Abstract
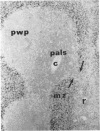
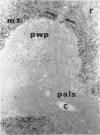
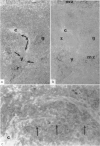
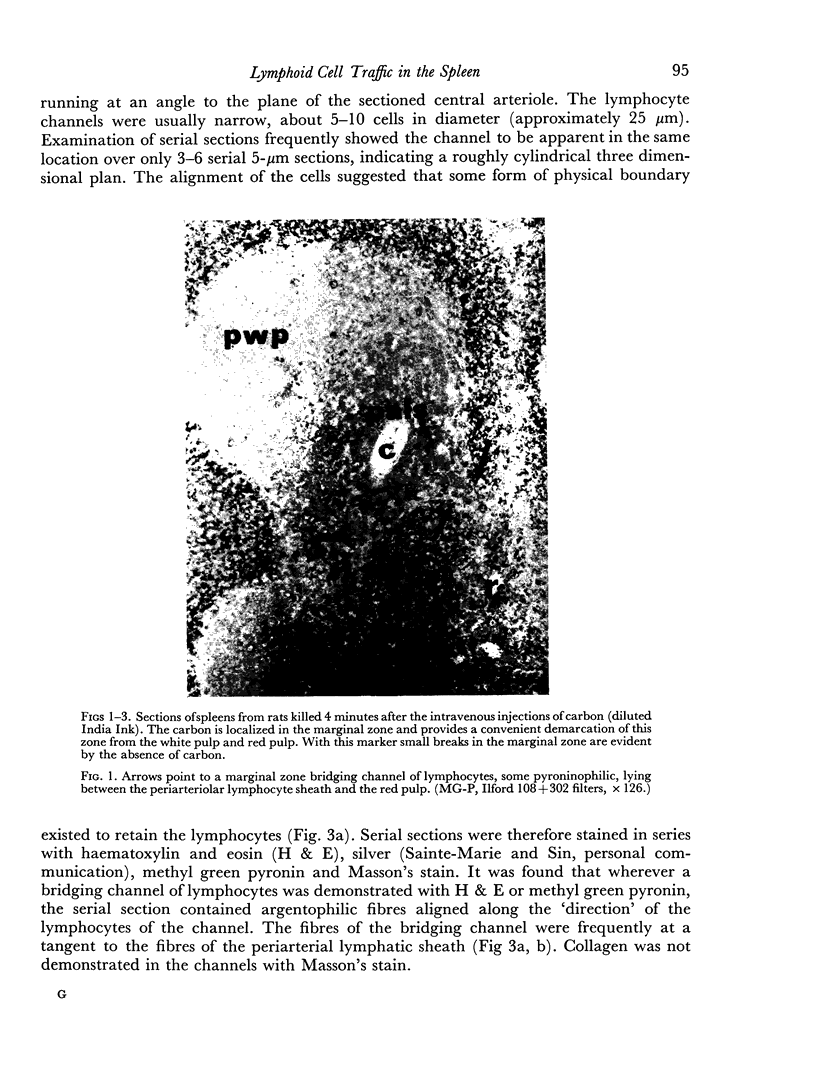
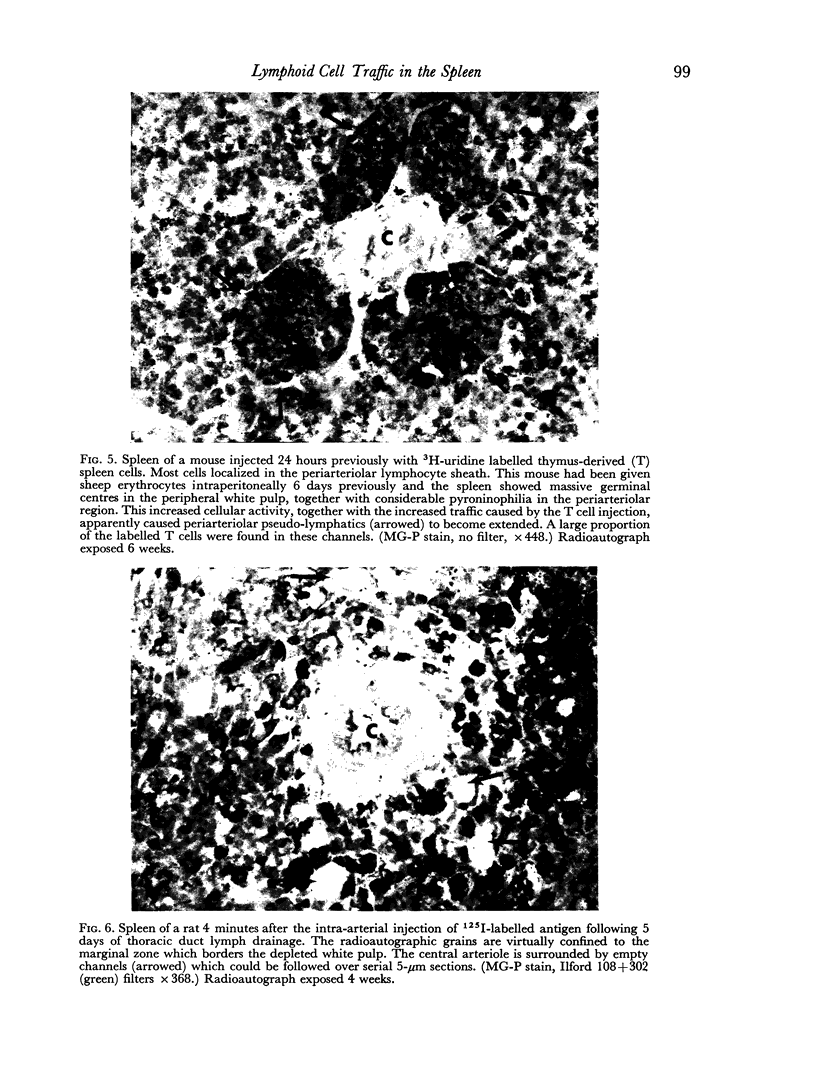
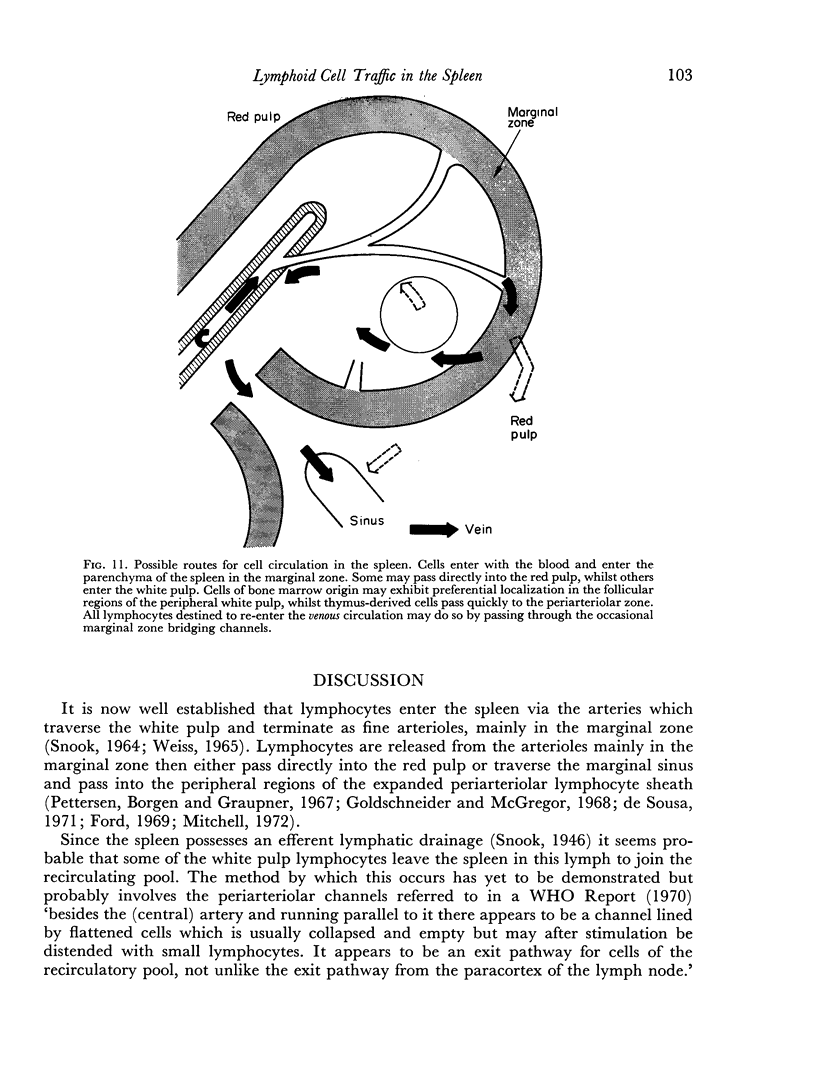
Histological evidence is presented for distinct, anatomically determined pathways in the spleen for cells in transit between the white pulp and the red pulp prior to entering the draining veins. In rats and mice these appear as narrow channels of lymphocytes which run between both the periarteriolar lymphatic sheath and the red pulp sinuses, and the peripheral white pulp and the red pulp sinuses, crossing the marginal zone in association with fine argentophilic fibres. These marginal zone bridging channels were found to contain labelled T or B cells 4 and 8 hours after injection which suggested that transit was occurring in the direction from white pulp to red pulp rather than the reverse.
