Abstract
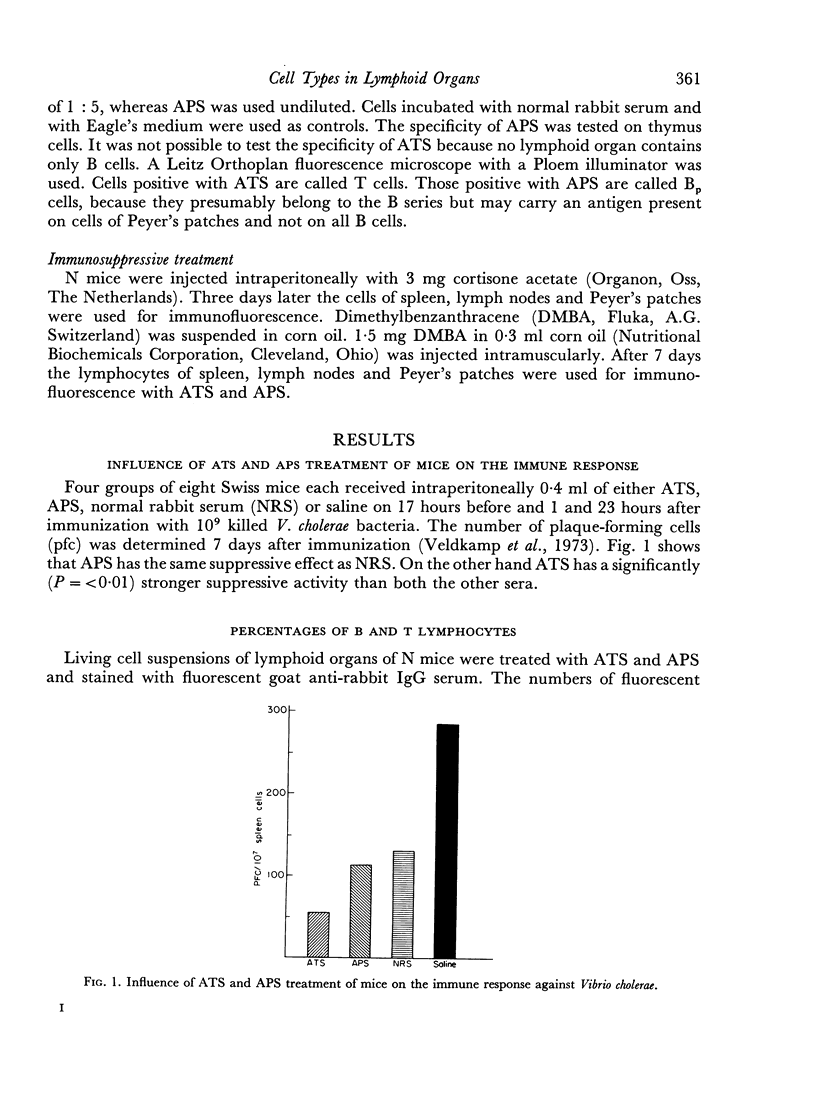
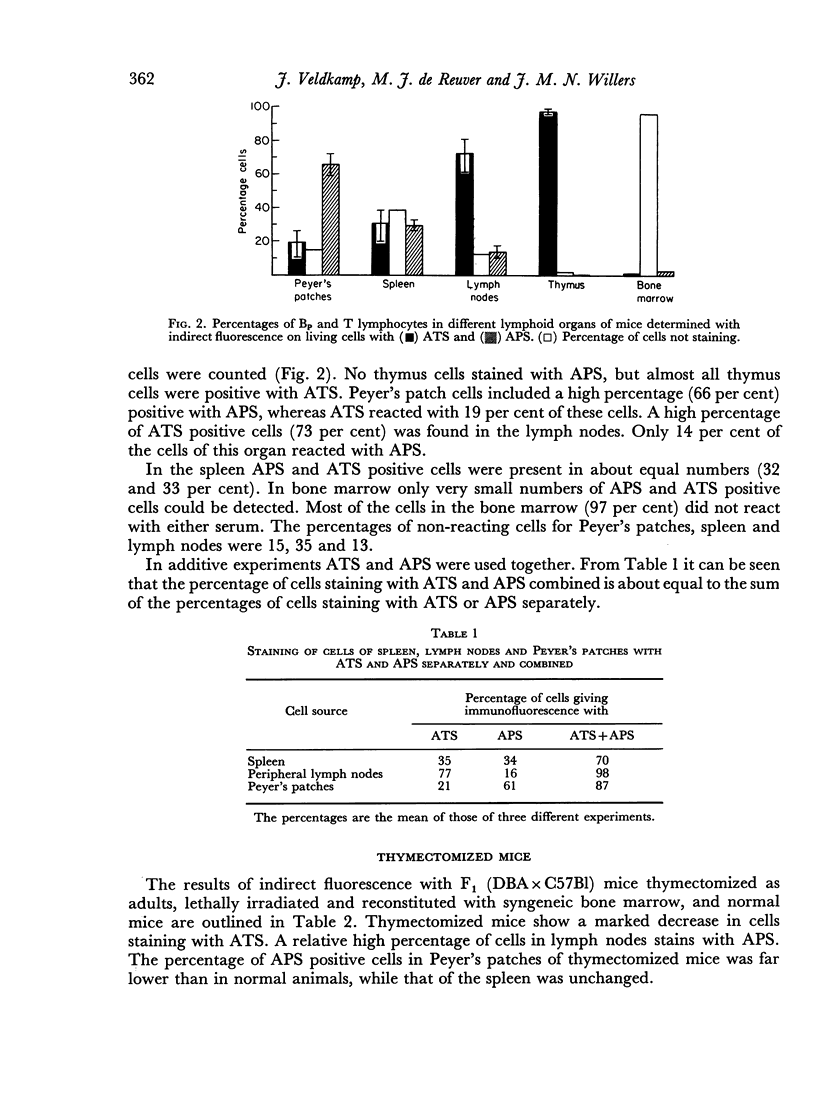
Anti-thymus serum (ATS) absorbed with Peyer's patch cells and anti-Peyer's patch serum (APS) absorbed with thymus cells were prepared. ATS treatment of mice resulted in a suppression of the immune response, measured with Vibrio cholerae as an antigen. APS did not have this effect. ATS and APS were used in the indirect fluorescence test to detect T and Bp cells. The cells staining with APS were called Bp cells to indicate that APS might only detect a special fraction of B cells (mature B cells). In Peyer's patches 66 per cent of the cells reacted with APS (Bp cells), whereas only 19 per cent cells reacted with ATS (T cells). The percentage of T cells in the lymph nodes was high (73 per cent), whereas only 14 per cent Bp cells were found in this organ. In the spleen almost equal numbers of Bp and T lymphocytes (32 per cent and 33 per cent) were detected. However, 35 per cent of the lymphocytes were non-reactive with either anti-serum. Bone marrow contained only small numbers of reactive cells (1 per cent T and 2 per cent Bp cells).
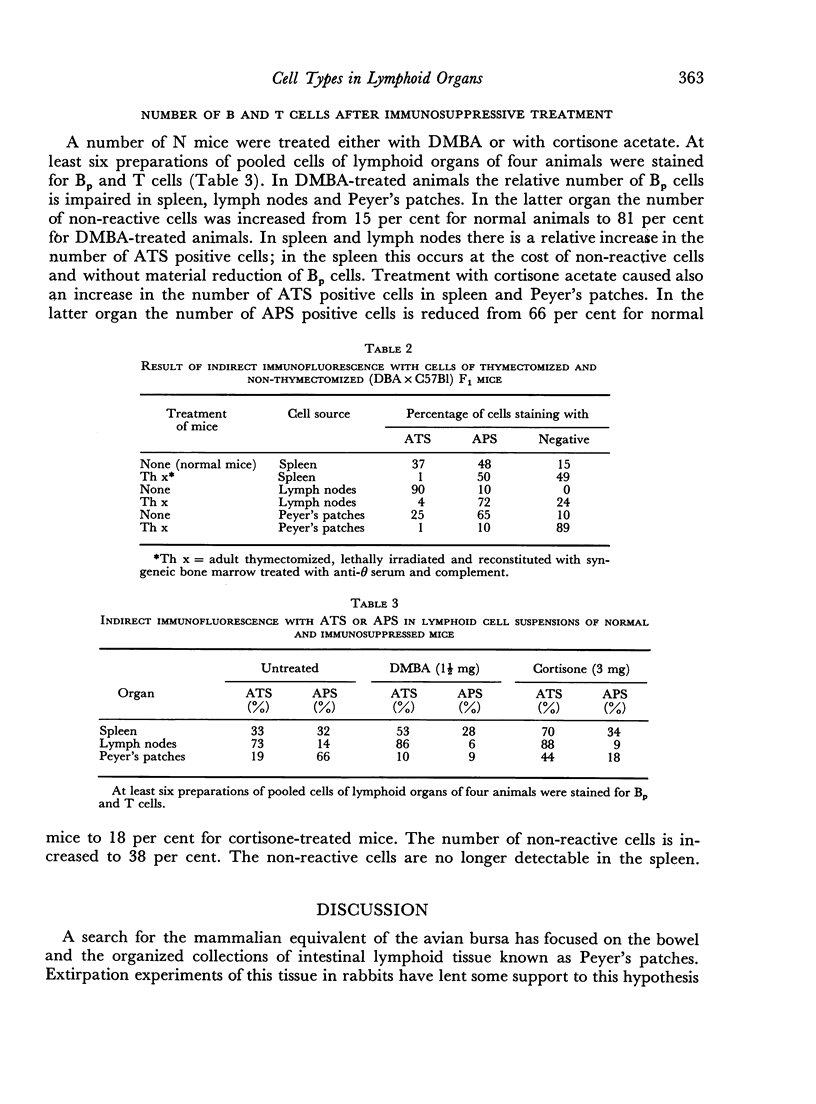
