Abstract
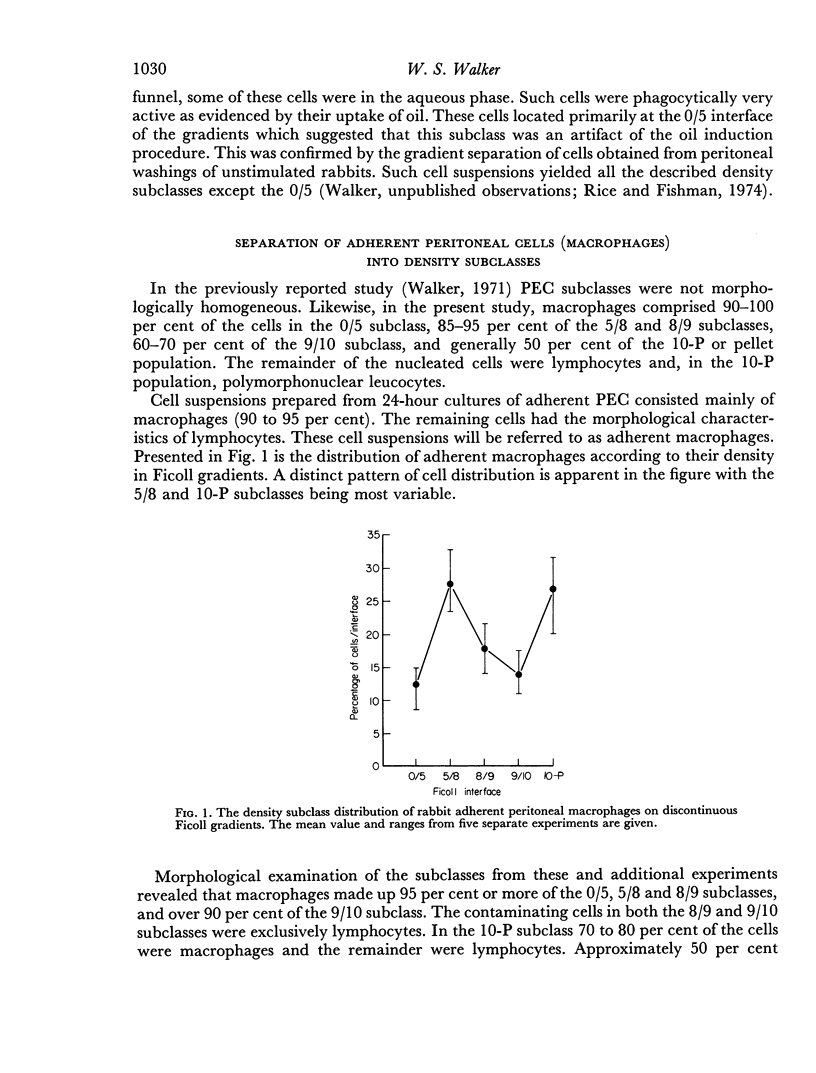
Cell suspensions prepared from 24-hour cultures of adherent peritoneal exudate cells from rabbits were separated into density subclasses on discontinuous gradients of Ficoll. Of the five subclasses obtained, macrophages comprised over 95 per cent of the cells in the two least dense subclasses and over 90 per cent of the cells in the subclasses of intermediate density. The most dense subclass contained approximately 80 per cent macrophages and 20 per cent lymphocytes.