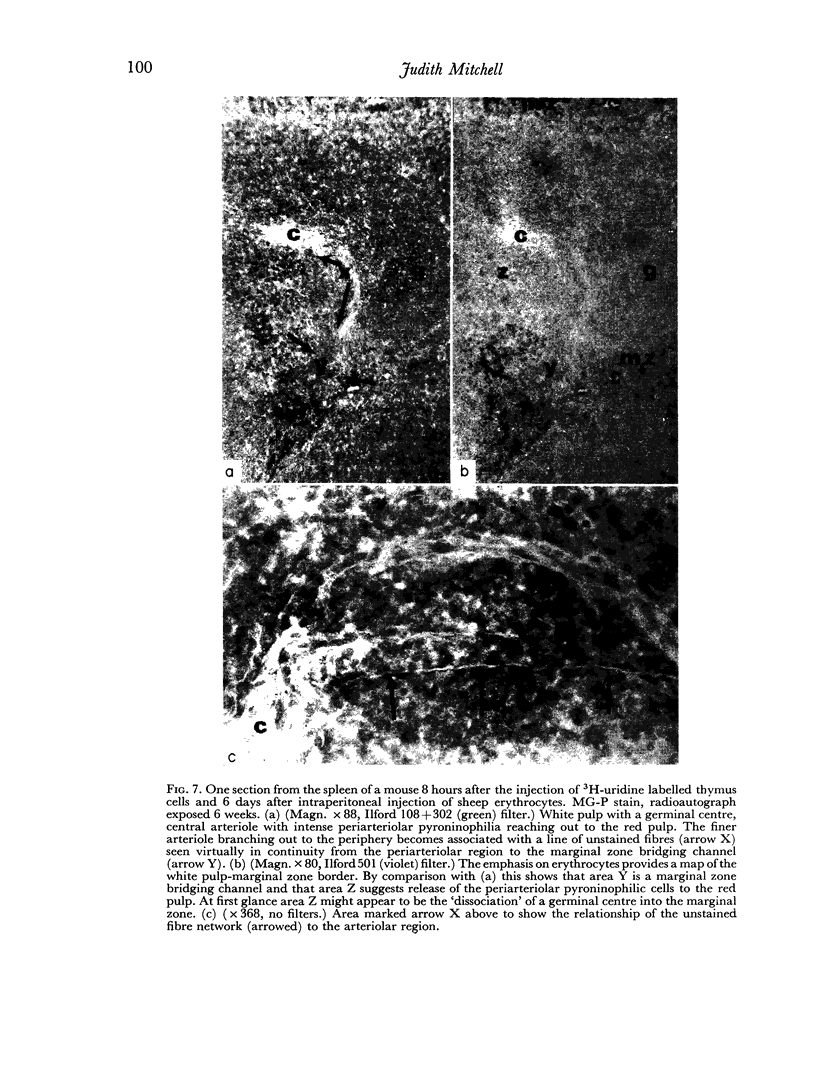
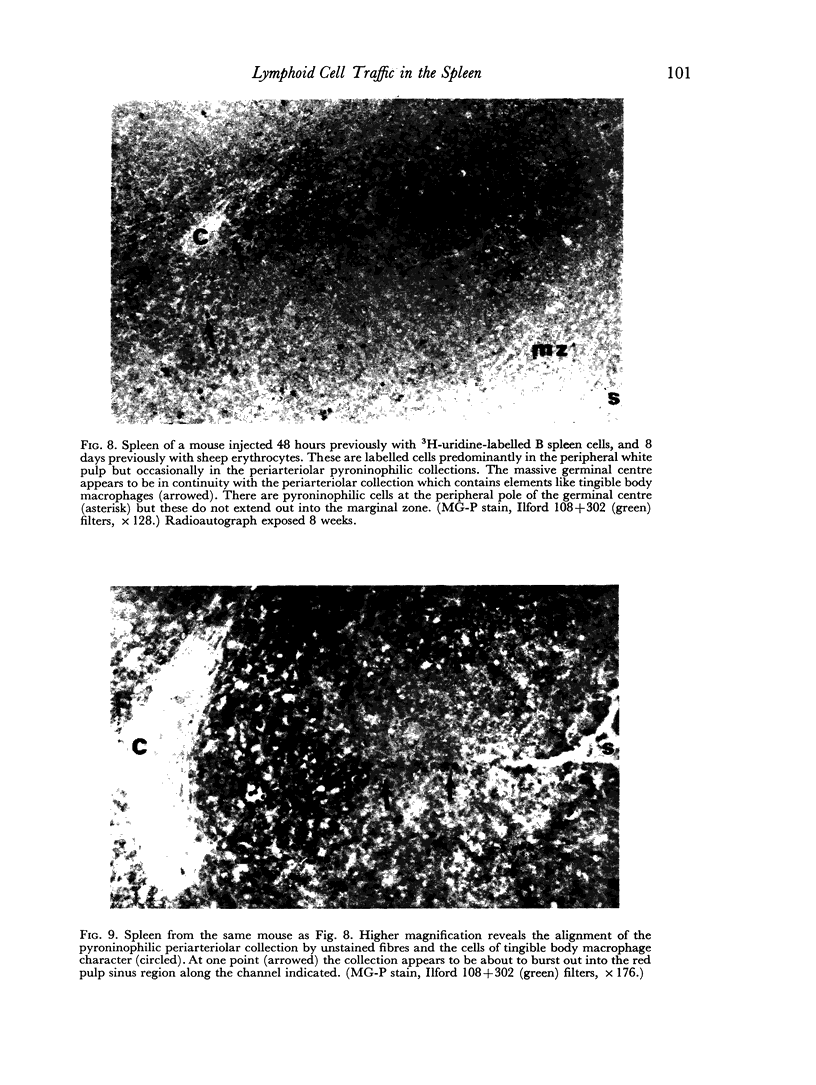
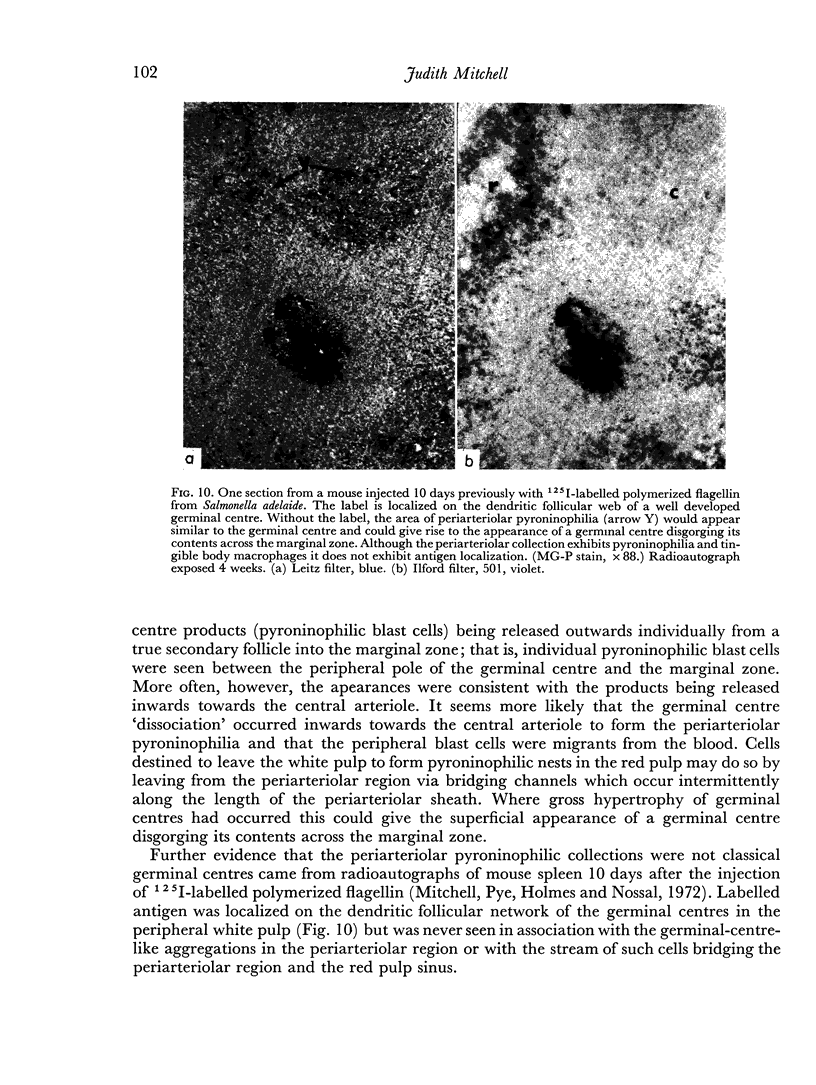
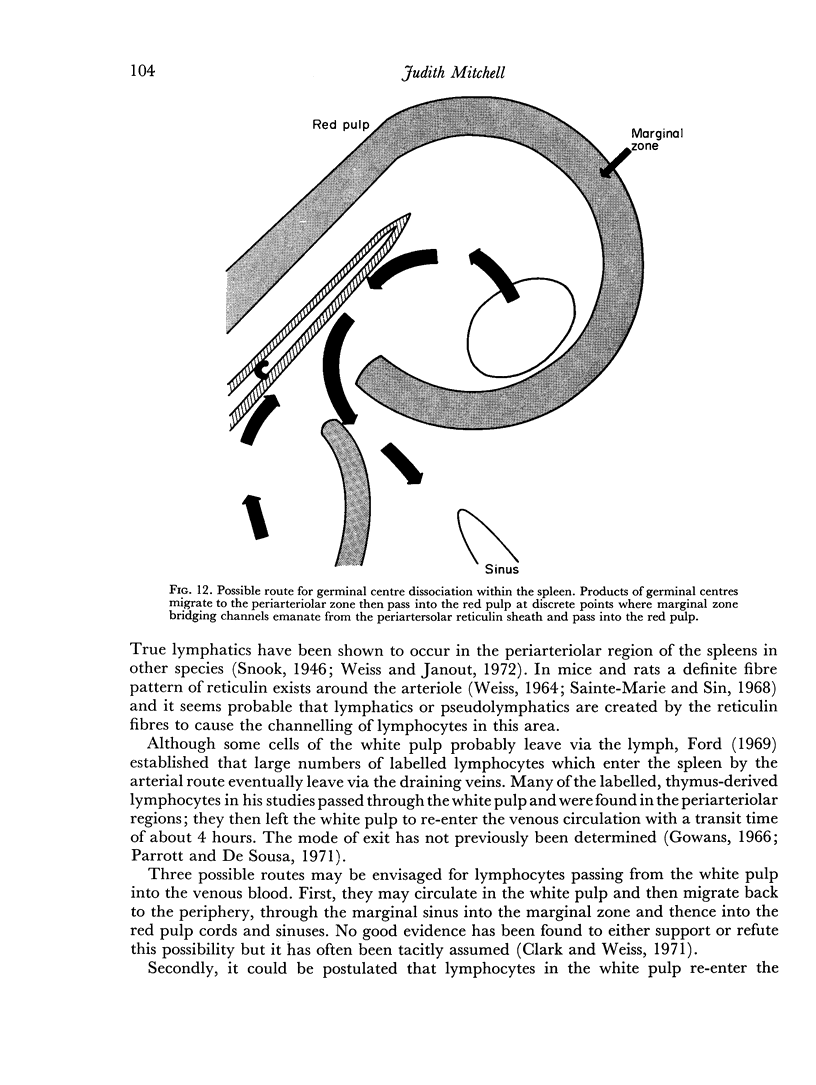
Additional histological evidence is given to suggest that, after antigenic stimulation, germinal centre dissociation occurs by release of the germinal centre cells towards the periarteriolar lymphatic sheath before they are shed into the red pulp through marginal zone bridges occurring in the periarteriolar region.
