Abstract
Whole serum or γ globulin derived from fowls, either susceptible or immune to coccidiosis (Eimeria tenella), was injected into fully susceptible fowls.
The serum proteins were given by intravenous or intraperitoneal injection and subsequent attempts were made to infect these fowls by giving either a moderate dose of sporulated oocysts per os or a suspension of viable merozoites per rectum.
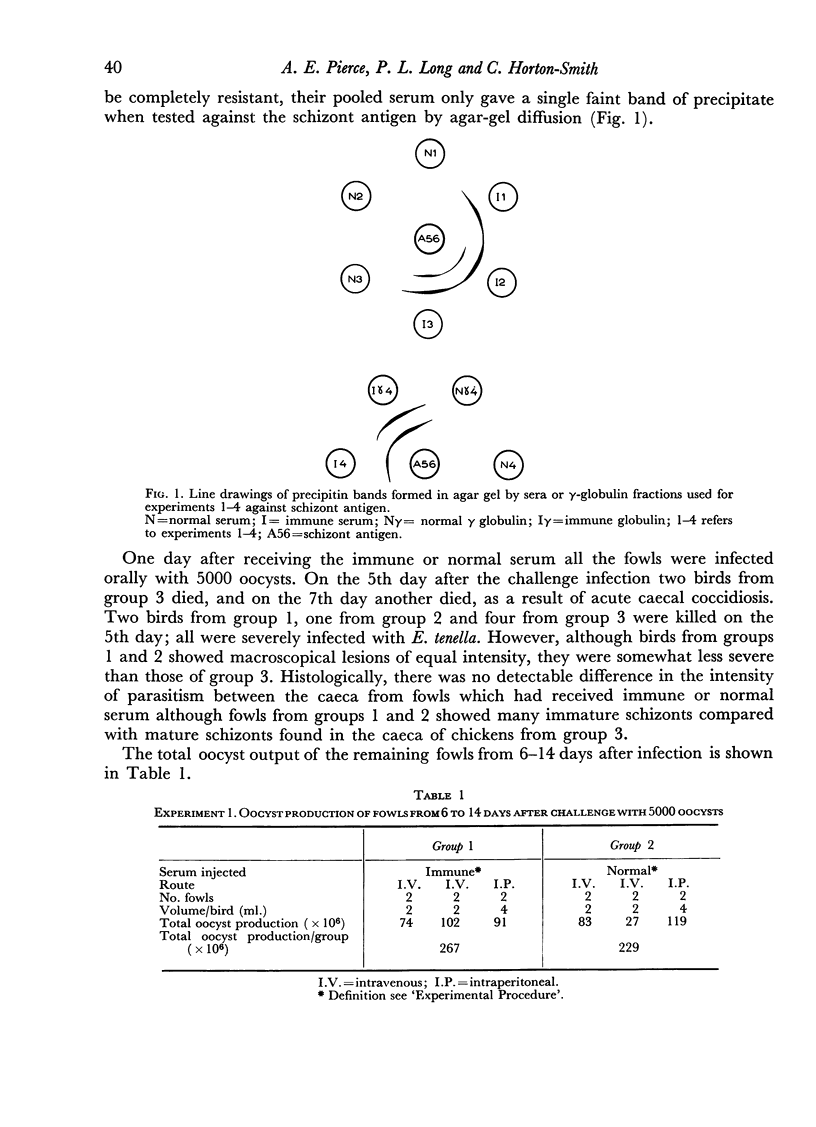
In the agar-gel double diffusion test, the serum from the donor birds, resistant to E. tenella, formed only weak lines of precipitate when reacting against an antigen prepared from the second schizont stage of the life cycle. However, the immune serum was considered satisfactory because the donor birds on challenge were immune.
