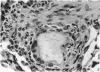
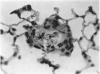
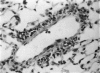
Abstract
A model for the study of granulomatous inflammation has been developed, employing bentonite particles (65 μm in diameter) coated with soluble antigens derived from Mycobacterium tuberculosis, Histoplasma capsulatum and Schistosoma mansoni. These particles are injected i v. into the micro-vasculature of the lungs of unsensitized mice or mice sensitized to the above antigens in a variety of ways. Uncoated particles or those coated with heterologous antigens elicit rapidly forming small foci of inflammatory cells composed mainly of macrophages. In contrast, particles coated with homologous antigens elicit large hypersensitivity-type granulomas composed of lymphocytes, macrophages, eosinophils and occasionally epithelioid cells. The occurrence of hypersensitivity-type granulomas correlates well with delayed footpad swelling also elicited by homologous antigen. In addition, the florid specific granulomatous inflammation is transferrable to syngeneic recipients with immune lymphoid cells but not with antiserum. Finally, when bare or heterologous antigen-coated particles are injected into sensitized mice which are simultaneously injected i.v. with homologous antigen, hypersensitivity granulomas appear around the particles. This bentonite model thus facilitates the study of both the foreign body and hypersensitivity granulomas and suggests mechanisms by which they might be formed.