The data are incorporated into a scheme of unidirectional lymphoid cell flow through the spleen. This proposes that the spleen is composed of many functionally discrete units in which the anatomical matrix, reflected by the reticulin fibre pattern, plays a major role. It further implies that the periarteriolar region of the spleen is not totally thymus dependent.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONGDON C. C., MAKINODAN T. Splenic white pulp alteration after antigen injection: relation to time of serum antibody production. Am J Pathol. 1961 Dec;39:697–709. [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Weiss L. Effects of a bacterial vaccine on the marginal zone of the spleen. Am J Anat. 1971 Sep;132(1):79–92. doi: 10.1002/aja.1001320109. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., McGregor D. D. Migration of lymphocytes and thymocytes in the rat. I. The route of migration from blood to spleen and lymph nodes. J Exp Med. 1968 Jan 1;127(1):155–168. doi: 10.1084/jem.127.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans J. L. Life-span, recirculation, and transformation of lymphocytes. Int Rev Exp Pathol. 1966;5:1–24. [PubMed] [Google Scholar]
- HANNA M. G., Jr AN AUTORADIOGRAPHIC STUDY OF THE GERMINAL CENTER IN SPLEEN WHITE PULP DURING EARLY INTERVALS OF THE IMMUNE RESPONSE. Lab Invest. 1964 Feb;13:95–104. [PubMed] [Google Scholar]
- Janout V., Weiss L. Deep splenic lymphatics in the marmot: an electron microscopic study. Anat Rec. 1972 Feb;172(2):197–219. doi: 10.1002/ar.1091720207. [DOI] [PubMed] [Google Scholar]
- Mitchell J., Abbot A. Antigens in immunity. XVI. A light and electron microscope study of antigen localization in the rat spleen. Immunology. 1971 Aug;21(2):207–224. [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. Antigens in immunity. XVII. The migration of antigen-binding, bone-marrow-derived and thymus-derived spleen cells in mice. Immunology. 1972 Feb;22(2):231–245. [PMC free article] [PubMed] [Google Scholar]
- Olson I. A. The kinetics of germinal center formation in lymph nodes responding to keyhole limpet hemocyanin. Exp Mol Pathol. 1971 Apr;14(2):139–150. doi: 10.1016/0014-4800(71)90060-8. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., De Sousa M. Thymus-dependent and thymus-independent populations: origin, migratory patterns and lifespan. Clin Exp Immunol. 1971 May;8(5):663–684. [PMC free article] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen J. C., Borgen D. F., Graupner K. C. A morphological and histochemical study of the primary and secondary immune responses in the rat spleen. Am J Anat. 1967 Sep;121(2):305–317. doi: 10.1002/aja.1001210209. [DOI] [PubMed] [Google Scholar]
- SNOOK T. STUDIES ON THE PERIFOLLICULAR REGION OF THE RAT'S SPLEEN. Anat Rec. 1964 Feb;148:149–159. doi: 10.1002/ar.1091480205. [DOI] [PubMed] [Google Scholar]
- Sainte-Marie G., Sin Y. M. Reticular fiber pattern in the rat spleen. Rev Can Biol. 1968 Sep;27(3):273–275. [PubMed] [Google Scholar]
- WEISS L. THE WHITE PULP OF THE SPLEEN. THE RELATIONSHIPS OF ARTERIAL VESSELS, RETICULUM AND FREE CELLS IN THE PERIARTERIAL LYMPHATIC SHEATH. Bull Johns Hopkins Hosp. 1964 Aug;115:99–172. [PubMed] [Google Scholar]
- de Sousa M. Kinetics of the distribution of thymus and marrow cells in the peripheral lymphoid organs of the mouse: ecotaxis. Clin Exp Immunol. 1971 Sep;9(3):371–380. [PMC free article] [PubMed] [Google Scholar]