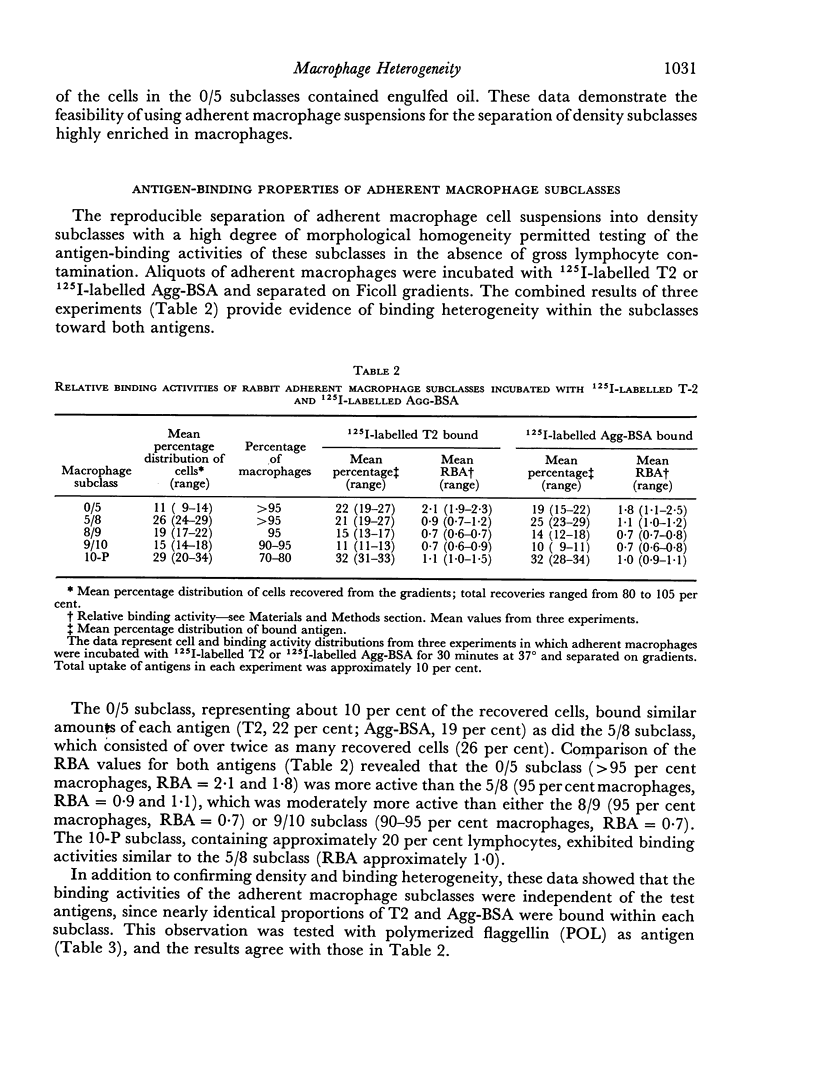
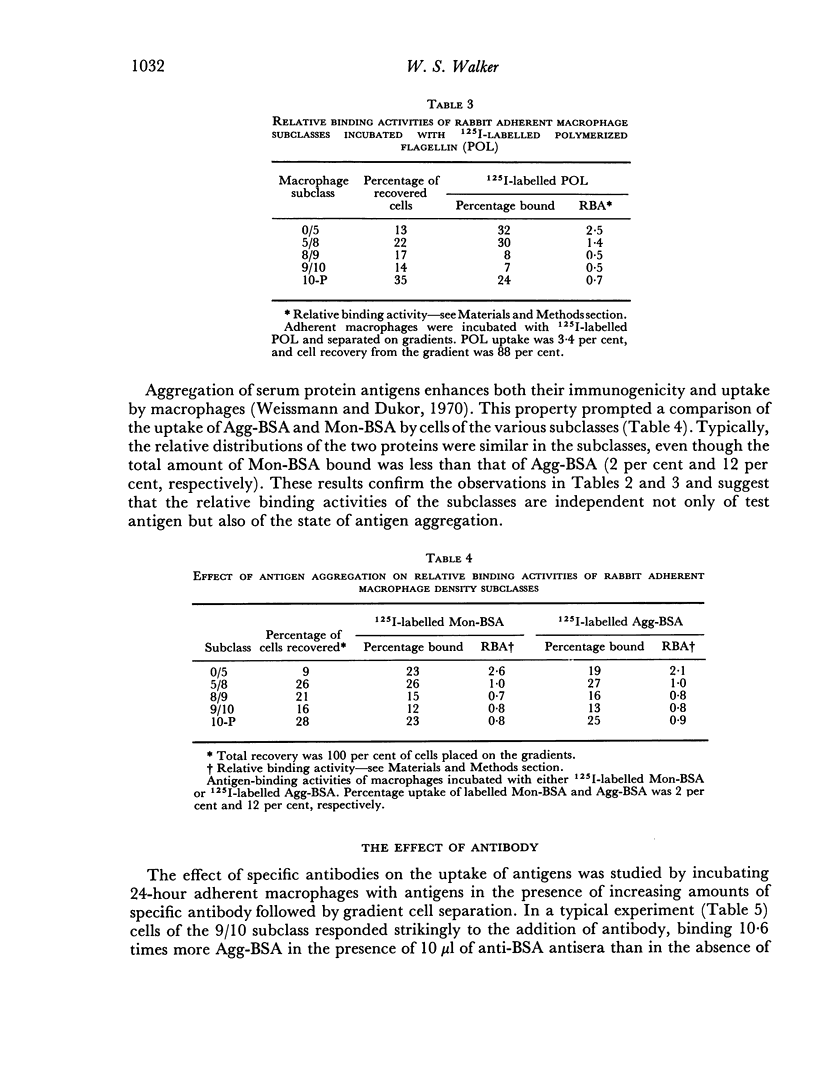
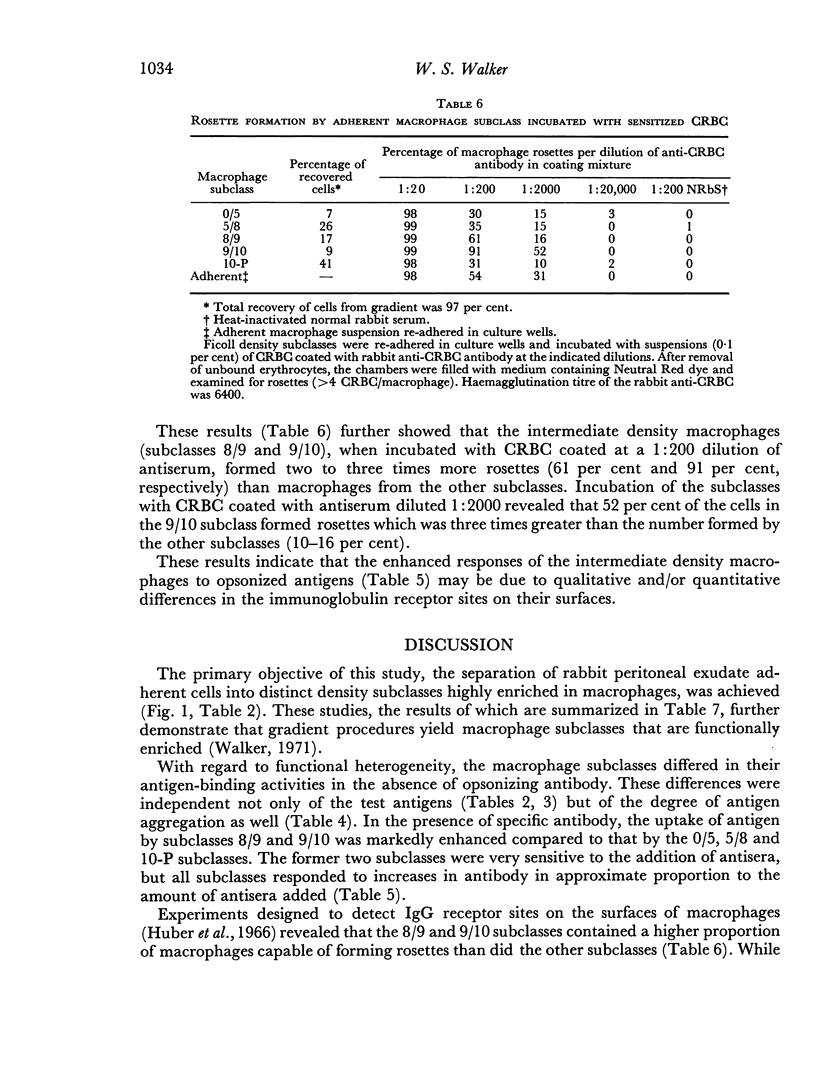
The antigen-binding properties of the subclasses were studied with a selected number of 125I-labelled antigens in the presence and absence of added antibodies. In the absence of antibody the subclasses differed from one another in antigen-binding activities; these differences were independent of both the test antigens and the state of antigen aggregation. In the presence of specific antibody, two macrophage subclasses bound substantially more antigen than the other subclasses. The enhanced responses of these subclasses were confirmed by studying the cells' capacity to form rosettes when incubated with sensitized chicken erythrocytes. The results indicated that macrophages in these two subclasses differed quantitatively and/or qualitatively from other macrophage subclasses with regard to immunoglobulin receptor sites.
