Abstract
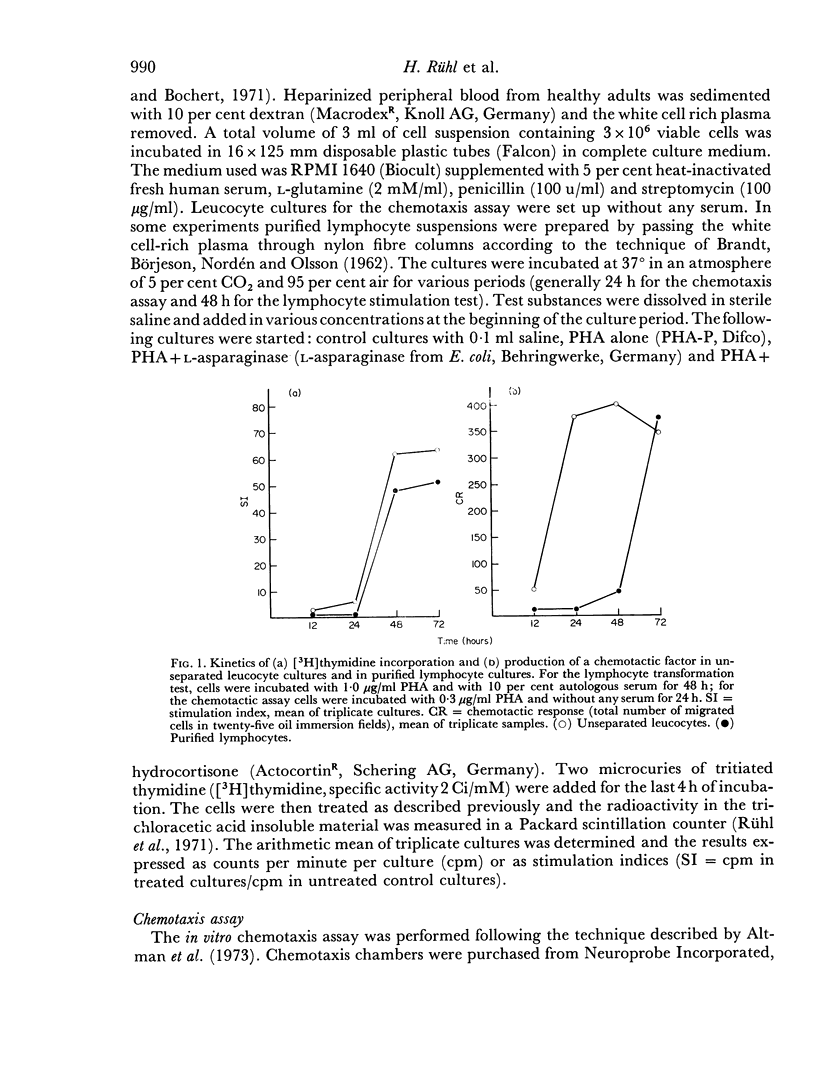
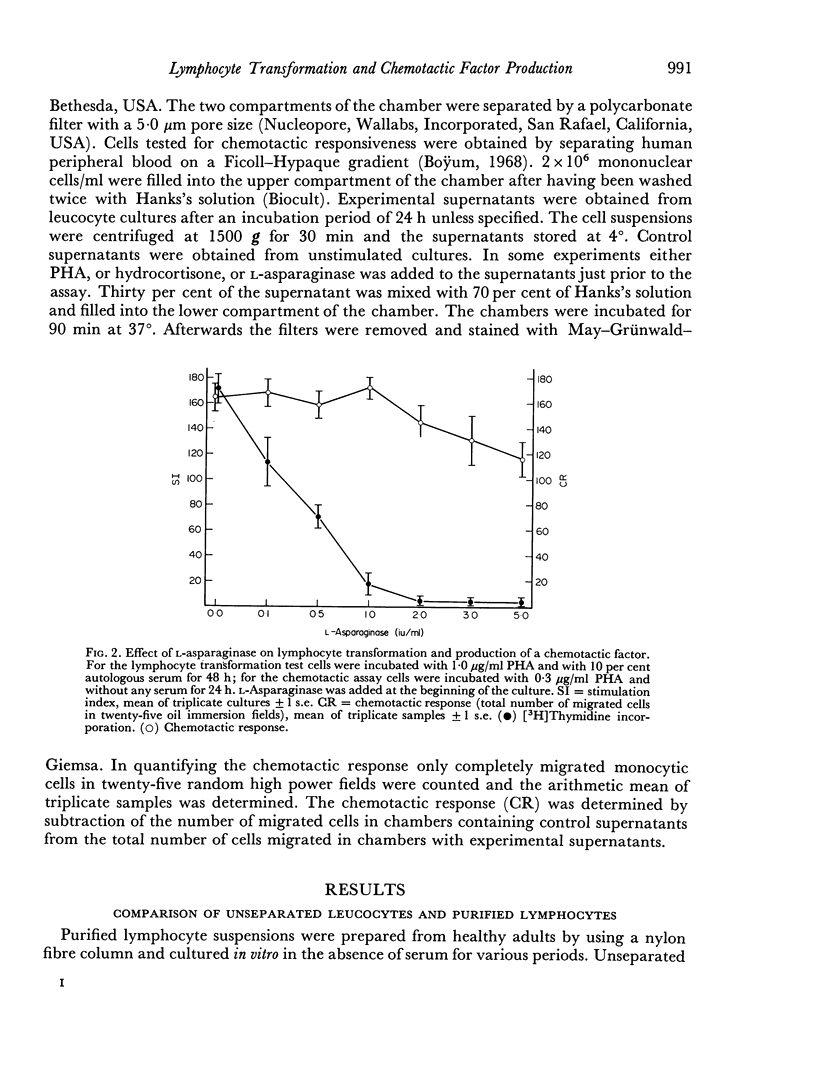
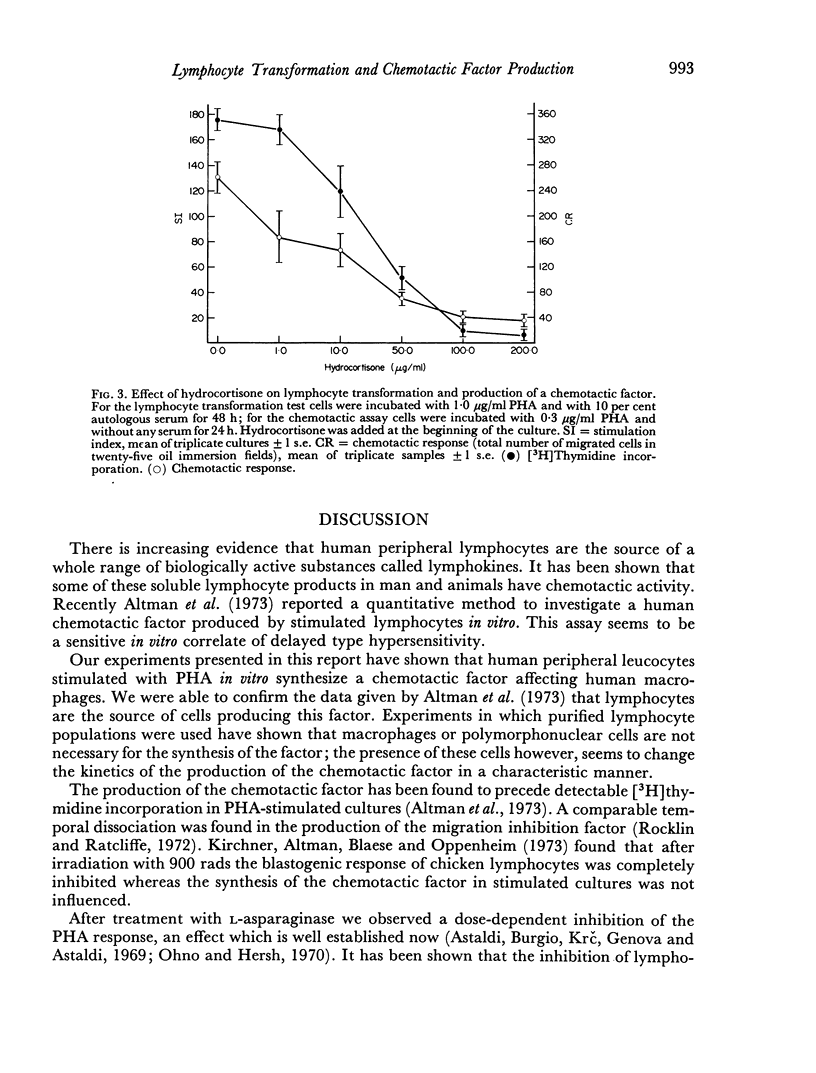
Human peripheral lymphocytes stimulated in vitro with PHA produce a soluble factor which is chemotactic for homologous monocytes. The synthesis of this factor was found to precede the blastogenic response as measured by [3H] thymidine incorporation. In cultures of unseparated leucocytes the maximum of chemotactic activity was detected after 24 hr, whereas in supernatants from purified lymphocyte suspensions the maximal synthesis occurs after 72 hr. High doses of L-asparaginase from E. coli which have been found to prevent lymphocyte transformation completely have no influence on the production of the chemotactic factor. Therefore it seems possible that the induction of DNA synthesis by PHA and its effect on the production of a chemotactic factor depend on different biochemical mechanisms. In contrast hydrocortisone leads to a dose-dependent