of IgE. Guinea-pig IgE antibody was able to persist in homologous skin for at least 46 days.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dobson C., Morseth D. J., Soulsby J. L. Immunoglobulin E-type antibodies induced by Ascaris suum infections in guinea pigs. J Immunol. 1971 Jan;106(1):128–133. [PubMed] [Google Scholar]
- JOHNSON A. G., GAINES S., LANDY M. Studies on the O antigen of Salmonella typhosa. V. Enhancement of antibody response to protein antigens by the purified lipopolysaccharide. J Exp Med. 1956 Feb 1;103(2):225–246. doi: 10.1084/jem.103.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIND P., JOHNSON A. G. Studies on the adjuvant action of bacterial endotoxins on antibody formation. I. Time limitation of enhancing effect and restoration of antibody formation in x-irradiated rabbits. J Immunol. 1959 May;82(5):415–427. [PubMed] [Google Scholar]
- Levine B. B., Chang H., Jr, Vaz N. M. The production of hapten-specific reaginic antibodies in the guinea pig. J Immunol. 1971 Jan;106(1):29–33. [PubMed] [Google Scholar]
- MERRITT K., JOHNSON A. G. STUDIES ON THE ADJUVANT ACTION OF BACTERIAL ENDOTOXINS ON ANTIBODY FORMATION. V. THE INFLUENCE OF ENDOTOXIN AND 5-FLUORO-2-DEOXYURIDINE ON THE PRIMARY ANTIBODY RESPONSE OF THE BALB MOUSE TO A PURIFIED PROTEIN ANTIGEN. J Immunol. 1963 Aug;91:266–272. [PubMed] [Google Scholar]
- MOTA I. Biological characterization of "mast cell sensitizing" antibodies. Life Sci. 1963 Jul;(7):465–474. doi: 10.1016/0024-3205(63)90134-6. [DOI] [PubMed] [Google Scholar]
- MOTA I. THE MECHANISM OF ANAPHYLAXIS. I. PRODUCTION AND BIOLOGICAL PROPERTIES OF 'MAST CELL SENSITIZING' ANTIBODY. Immunology. 1964 Nov;7:681–699. [PMC free article] [PubMed] [Google Scholar]
- Mota I. Biological characterization of mouse 'early' antibodies. Immunology. 1967 Mar;12(3):343–348. [PMC free article] [PubMed] [Google Scholar]
- Mota I., Peixoto J. M. A skin-sensitizing and thermolabile antibody in the mouse. Life Sci. 1966 Sep;5(18):1723–1728. doi: 10.1016/0024-3205(66)90108-1. [DOI] [PubMed] [Google Scholar]
- Mota I., Perini A. A heat labile mercaptoethanol susceptible homocytotropic antibody in the guinea pig. Life Sci II. 1970 Aug 22;9(16):923–930. doi: 10.1016/0024-3205(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Möller G., Andersson J., Sjöberg O. Lipopolysaccharides can convert heterologous red cells into thymus-independent antigens. Cell Immunol. 1972 Aug;4(4):416–424. doi: 10.1016/0008-8749(72)90043-3. [DOI] [PubMed] [Google Scholar]
- OVARY Z., BENACERRAF B., BLOCH K. J. Properties of guinea pig 7S antibodies. II. Identification of antibodies involved in passive cutaneous and systemic anaphylaxis. J Exp Med. 1963 Jun 1;117:951–964. doi: 10.1084/jem.117.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. 3. Effect of thymectomy and splenectomy. J Immunol. 1971 Apr;106(4):1019–1025. [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. VI. Inhibitory effect of thymocytes on the homocytotropic antibody response. J Immunol. 1971 Dec;107(6):1682–1689. [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Jaroslow B. N. Enhancement by the adjuvant, endotoxin, of an immune response induced in vitro. Immunology. 1970 Sep;19(3):387–399. [PMC free article] [PubMed] [Google Scholar]
- Parish W. E. Homologous serum passive cutaneous anaphylaxis in guinea pigs mediated by two gamma-1 or gamma-1-type heat-stable globulins and a non-gamma-1 heat-labile reagin. J Immunol. 1970 Nov;105(5):1296–1298. [PubMed] [Google Scholar]
- Perini A., Mota I. Heterogeneity of guinea-pig homocytotropic antibodies. Immunology. 1972 May;22(5):915–923. [PMC free article] [PubMed] [Google Scholar]
- Sjöberg O. Antigen-binding cells in mice immune or tolerant to Escherichia coli polysaccharide. J Exp Med. 1971 May 1;133(5):1015–1025. doi: 10.1084/jem.133.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Okumura K. Regulation of homocytotropic antibody formation in the rat. I. Feed-back regulation by passively administered antibody. J Immunol. 1971 Apr;106(4):1002–1011. [PubMed] [Google Scholar]
- Vaz N. M., Vaz E. M., Levine B. B. Relationship between histocompatibility (H-2) genotype and immune responsiveness to low doses of ovalbumin in the mouse. J Immunol. 1970 Jun;104(6):1572–1574. [PubMed] [Google Scholar]
- Zvaifler N. J., Becker E. L. Rabbit anaphylactic antibody. J Exp Med. 1966 May 1;123(5):935–950. doi: 10.1084/jem.123.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]


