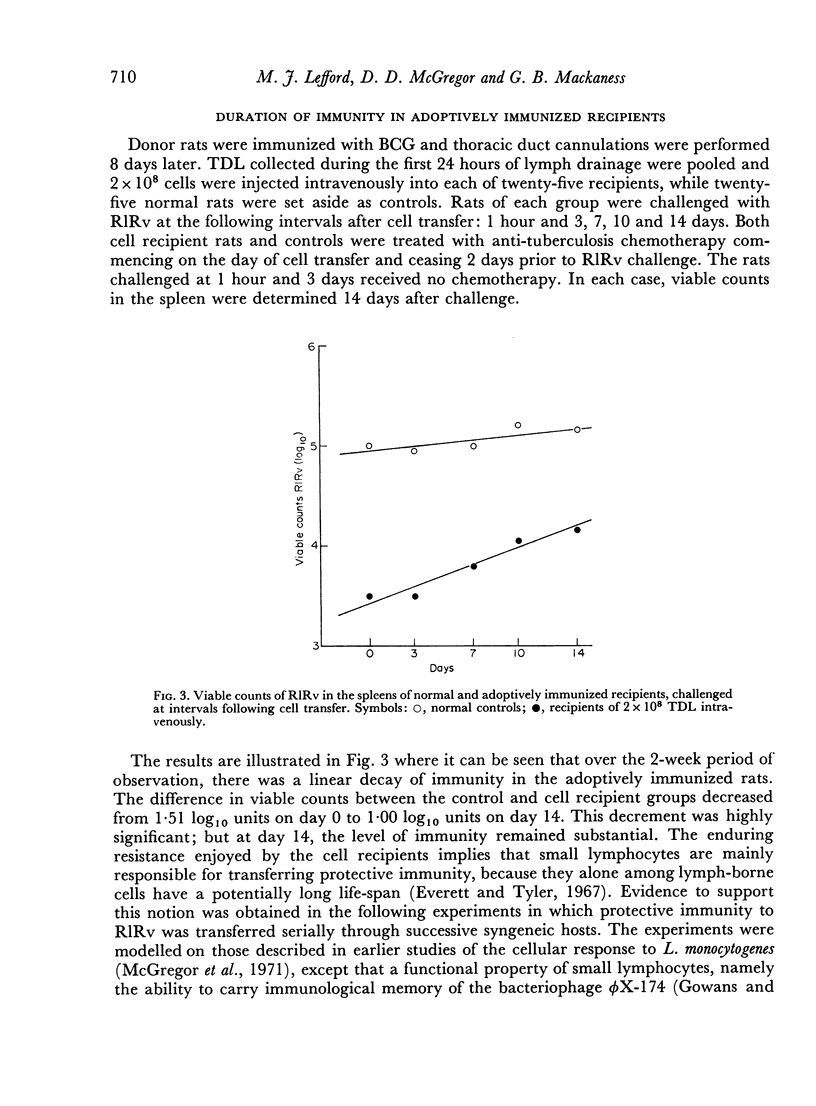
Abstract
Following immunization of rats with BCG there was added to thoracic duct lymph a population of sensitized lymphocytes which conferred adoptive immunity to tuberculosis upon syngeneic recipients. At an early stage of immunization, 5 days after BCG inoculation, the sensitized cells were large and medium lymphocytes as evidenced by their sensitivity to vinblastine. On day 8, the sensitized cell population was partially resistant to vinblastine and by day 28 it was totally resistant. The vinblastine resistant cells have the functional properties of small lymphocytes, since they recirculate freely from the blood to lymph, and can confer long lasting immunity upon recipient rats.