Full text
PDF







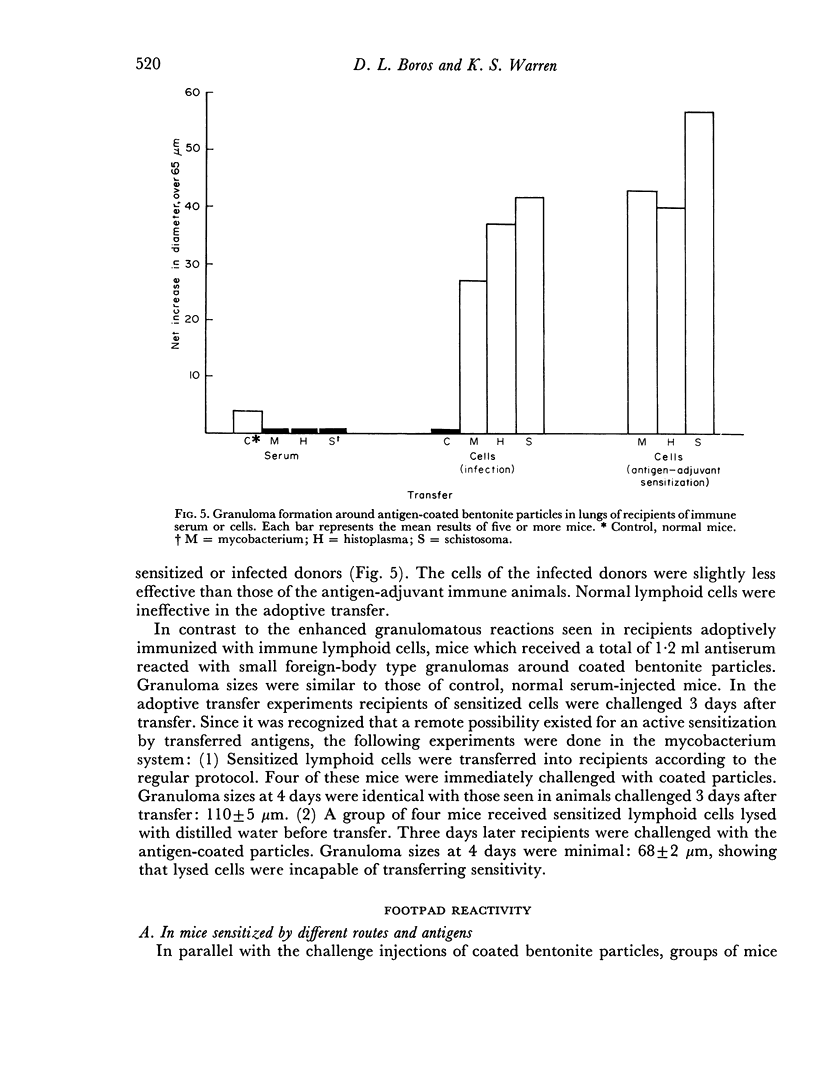
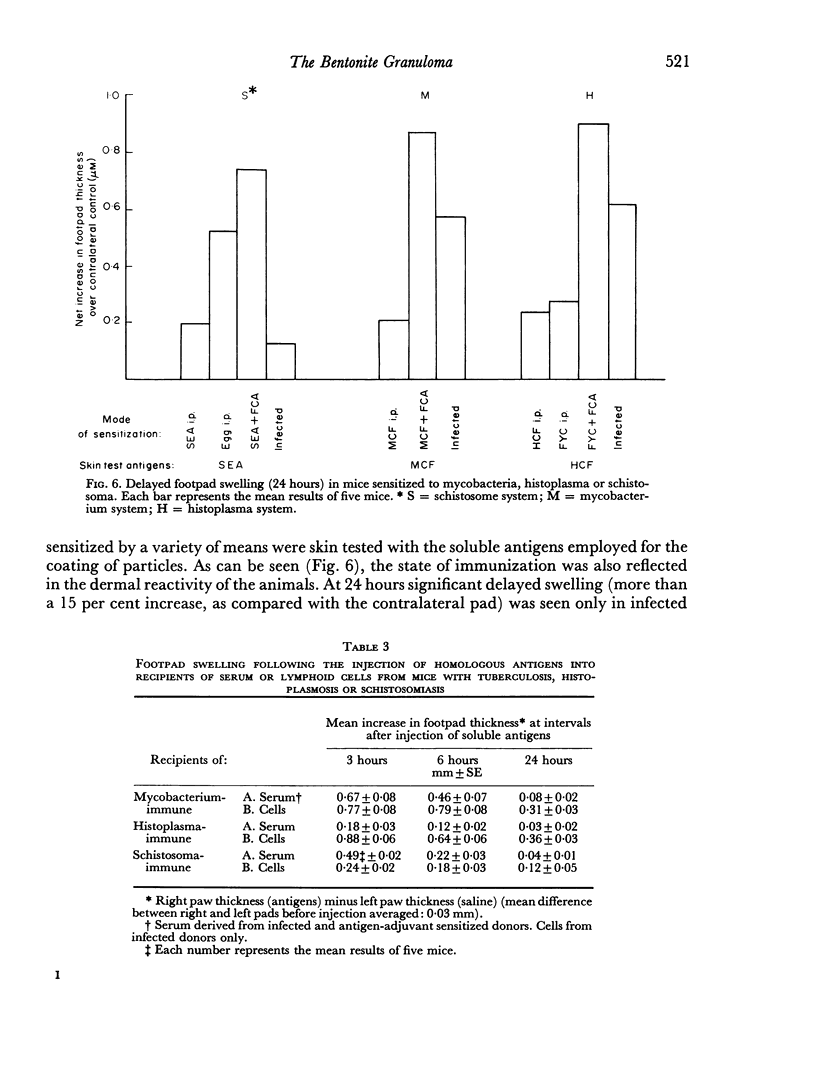
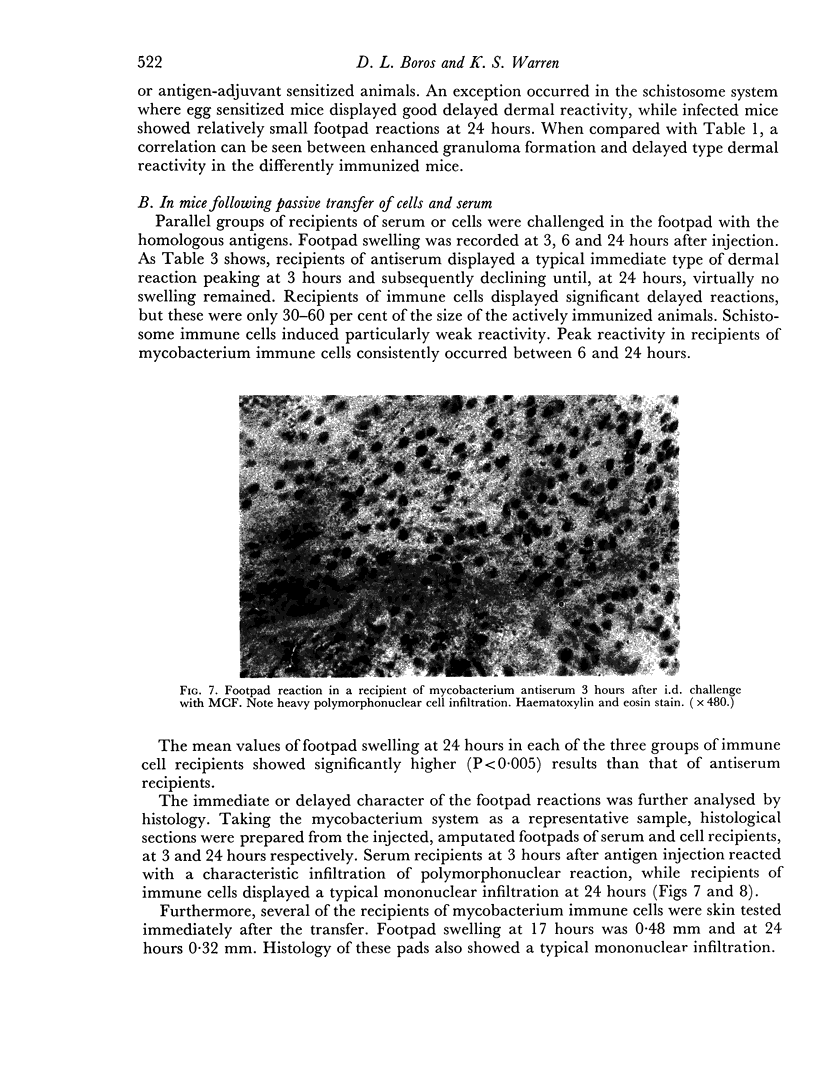

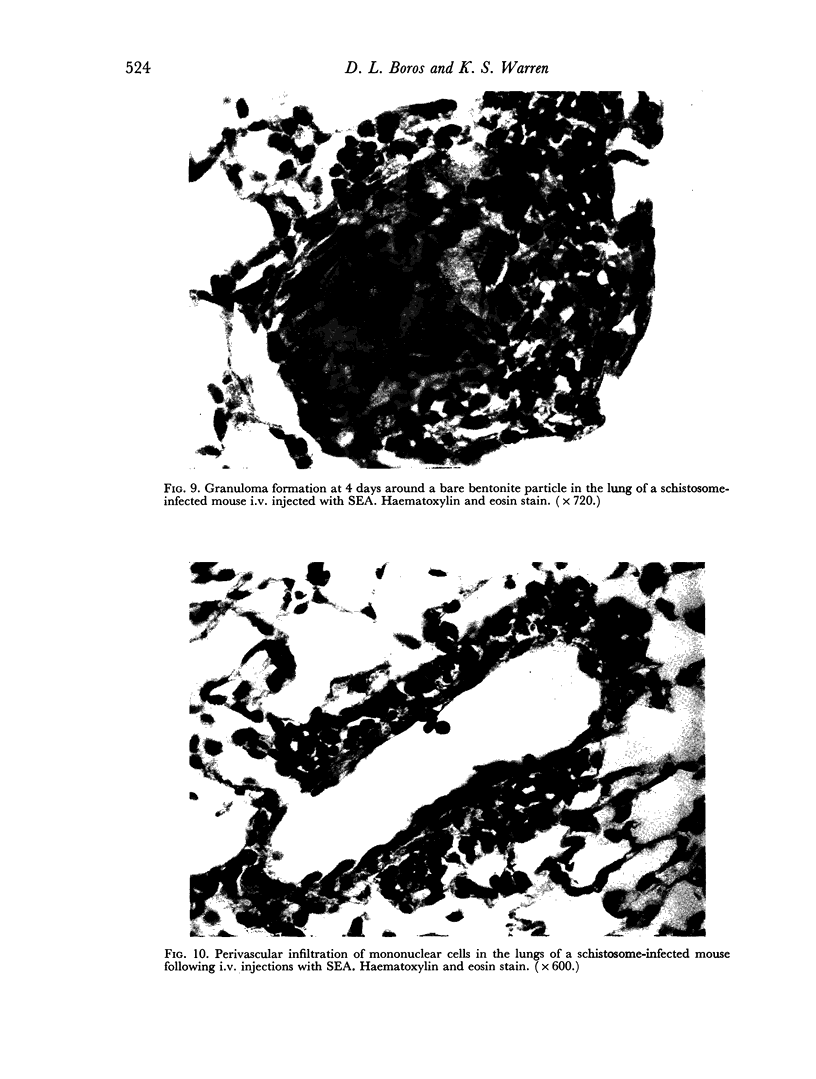





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYDEN S. V. The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. J Exp Med. 1951 Feb;93(2):107–120. doi: 10.1084/jem.93.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basten A., Beeson P. B. Mechanism of eosinophilia. II. Role of the lymphocyte. J Exp Med. 1970 Jun 1;131(6):1288–1305. doi: 10.1084/jem.131.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Bloom B. R. In vitro approaches to the mechanism of cell-mediated immune reactions. Adv Immunol. 1971;13:101–208. doi: 10.1016/s0065-2776(08)60184-4. [DOI] [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970 Sep 1;132(3):488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros D. L., Warren K. S. Specific granulomatous hypersensitivity elicited by bentonite particles coated with soluble antigens from schistosome eggs and turcle bacilli. Nature. 1971 Jan 15;229(5281):200–201. doi: 10.1038/229200a0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Ward P. A. In vitro and in vivo activity of a lymphocyte and immune complex-dependent chemotactic factor for eosinophils. J Exp Med. 1971 Jan 1;133(1):133–146. doi: 10.1084/jem.133.