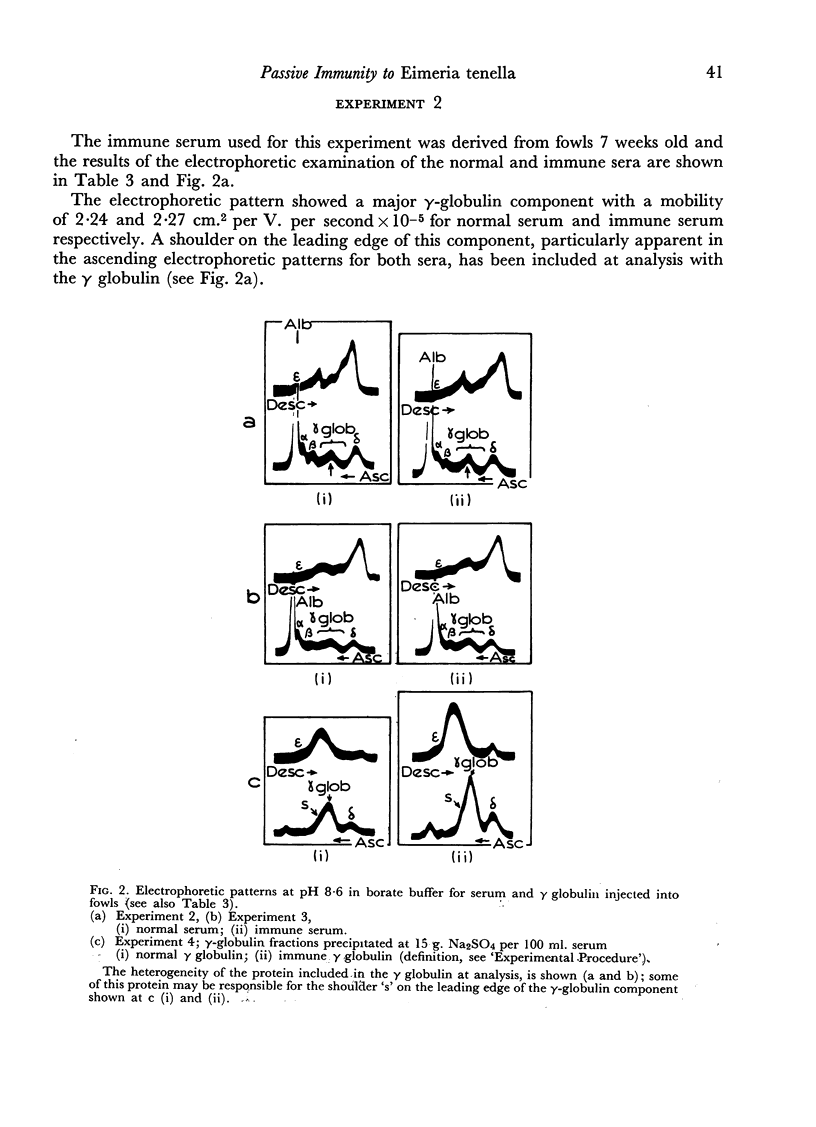
Although relatively large amounts of γ globulin were injected into the susceptible fowls (up to 0.88 g. per kg. body weight) and subsequently only mild infections were given, no passively acquired resistance was shown either from the results of the oocyst counts on the faeces or by a macrosopic or microscopic examination of the caeca.
These results are discussed in relation to earlier studies; they show that passively acquired serum antibody at these dose levels did not provide protection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS W. C., CHALLEY J. R. Resistance of birds to challenge with Eimeria tenella. Exp Parasitol. 1959 Dec;8:515–526. doi: 10.1016/s0014-4894(59)80001-1. [DOI] [PubMed] [Google Scholar]
- BUXTON A. On the transference of bacterial antibodies from the hen to the chick. J Gen Microbiol. 1952 Nov;7(3-4):268–286. doi: 10.1099/00221287-7-3-4-268. [DOI] [PubMed] [Google Scholar]
- HORTON-SMITH C., BEATTIE J., LONG P. L. Resistance to Eimeria tenella and its transference from one caecum to the other in individual fowls. Immunology. 1961 Apr;4:111–121. [PMC free article] [PubMed] [Google Scholar]
- HORTON-SMITH C., LONG P. L. Studies in histomoniasis. I. The infection of chickens (Gallus gallus) with histomonad suspensions. Parasitology. 1956 May;46(1-2):79–90. [PubMed] [Google Scholar]
- HORTON-SMITH C., LONG P. L. The effects of different anticoccidial agents on the intestinal coccidioses of the fowl. J Comp Pathol. 1959 Apr;69(2):192–207. doi: 10.1016/s0368-1742(59)80018-7. [DOI] [PubMed] [Google Scholar]
- KOSHLAND M. E. The origin of fecal antibody and its relationship to immunization with adjuvant. J Immunol. 1953 Apr;70(4):359–365. [PubMed] [Google Scholar]
- LEVINE P. P., FABRICANT J. Susceptibility to Newcastle infection of chicks with congenital serum antibodies. Cornell Vet. 1950 Apr;40(2):213–225. [PubMed] [Google Scholar]
- LONG P. L., ROSE M. E. Attempted transfer of resistance to Eimeria tenella infections from domestic hens to their progeny. Exp Parasitol. 1962 Apr;12:75–81. [PubMed] [Google Scholar]
- PIERCE A. E., LONG P. L., HORTON-SMITH C. Immunity to Eimeria tenella in young fowls (Gallus domesticus). Immunology. 1962 Jan;5:129–152. [PMC free article] [PubMed] [Google Scholar]
- ROSE M. E., LONG P. L. Immunity to four species of Eimeria in fowls. Immunology. 1962 Jan;5:79–92. [PMC free article] [PubMed] [Google Scholar]


