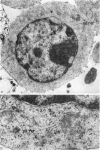
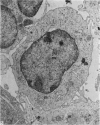
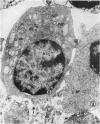
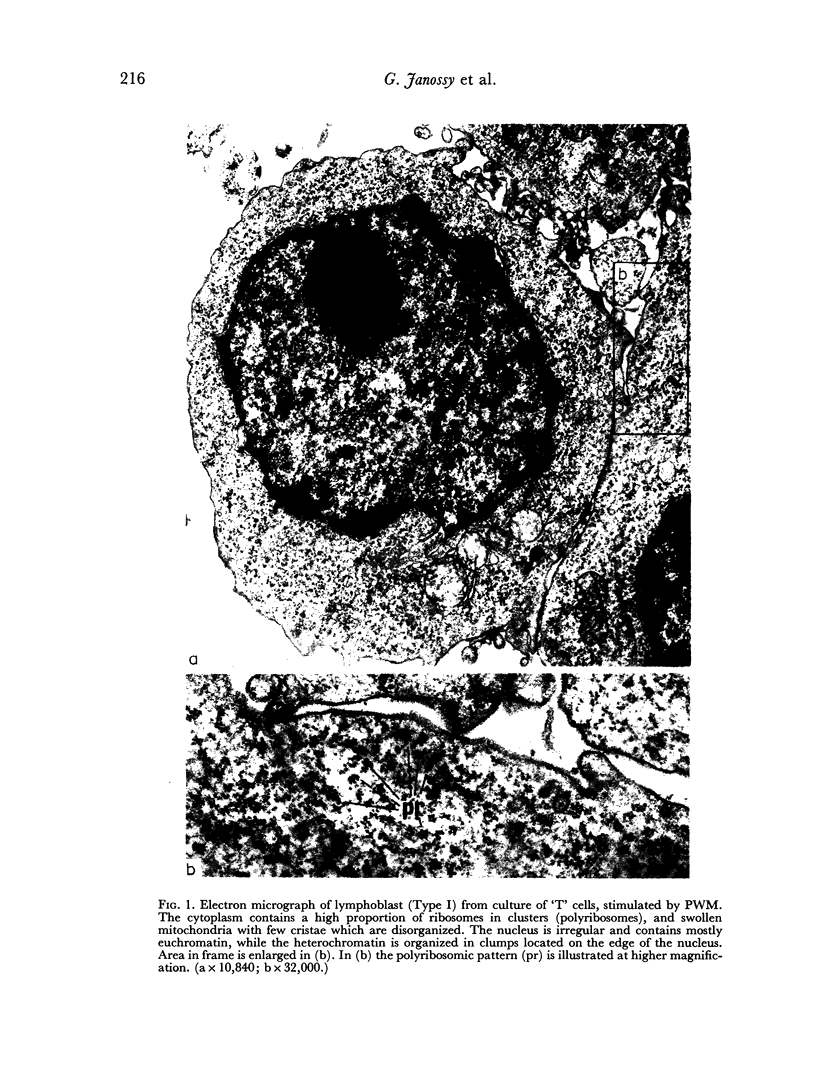
Abstract
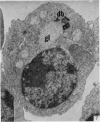
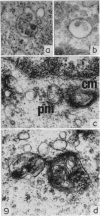
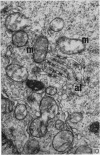
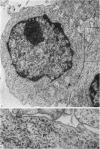
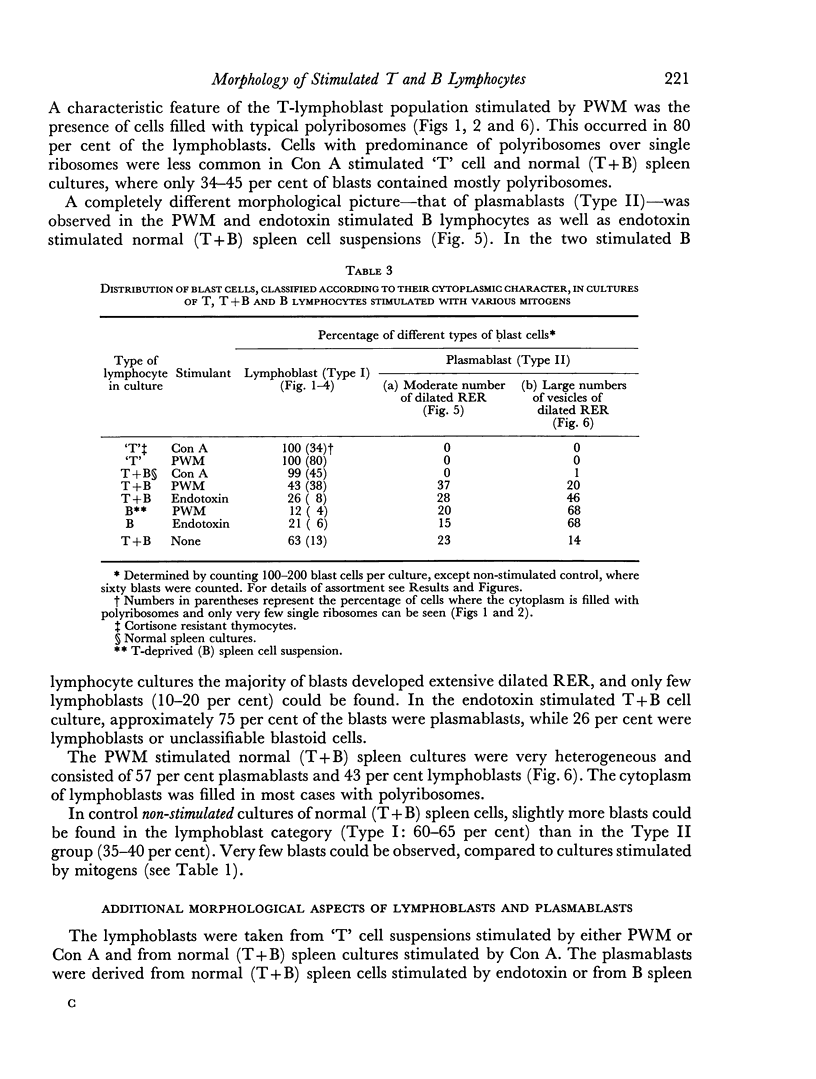
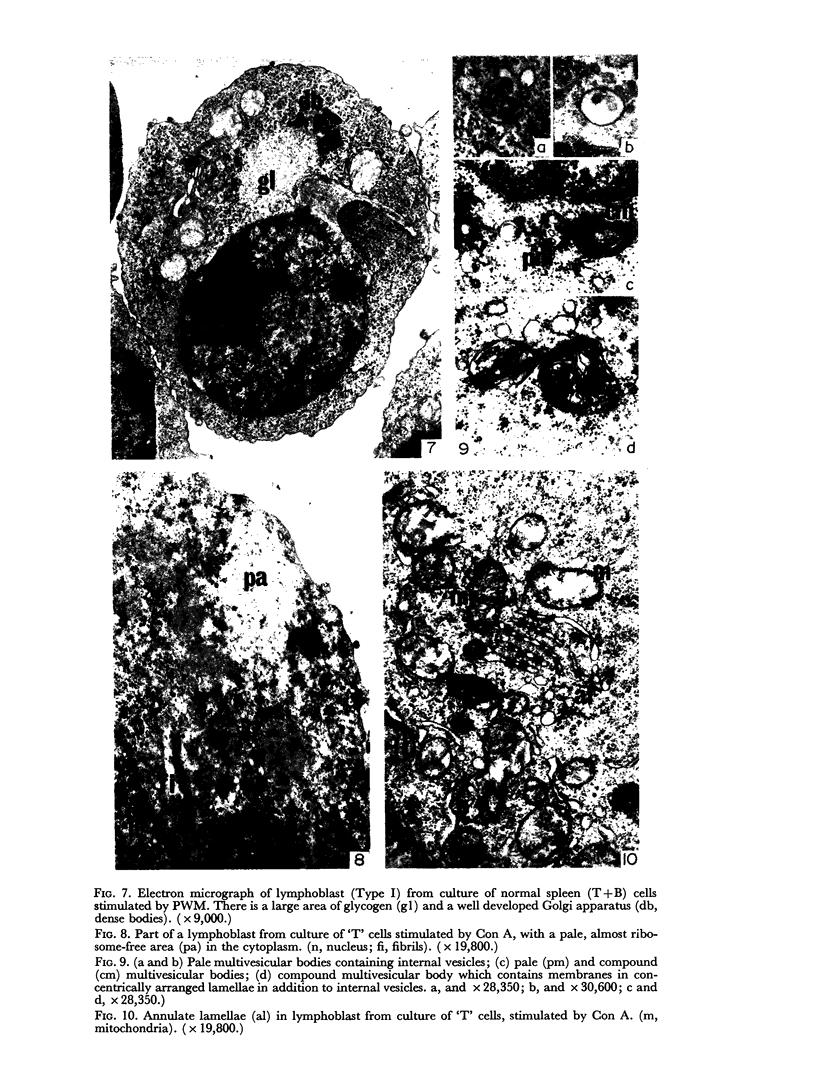
Thymocytes from cortisone-treated mice (`T' cells), `B' spleen cells (B lymphocytes from thymectomized, irradiated, marrow reconstituted mice) and normal spleen (T + B) cells were examined by electron microscopy after 60 hours stimulation by Concanavalin A (a T cell specific mitogen), endotoxin (B cell specific mitogen), and pokeweed mitogen (which stimulates both T and B cells). Stimulation of T cells by Con A or PWM induced the appearance of lymphoblasts (Type I) and only PWM or endotoxin stimulated B cells developed `plasmablast' features (dilated, vesicular rough endoplasmic reticulum; Type II). A few stimulated B cells also had lymphoblast morphology. Large cells from normal (T + B) spleen stimulated by PWM were heterogeneous consisting of 55–60 per cent plasmablasts and 40–45 per cent lymphoblasts. It was concluded that the ultrastructure of stimulated lymphocytes depended on whether T or B cells were stimulated and not primarily on the mitogen used. In general, the response evoked by mitogens paralleled at the ultrastructural level that induced by antigens. It was also found that multivesicular bodies and glycogen particles occurred predominantly in the cytoplasm of stimulated T cells (lymphoblasts).
Full text
PDF




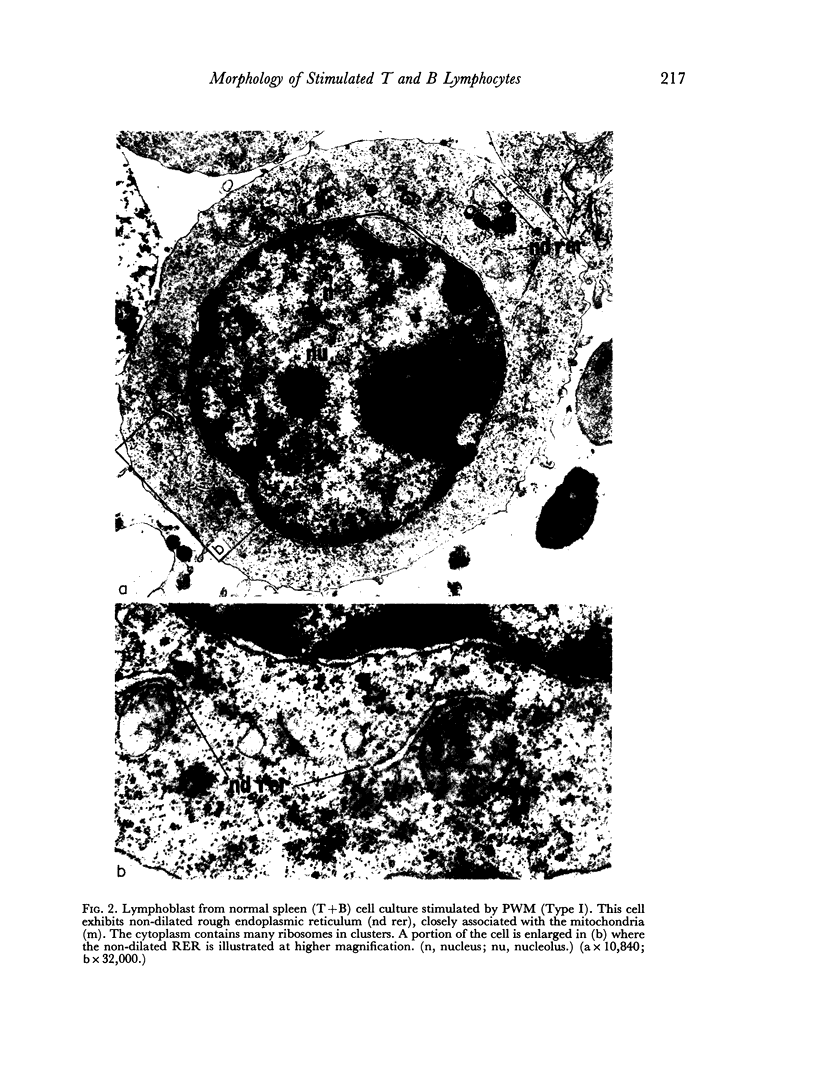
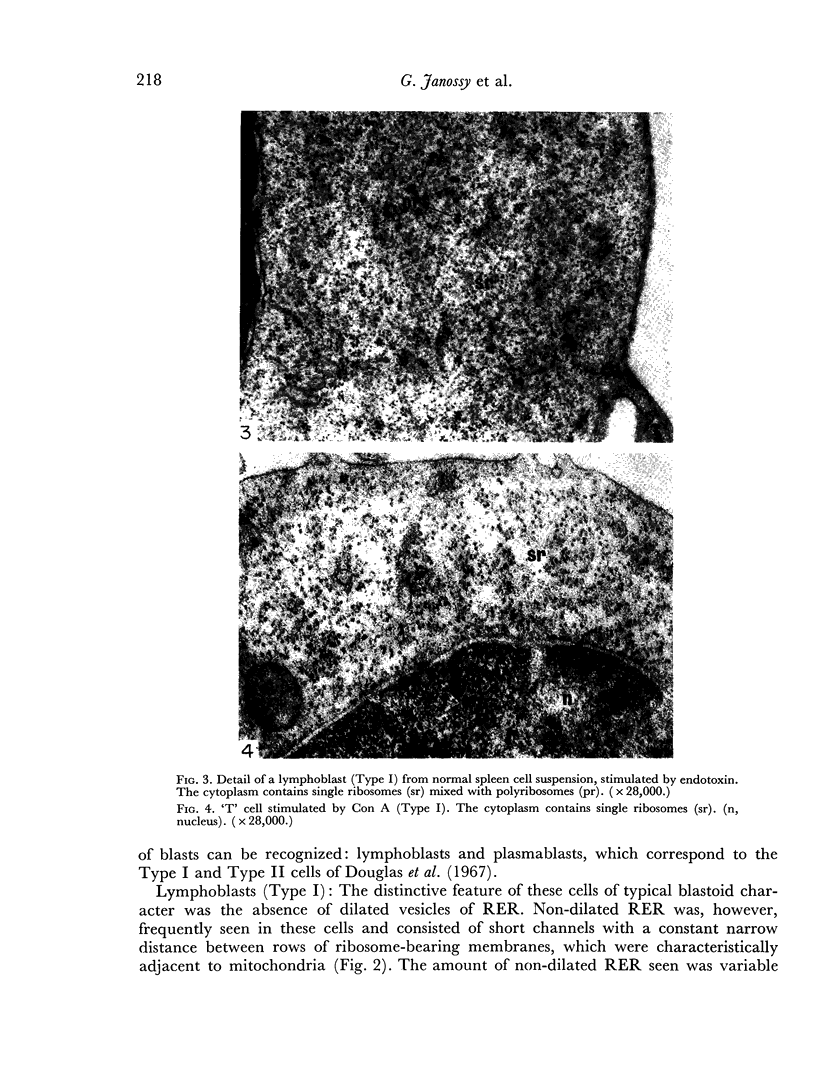
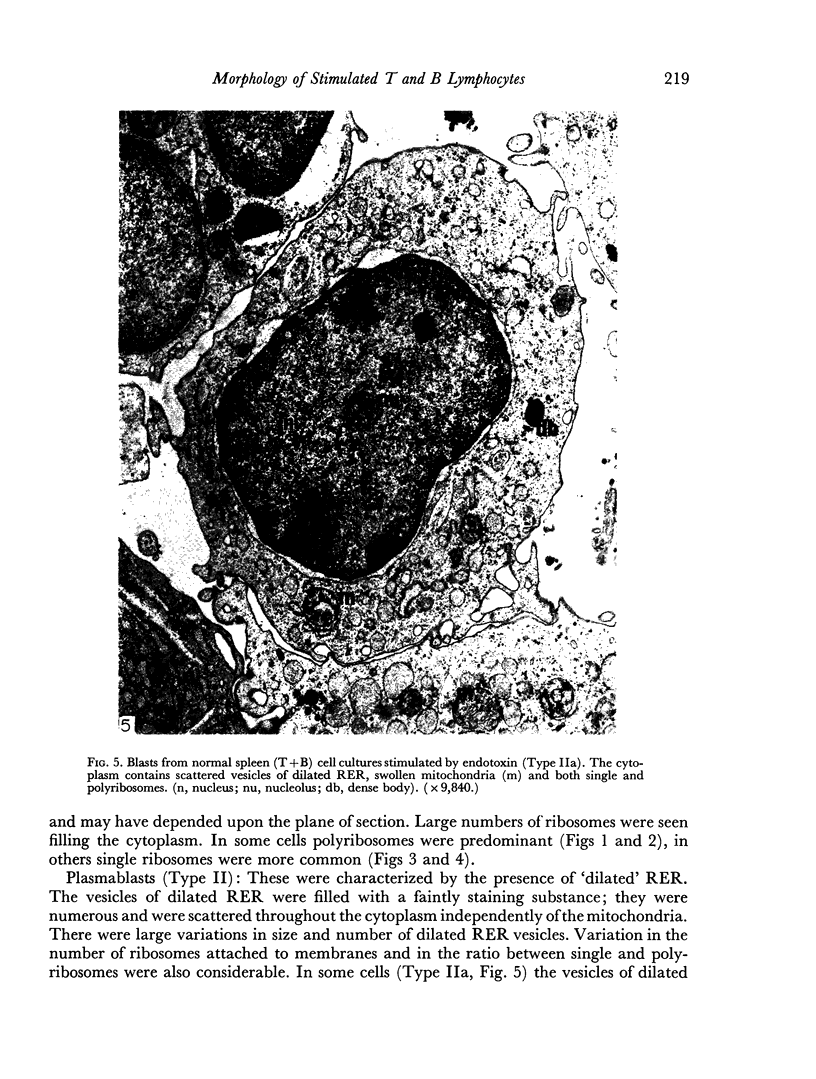
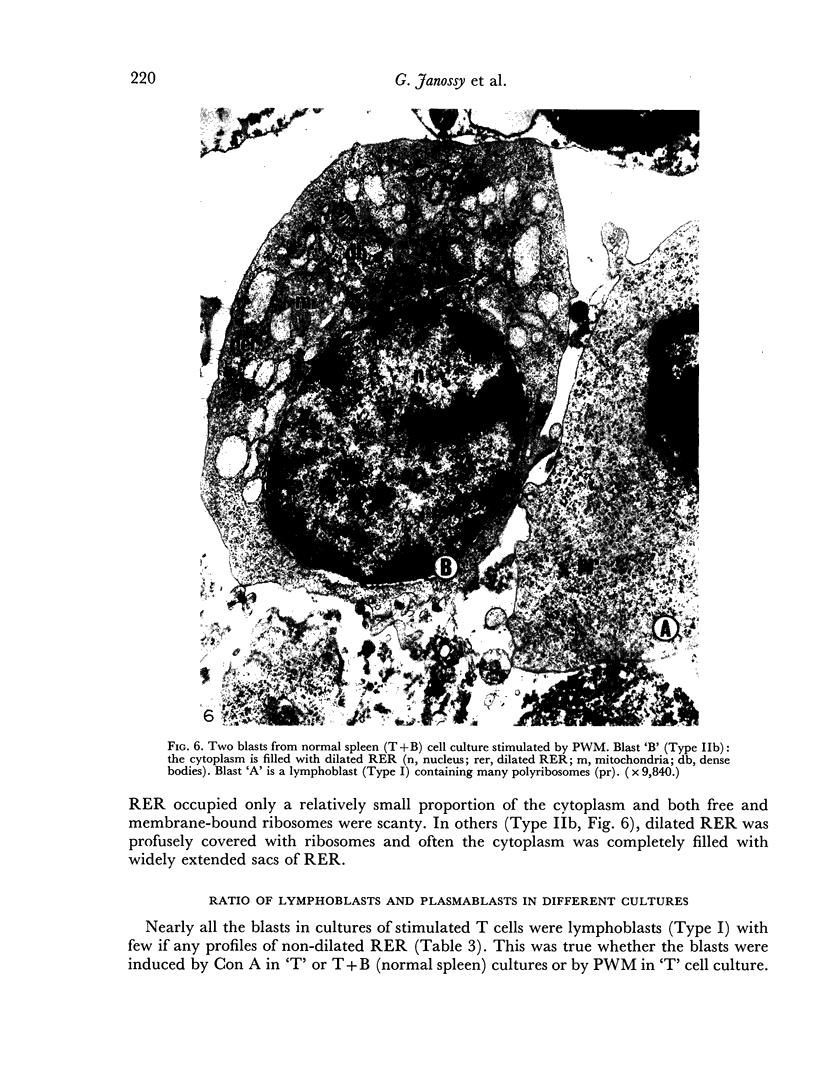





