Abstract
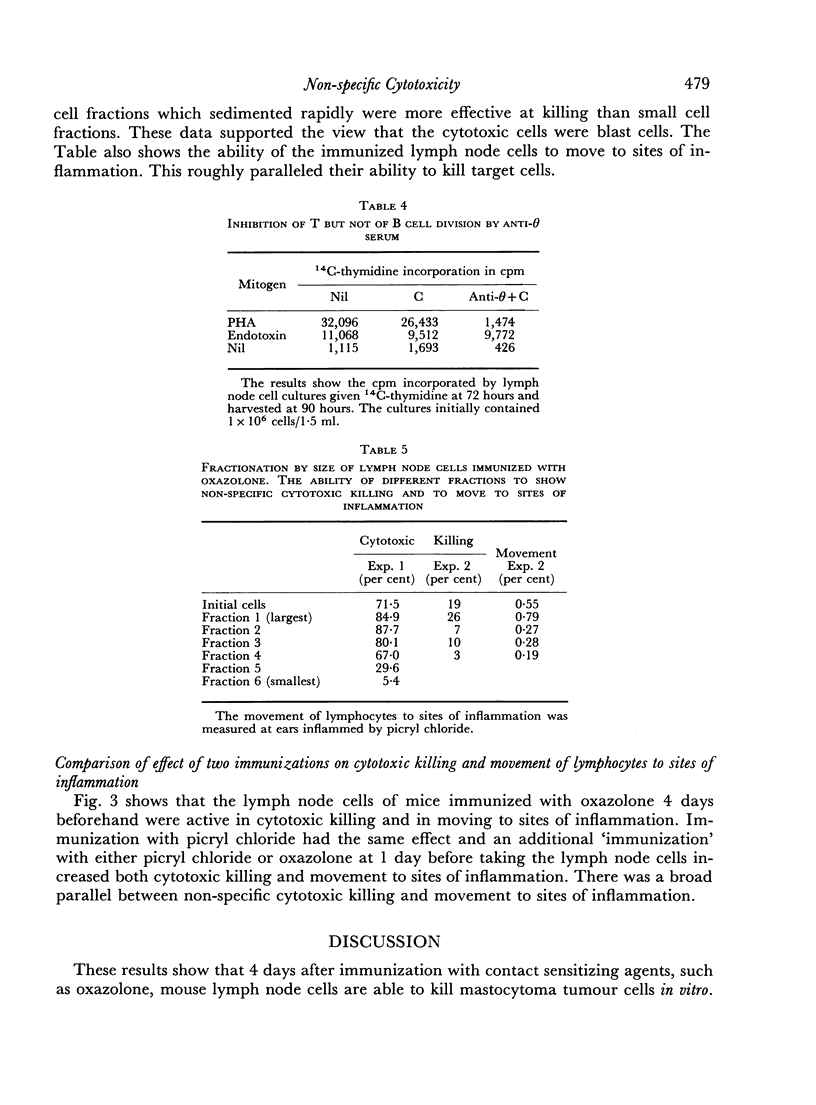
CBA mice were immunized by painting the skin with the contact sensitizing agent `oxazolone'. The draining lymph nodes were taken at 4 days and mixed with 51Cr-labelled mastocytoma tumour cells. Cytotoxic killing was assessed by the release of 51Cr. Little killing occurred in the absence of phytohaemagglutinin. However, immunized but not normal lymph node cells killed the mastocytoma cells providing phytohaemagglutinin (PHA) was present during the killing reaction. Analysis by the `one hit' hypothesis suggested that up to 14 per cent of the lymphocytes in immunized lymph nodes were effector or killer cells.