The data imply that protective immunity against the tubercle bacillus is transferred by a family of newly-formed lymphocytes, the most immature of which are immunoblasts or large lymphocytes. As the response matures, sensitized small lymphocytes are delivered to the circulation and comprise an increasing proportion of the protective cell population.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad M. A. Suppression of delayed hypersensitivity by antigen and antibody. Is a common precursor cell responsible for both delayed hypersensitivity and antibody formation? Immunology. 1968 Aug;15(2):159–171. [PMC free article] [PubMed] [Google Scholar]
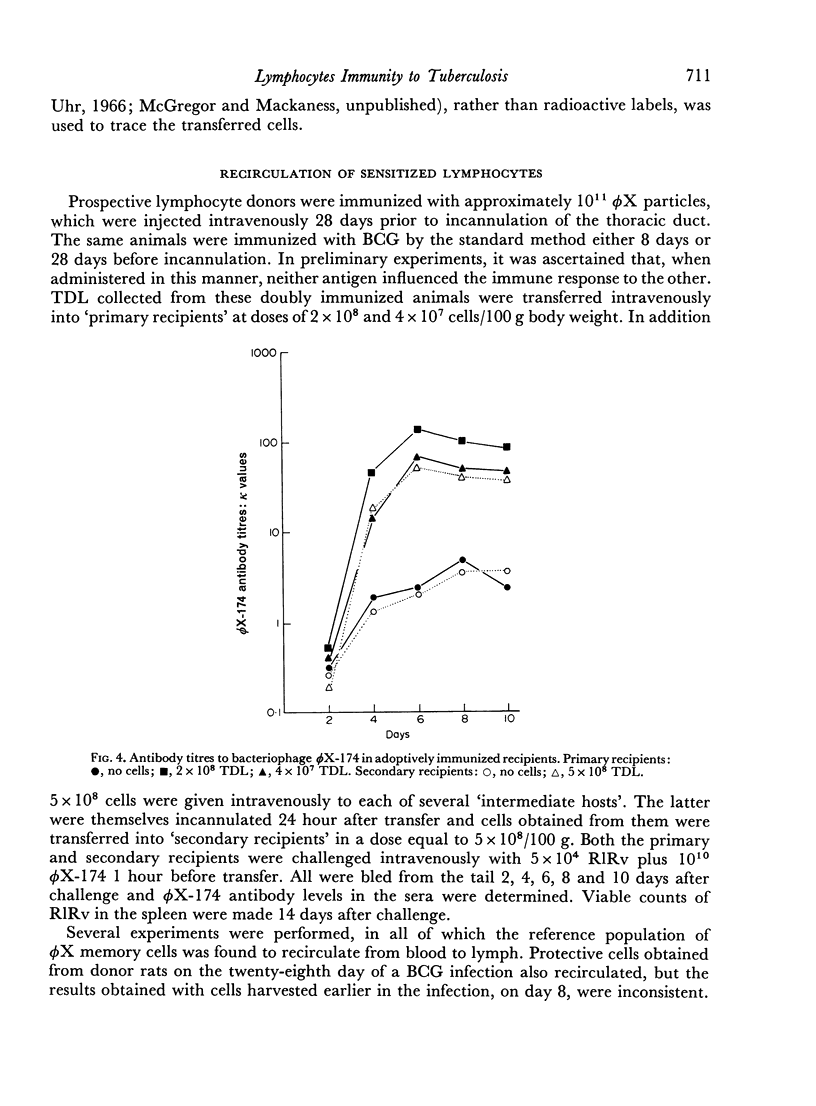
- Baldwin R. W., Price M. R., Robins R. A. Blocking of lymphocyte-mediated cytotoxicity for rat hepatoma cells by tumour-specific antigen-antibody complexes. Nat New Biol. 1972 Aug 9;238(84):185–186. doi: 10.1038/newbio238185a0. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Owen A. A., Becker A. J., Till J. E. Effects of vinblastine on the proliferative capacity of L cells and their progress through the division cycle. Cancer Res. 1965 Sep;25(8):1232–1237. [PubMed] [Google Scholar]
- CAFFREY R. W., RIEKE W. O., EVERETT N. B. Radioautographic studies of small lymphocytes in the thoracic duct of the rat. Acta Haematol. 1962;28:145–154. doi: 10.1159/000207257. [DOI] [PubMed] [Google Scholar]
- Delorme E. J., Hodgett J., Hall J. G., Alexander P. The cellular immune response to primary sarcomata in rats. I. The significance of large basophilic cells in the thoracic duct lymph following antigenic challenge. Proc R Soc Lond B Biol Sci. 1969 Nov 18;174(1035):229–236. doi: 10.1098/rspb.1969.0089. [DOI] [PubMed] [Google Scholar]
- Everett N. B., Tyler R. W. Lymphopoiesis in the thymus and other tissues: functional implications. Int Rev Cytol. 1967;22:205–237. doi: 10.1016/s0074-7696(08)61836-7. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959 Apr 23;146(1):54–69. doi: 10.1113/jphysiol.1959.sp006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Cohen P., Hencin R., Liebhaber S. A. Suppressor T cells. J Immunol. 1972 Mar;108(3):586–590. [PubMed] [Google Scholar]