These studies demonstrate the feasibility of using gradient separation procedures to obtain functionally distinct subclasses of cells rich in macrophages. The availability of well-defined macrophage populations should permit more precise studies of macrophage functions in immunity.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., NOSSAL G. J., PYE J., ABBOT A. ANTIGENS IN IMMUNITY. I. PREPARATION AND PROPERTIES OF FLAGELLAR ANTIGENS FROM SALMONELLA ADELAIDE. Aust J Exp Biol Med Sci. 1964 Jun;42:267–282. [PubMed] [Google Scholar]
- Adler F. L., Fishman M., Dray S. Antibody formation initiated in vitro. 3. Antibody formation and allotypic specificity directed by ribonucleic acid from peritoneal exudate cells. J Immunol. 1966 Oct;97(4):554–558. [PubMed] [Google Scholar]
- Arend W. P., Mannik M. The macrophage receptor for IgG: number and affinity of binding sites. J Immunol. 1973 Jun;110(6):1455–1463. [PubMed] [Google Scholar]
- Berken A., Benacerraf B. Properties of antibodies cytophilic for macrophages. J Exp Med. 1966 Jan 1;123(1):119–144. doi: 10.1084/jem.123.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- FISHMAN M. Antibody formation in vitro. J Exp Med. 1961 Dec 1;114:837–856. doi: 10.1084/jem.114.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M. Cell interactions in the immune response in vitro. II. The requirement for macrophages in lymphoid cell collaboration. J Exp Med. 1972 May 1;135(5):1049–1058. doi: 10.1084/jem.135.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M. Cell interactions in the immune response in vitro. V. Specific collaboration via complexes of antigen and thymus-derived cell immunoglobulin. J Exp Med. 1972 Oct 1;136(4):737–760. doi: 10.1084/jem.136.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Christie G. H., Boak J. L., Kinsky R. G. Peritoneal and alveolar macrophages derived from lymphocyte populations during graft-versus-host reaction. Br J Exp Pathol. 1969 Oct;50(5):448–455. [PMC free article] [PubMed] [Google Scholar]
- Huber H., Douglas S. D., Fudenberg H. H. The IgG receptor: an immunological marker for the characterization of mononuclear cells. Immunology. 1969 Jul;17(1):7–21. [PMC free article] [PubMed] [Google Scholar]
- Kölsch E., Mitchison N. A. The subcellular distribution of antigen in macrophages. J Exp Med. 1968 Nov 1;128(5):1059–1079. doi: 10.1084/jem.128.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu R. W., Eddleston A. L., Hadden J. W., Good R. A. Mechanism of action of migration inhibitory factor (MIF). I. Evidence for a receptor for MIF present on the peritoneal macrophage but not on the alveolar macrophage. J Exp Med. 1972 Sep 1;136(3):589–603. doi: 10.1084/jem.136.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mitchison N. A. The immunogenic capacity of antigen taken up by peritoneal exudate cells. Immunology. 1969 Jan;16(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. Receptor for monomeric IgM on guinea-pig splenic macrophages. Nature. 1973 Jun 29;243(5409):527–528. doi: 10.1038/243527a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Cerottini J. C. The immunogenicity of antigen bound to the plasma membrane of macrophages. J Exp Med. 1970 Apr 1;131(4):711–725. doi: 10.1084/jem.131.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker W. S., Liu C. T., Adler F. L. Detection of anti-phage antibody by passive hemagglutination. J Immunol. 1969 Nov;103(5):907–916. [PubMed] [Google Scholar]
- Weissmann G., Dukor P. The role of lysosomes in immune responses. Adv Immunol. 1970;12:283–331. doi: 10.1016/s0065-2776(08)60172-8. [DOI] [PubMed] [Google Scholar]
- Wolberg G., Liu C. T., Adler F. L. Studies on passive hemagglutination. I. Titration of "early" and "late" antibodies with tanned red cells sensitized with native or denatured bovine serum albumin. J Immunol. 1969 Nov;103(5):879–890. [PubMed] [Google Scholar]
- Zembala M., Asherson G. L. The rapid purification of peritoneal exudate macrophages by ficoll (polysucrose) density gradient centrifugation. Immunology. 1970 Oct;19(4):677–681. [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