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRE SCHWARTZ J. THE MORPHOLOGIC RESPONSES OF THE LYMPHOID SYSTEM TO HOMOGRAFTS. 3. ELECTRON MICROSCOPY STUDY. Blood. 1964 Aug;24:113–133. [PubMed] [Google Scholar]
- Biberfeld P. Endocytosis and lysosome formation in blood lymphocytes transformed by phytohemagglutinin. J Ultrastruct Res. 1971 Oct;37(1):41–68. doi: 10.1016/s0022-5320(71)80040-0. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Svedmyr E. In vitro stimulation of mouse thymus cells by PHA and allogeneic cells. Cell Immunol. 1971 Aug;2(4):285–299. doi: 10.1016/0008-8749(71)90063-3. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Cellular and humoral response to transplantation antigens. I. Development of alloantibody-forming cells and cytotoxic lymphocytes in the graft-versus-host reaction. J Exp Med. 1971 Aug 1;134(2):553–564. doi: 10.1084/jem.134.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessin L. N., Börjeson J., Welsh P. D., Douglas S. D., Cooper H. L. Studies on human peripheral blood lymphocytes in vitro. II. Morphological and biochemical studies on the transformation of lymphocytes by pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):873–884. doi: 10.1084/jem.124.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessin L. N., Börjeson J., Welsh P. D., Douglas S. D., Cooper H. L. Studies on human peripheral blood lymphocytes in vitro. II. Morphological and biochemical studies on the transformation of lymphocytes by pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):873–884. doi: 10.1084/jem.124.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- De Petris S., Karlsbad G., Pernis B., Turk J. L. Ultrastructure of cells present in lymph nodes during the development of contact sensitivity. Int Arch Allergy Appl Immunol. 1966;29(2):112–130. doi: 10.1159/000229693. [DOI] [PubMed] [Google Scholar]
- Douglas S. D., Hoffman P. F., Borjeson J., Chessin L. N. Studies on human peripheral blood lymphocytes in vitro. 3. Fine structural features of lymphocyte transformation by pokeweed mitogen. J Immunol. 