- Gowans J. L., Uhr J. W. The carriage of immunological memory by small lymphocytes in the rat. J Exp Med. 1966 Nov 1;124(5):1017–1030. doi: 10.1084/jem.124.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylko E., Mackaness G. B. The kinetics of lymphoid cell proliferation in the tuberculous mouse spleen. Cell Immunol. 1972 Sep;5(1):148–170. doi: 10.1016/0008-8749(72)90092-5. [DOI] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Immunological enhancement as studied by cell culture techniques. Annu Rev Microbiol. 1970;24:373–398. doi: 10.1146/annurev.mi.24.100170.002105. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D., Mackaness G. B. The mediator of cellular immunity. II. Migration of immunologically committed lymphocytes into inflammatory exudates. J Exp Med. 1971 Feb 1;133(2):400–409. doi: 10.1084/jem.133.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T., Mackaness G. B. The mediator of cellular immunity. I. The life-span and circulation dynamics of the immunologically committed lymphocyte. J Exp Med. 1971 Feb 1;133(2):389–399. doi: 10.1084/jem.133.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Logie P. S. The mediator of cellular immunity. VI. Effect of the antimitotic drug vinblastine on the mediator of cellular resistance to infection. J Exp Med. 1973 Mar 1;137(3):660–674. doi: 10.1084/jem.137.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Mackaness G. B., Elliott R. W. The histogenesis of immunologically committed lymphocytes. Cell Immunol. 1972 Apr;3(4):680–694. doi: 10.1016/0008-8749(72)90130-x. [DOI] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALMER C. G., LIVENGOOD D., WARREN A. K., SIMPSON P. J., JOHNSON I. S. The action of the vincaleukolastine on mitosis in vitro. Exp Cell Res. 1960 Jun;20:198–201. doi: 10.1016/0014-4827(60)90234-2. [DOI] [PubMed] [Google Scholar]
- ROBINSON S. H., BRECHER G., LOURIE I. S., HALEY J. E. LEUKOCYTE LABELING IN RATS DURING AND AFTER CONTINUOUS INFUSION OF TRITIATED THYMIDINE: IMPLICATIONS FOR LYMPHOCYTE LONGEVITY AND DNA REUTILIZATION. Blood. 1965 Sep;26:281–295. [PubMed] [Google Scholar]
- Smith J. B., Pedersen N. C., Morris B. The role of the lymphatic system in inflammatory responses. Ser Haematol. 1970;3(2):17–61. [PubMed] [Google Scholar]
- UHR J. W., FINKELSTEIN M. S., BAUMANN J. B. Antibody formation. III. The primary and secondary antibody response to bacteriophage phi X 174 in guinea pigs. J Exp Med. 1962 Mar 1;115:655–670. doi: 10.1084/jem.115.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]