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL T. M. OBSERVATIONS ON THE ANTIBODY RESPONSE OF RABBITS TO MYCOBACTERIAL ANTIGENS. J Immunol. 1965 Jul;95:100–108. [PubMed] [Google Scholar]
- DIETRICH F. M., NORDIN A. A., BLOCH H. Delayed hypersensitivity in tuberculous mice. II. Generalized tuberculin reaction and foot pad tests. Int Arch Allergy Appl Immunol. 1962;20:129–142. [PubMed] [Google Scholar]
- Daniel T. M., Ferguson L. E. Purification and Characterization of Two Proteins from Culture Filtrates of Mycobacterium tuberculosis H(37)Ra Strain. Infect Immun. 1970 Feb;1(2):164–168. doi: 10.1128/iai.1.2.164-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S., Stenger R. J. Increased incidence of hepatoma in mice with chronic schistosomiasis mansoni treated with a carcinogen. Am J Pathol. 1967 Sep;51(3):307–321. [PMC free article] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. 3. Heterologous antilymphocyte serum. Am J Pathol. 1968 Mar;52(3):613–631. [PMC free article] [PubMed] [Google Scholar]
- Domingo E. O., Warren K. S. The inhibition of granuloma formation around Schistosoma mansoni eggs. II. Thymectomy. Am J Pathol. 1967 Nov;51(5):757–767. [PMC free article] [PubMed] [Google Scholar]
- Epstein W. L. Granulomatous hypersensitivity. Prog Allergy. 1967;11:36–88. [PubMed] [Google Scholar]