1967 Jan;98(1):17–30. [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S., Janossy G. Lymphocyte activation. 3. Binding sites for phytomitogens on lymphocyte subpopulations. Clin Exp Immunol. 1972 Mar;10(3):537–554. [PMC free article] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Gudat F. G., Harris T. N., Harris S., Hummeler K. Studies on antibody-producing cells. 3. Identification of young plaque-forming cells by thymidine- 3 H labeling. J Exp Med. 1971 Nov 1;134(5):1155–1169. doi: 10.1084/jem.134.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudat F. G., Harris T. N., Harris S., Hummeler K. Studies on antibody-producing cells. I. Ultrastructure of 19S and 7S antibody-producing cells. J Exp Med. 1970 Sep 1;132(3):448–474. doi: 10.1084/jem.132.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. N., Hummeler K., Harris S. Electron microscopic observations on antibody-producing lymph node cells. J Exp Med. 1966 Jan 1;123(1):161–172. doi: 10.1084/jem.123.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummeler K., Harris T. N., Harris S., Farber M. B. Studies on antibody-producing cells. IV. Ultrastructue of plaque-forming cells of rabbit lymph. J Exp Med. 1972 Mar 1;135(3):491–502. doi: 10.1084/jem.135.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. II. discriminating stimulation of lymphocyte subpopulations by phytomitogens and heterologous antilymphocyte sera. Clin Exp Immunol. 1972 Mar;10(3):525–536. [PMC free article] [PubMed] [Google Scholar]
- Leduc E. H., Avrameas S., Bouteille M. Ultrastructural localization of antibody in differentiating plasma cells. J Exp Med. 1968 Jan 1;127(1):109–118. doi: 10.1084/jem.127.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. THE FINE STRUCTURE OF THE LYMPHOID TISSUE DURING ANTIBODY FORMATION. Exp Mol Pathol. 1965 Apr;28:155–188. doi: 10.1016/0014-4800(65)90031-6. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):821–837. doi: 10.1084/jem.128.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins W. D., Karnovsky M. J., Unanue E. R. An ultrastructural study of lymphocytes with surface-bound immunoglobulin. J Exp Med. 1972 Feb 1;135(2):267–276. doi: 10.1084/jem.135.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Pick E., Turk J. L. The biological activities of soluble lymphocyte products. Clin Exp Immunol. 1972 Jan;10(1):1–23. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Stobo J. D., Rosenthal A. S., Paul W. E. Functional heterogeneity of murine lymphoid cells. I. Responsiveness to and surface binding of concanavalin A and phytohemagglutinin. J Immunol. 1972 Jan;108(1):1–17. [PubMed] [Google Scholar]
- Takahashi T., Old L. J., McIntire K. R., Boyse E. A. Immunoglobulin and other surface antigens of cells of the immune system. J Exp Med. 1971 Oct 1;134(4):815–832. doi: 10.1084/jem.134.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]