Treatment of mice with dimethylbenzanthracene (DMBA) caused an increase in the number of non-reactive cells in Peyer's patches and a dramatic decrease in the number of Bp cells. The relative increase of the number of T cells in spleen and lymph nodes was not found in Peyer's patches. Cortisone acetate seems also to act primarily on B cells, especially on those of Peyer's patches. In this case also an increase in the proportion of T cells in the spleen was detected.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Fschbach M., Claman H. N. Hydrocortisne resistance of graft vs host activity in mouse thymus, spleen and bone marrow. J Immunol. 1970 Nov;105(5):1146–1150. [PubMed] [Google Scholar]
- Kerckhaert J. A., Benner R., Willers J. M. Cells involved in the graft-versus-host reaction in vitro. Immunology. 1973 Jul;25(1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Perey D. Y., Frommel D., Hong R., Good R. A. The mammalian homologue of the avian bursa of Fabricius. II. Extirpation, lethal x-irradiation, and reconstitution in rabbits. Effects on humoral immune responses, immunoglobulins, and lymphoid tissues. Lab Invest. 1970 Mar;22(3):212–227. [PubMed] [Google Scholar]
- Potworowski E. F. T and B lymphocytes. Organ and age distribution in the chicken. Immunology. 1972 Aug;23(2):199–204. [PMC free article] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino E., Colon S., Grey H. M., Unanue E. R. Immunoglobulins on the surface of lymphocytes. I. Distribution and quantitation. J Exp Med. 1971 Jan 1;133(1):156–167. doi: 10.1084/jem.133.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Nase S., Mitchison N. A. Mouse specific bone marrow-derived lymphocyte antigen as a marker for thymus-independent lymphocytes. Nature. 1971 Mar 5;230(5288):50–51. doi: 10.1038/230050a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Sternberg M., Taylor R. B. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970 Feb 7;225(5232):553–554. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- Reif A. E., Allen J. M. Mouse thymic iso-antigens. Nature. 1966 Jan 29;209(5022):521–523. doi: 10.1038/209521b0. [DOI] [PubMed] [Google Scholar]
- Rowland G. F., Hurd C. M. Target lymphoid cell population of carcinogen induced immunodepression in mice. Nature. 1970 Jul 11;227(5254):167–168. doi: 10.1038/227167a0. [DOI] [PubMed] [Google Scholar]
- Schlesinger M., Yron I. Antigenic changes in lymph-node cells after administration of antiserum to thymus cells. Science. 1969 Jun 20;164(3886):1412–1413. doi: 10.1126/science.164.3886.1412. [DOI] [PubMed] [Google Scholar]
- Veldkamp J., van der Gaag R., Willers J. M. The role of Peyer's patch cells in antibody formation. Immunology. 1973 Nov;25(5):761–771. [PMC free article] [PubMed] [Google Scholar]