inhibition of both DNA synthesis and chemotactic response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. C., Snyderman R., Oppenheim J. J., Mergenhagen S. E. A human mononuclear leukocyte chemotactic factor: characterization, specificity and kinetics of production by homologous leukocytes. J Immunol. 1973 Mar;110(3):801–810. [PubMed] [Google Scholar]
- BRANDT L., BORJESON J., NORDEN A., OLSSON I. Separation of lymphocytes from peripheral blood by means of a glass wool column. A method for in vitro culture of lymphocytes. Acta Med Scand. 1962 Oct;172:459–462. doi: 10.1111/j.0954-6820.1962.tb07179.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Caron G. A. Prednisolone inhibition of DNA synthesis by human lymphocytes induced in 1itro by phytohaemagglutinin. Int Arch Allergy Appl Immunol. 1967;32(2):191–200. doi: 10.1159/000229927. [DOI] [PubMed] [Google Scholar]
- Elrod L. M., Schrek R. In vitro reactions of lymphocytes from normal mice and from viral and x-ray-induced lymphomas. Cancer Res. 1966 Jul;26(7):1324–1329. [PubMed] [Google Scholar]
- NOWELL P. C. Inhibition of human leukocyte mitosis by prednisolone in vitro. Cancer Res. 1961 Dec;21:1518–1521. [PubMed] [Google Scholar]
- Ohno R., Hersh E. M. The inhibition of lymphocyte blastogenesis by L-asparaginase. Blood. 1970 Feb;35(2):250–262. [PubMed] [Google Scholar]
- Pick E., Turk J. L. The biological activities of soluble lymphocyte products. Clin Exp Immunol. 1972 Jan;10(1):1–23. [PMC free article] [PubMed] [Google Scholar]
- Rühl H., Kirchner H., Bochert G. Kinetics of the Zn 2+ - stimulation of human peripheral lmphocytes in vitro. Proc Soc Exp Biol Med. 1971 Jul;137(3):1089–1092. [PubMed] [Google Scholar]
- Schlag W. Die Wirkung der L-Asparaginase aus E. coli auf die Makrophagen-Wanderungs-Hemmung ud die Lymphocyten-Transformation durch Tuberculin. Klin Wochenschr. 1971 Sep 15;49(18):1030–1031. doi: 10.1007/BF01487734. [DOI] [PubMed] [Google Scholar]