- HILL G. B., CAMPBELL C. C. A further evaluation of histoplasmin and yeast phase antigen of Histoplasma capsulatum in the complement fixation test. J Lab Clin Med. 1956 Aug;48(2):255–263. [PubMed] [Google Scholar]
- Kellermeyer R. W., Warren K. S. The role of chemical mediators in the inflammatory response induced by foreign bodies: comparison with the schistosome egg granuloma. J Exp Med. 1970 Jan 1;131(1):21–39. doi: 10.1084/jem.131.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Smith T. M., Lucia H. L., Doughty B. L., von Lichtenberg F. C. The role of phospholipids in schistosome granulomas. J Infect Dis. 1971 Jun;123(6):629–639. doi: 10.1093/infdis/123.6.629. [DOI] [PubMed] [Google Scholar]
- Spector W. G. The granulomatous inflammatory exudate. Int Rev Exp Pathol. 1969;8:1–55. [PubMed] [Google Scholar]
- Uhr J. W. Delayed hypersensitivity. Physiol Rev. 1966 Jul;46(3):359–419. doi: 10.1152/physrev.1966.46.3.359. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. Leukotactic factor produced by sensitized lymphocytes. Science. 1969 Mar 7;163(3871):1079–1081. doi: 10.1126/science.163.3871.1079. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Benacerraf B., McCluskey R. T., Vassalli P. The effect of intravenous antigen on circulating monocytes in animals with delayed hypersensitivity. J Immunol. 1969 Apr;102(4):804–811. [PubMed] [Google Scholar]