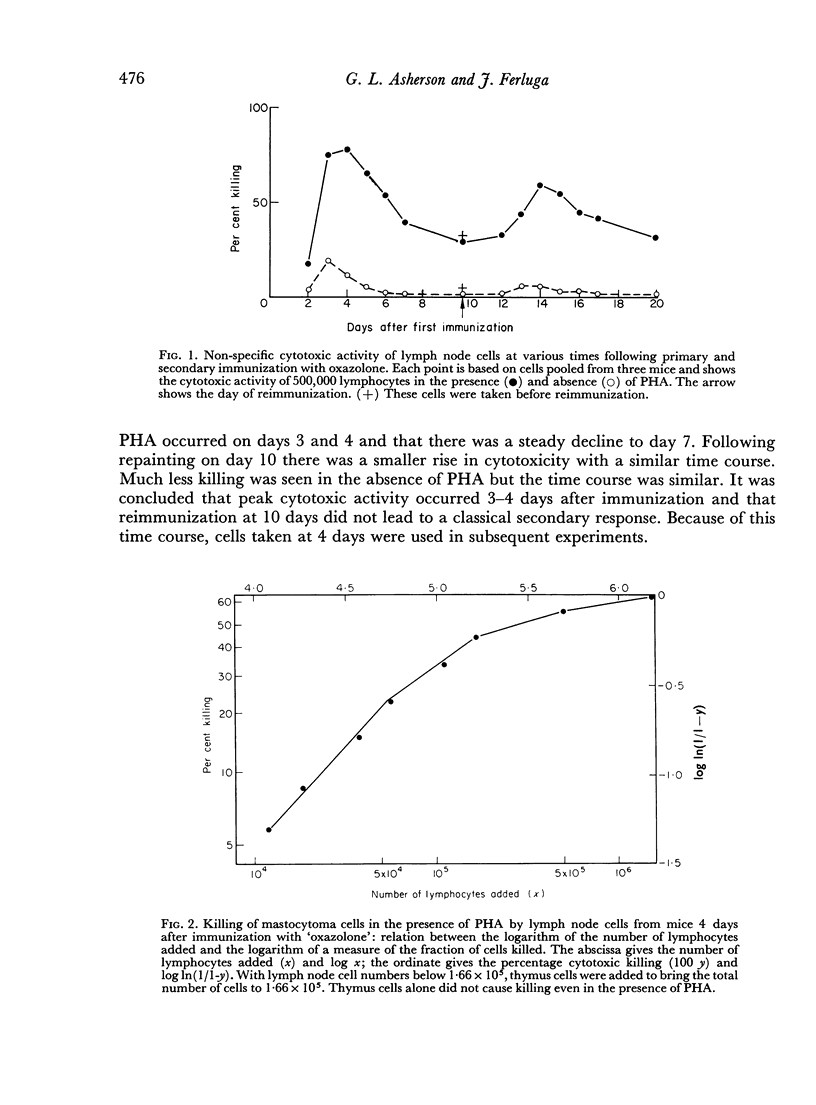
The cytotoxicity was non-specific. The evidence for this was that DBA/2 lymph nodes immunized with oxazolone killed DBA/2 mastocytoma cells. The killing reaction did not require macrophages but was due to large cells. These were shown to be θ-positive in carefully controlled experiments. The non-specific cytotoxicity was greatest 4 days after immunization with oxazolone. Reimmunization of the mice on day 10 did not cause a classical secondary response, as assessed by non-specific cytotoxic killing. Instead the time course resembled the primary while the magnitude of killing was slightly reduced.
There were several similarities between non-specific cytotoxic cells and the large pyroninophilic cells and the cells which move to sites of inflammation which are found in lymph nodes immunized with contact sensitizing agents. These similarities included θ-positivity, large size and peak occurrence 4 days after immunization. It is postulated that non-specific cytotoxicity and movement to sites of inflammation are due to blasts or their immediate descendants which originated from T lymphocytes.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Hall J. G. The role of immunoblasts in host resistance and immunotherapy of primary sarcomata. Adv Cancer Res. 1970;13:1–37. doi: 10.1016/s0065-230x(08)60162-1. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Allwood G. G. Inflammatory lymphoid cells. Cells in immunized lymph nodes that move to sites of inflammation. Immunology. 1972 Mar;22(3):493–502. [PMC free article] [PubMed] [Google Scholar]
- Asherson G. L., Allwood G. G., Mayhew B. Contact sensitivity in the mouse. XI. Movement of T blasts in the draining lymph nodes to sites of inflammation. Immunology. 1973 Sep;25(3):485–494. [PMC free article] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Rudolf H., Chapuis B. Studies of allograft immunity in mice. I. Induction, development and in vitro assay of cellular immunity. Immunology. 1970 Apr;18(4):501–515. [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature. 1970 Dec 26;228(5278):1308–1309. doi: 10.1038/2281308a0. [DOI] [PubMed] [Google Scholar]
- Denham S., Grant C. K., Hall J. G., Alexander P. The occurrence of tow types of cytotoxic lymphoid cells in mice immunised with allogeneic tumour cells. Transplantation. 1970 Apr;9(4):366–382. [PubMed] [Google Scholar]
- Dennert G., Lennox E. Cell interactions in humoral and cell-mediated immunity. Nat New Biol. 1972 Jul 26;238(82):114–116. doi: 10.1038/newbio238114a0. [DOI] [PubMed] [Google Scholar]
- Evans R., Alexander P. Rendering macrophages specifically cytotoxic by a factor released from immune lymphoid cells. Transplantation. 1971 Sep;12(3):227–229. doi: 10.1097/00007890-197109000-00015. [DOI] [PubMed] [Google Scholar]
- Ferluga J., Asherson G. L., Becker E. L. The effect of organophosphorus inhibitors, p-nitrophenol and cytochalasin B on cytotoxic killing of tumour cells by immune spleen cells, and the effect of shaking. Immunology. 1972 Oct;23(4):577–590. [PMC free article] [PubMed] [Google Scholar]
- GOWANS J. L., McGREGOR D. D., COWEN D. M. Initiation of immune responses by small lymphocytes. Nature. 1962 Nov 17;196:651–655. doi: 10.1038/196651a0. [DOI] [PubMed] [Google Scholar]
- Henney C. S. Quantitation of the cell-mediated immune response. I. The number of cytolytically active mouse lymphoid cells induced by immunization with allogeneic mastocytoma cells. J Immunol. 1971 Dec;107(6):1558–1566. [PubMed] [Google Scholar]
- Hogg N. M., Greaves M. F. Antigen-binding thymus-derived lymphocytes. I. Rapid method for isolation of theta-positive antigen-stimulated cells. Immunology. 1972 Jun;22(6):959–965. [PMC free article] [PubMed] [Google Scholar]
- Holm G., Perlmann P. Cytotoxic potential of stimulated human lymphocytes. J Exp Med. 1967 Apr 1;125(4):721–736. doi: 10.1084/jem.125.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy W. E., Nelson D. S. Delayed-type hypersensitivity in mice after skin and tumour allografts and tumour isografts. Nature. 1969 Jun 7;222(5197):1001–1003. doi: 10.1038/2221001a0. [DOI] [PubMed] [Google Scholar]
- Klein E. Hypersensitivity reactions at tumor sites. Cancer Res. 1969 Dec;29(12):2351–2362. [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D. The mediator of cellular immunity. 3. Lymphocyte traffic from the blood into the inflamed peritoneal cavity. J Exp Med. 1971 Apr 1;133(4):864–876. doi: 10.1084/jem.133.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewi G. Non-lymphoid cytoxic killing. Clin Sci. 1971 Aug;41(2):3P–3P. doi: 10.1042/cs041003p. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Harding B. The role of immunoglobulins in lymphocyte-mediated cell damage, in vitro. II. The mechanism of target cell damage by lymphoid cells from immunized rats. Immunology. 1970 Mar;18(3):405–412. [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Brunner K. T., Sprent J., Russell P. J., Mitchell G. F. Thymus-derived cells as killer cells in cell-mediated immunity. Transplant Proc. 1971 Mar;3(1):915–917. [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Sprent J., Miller J. F. Interaction of thymus lymphocytes with histoincompatible cells. II. Recirculating lymphocytes derived from antigen-activated thymus cells. Cell Immunol. 1972 Mar;3(3):385–404. doi: 10.1016/0008-8749(72)90245-6. [DOI] [PubMed] [Google Scholar]
- Stavy L., Treves A. J., Feldman M. Capacity of thymic cells to effect target cell lysis following treatment with concanavalin A. Cell Immunol. 1972 Apr;3(4):623–628. doi: 10.1016/0008-8749(72)90124-4. [DOI] [PubMed] [Google Scholar]
- Turk J. L. Cytology of the induction of hypersensitivity. Br Med Bull. 1967 Jan;23(1):3–8. doi: 10.1093/oxfordjournals.bmb.a070511. [DOI] [PubMed] [Google Scholar]
- Volkman A. The origin and turnover of mononuclear cells in peritoneal exudates in rats. J Exp Med. 1966 Aug 1;124(2):241–254. doi: 10.1084/jem.124.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]


