Abstract
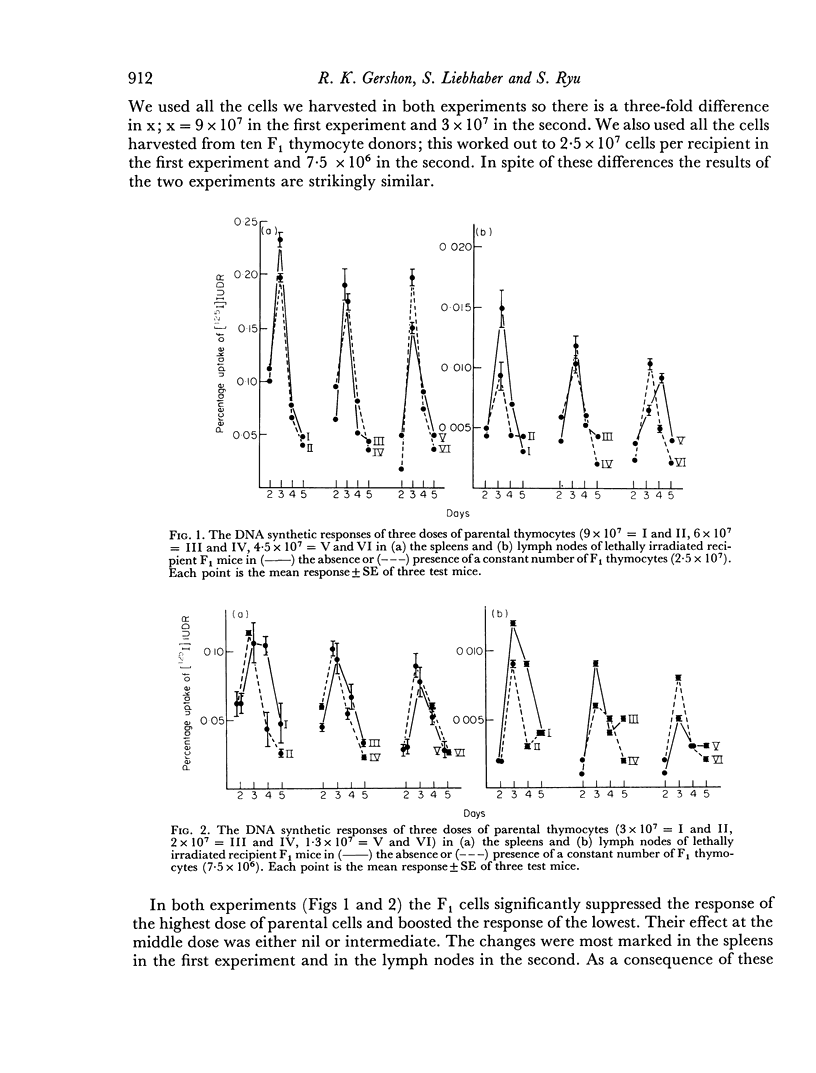
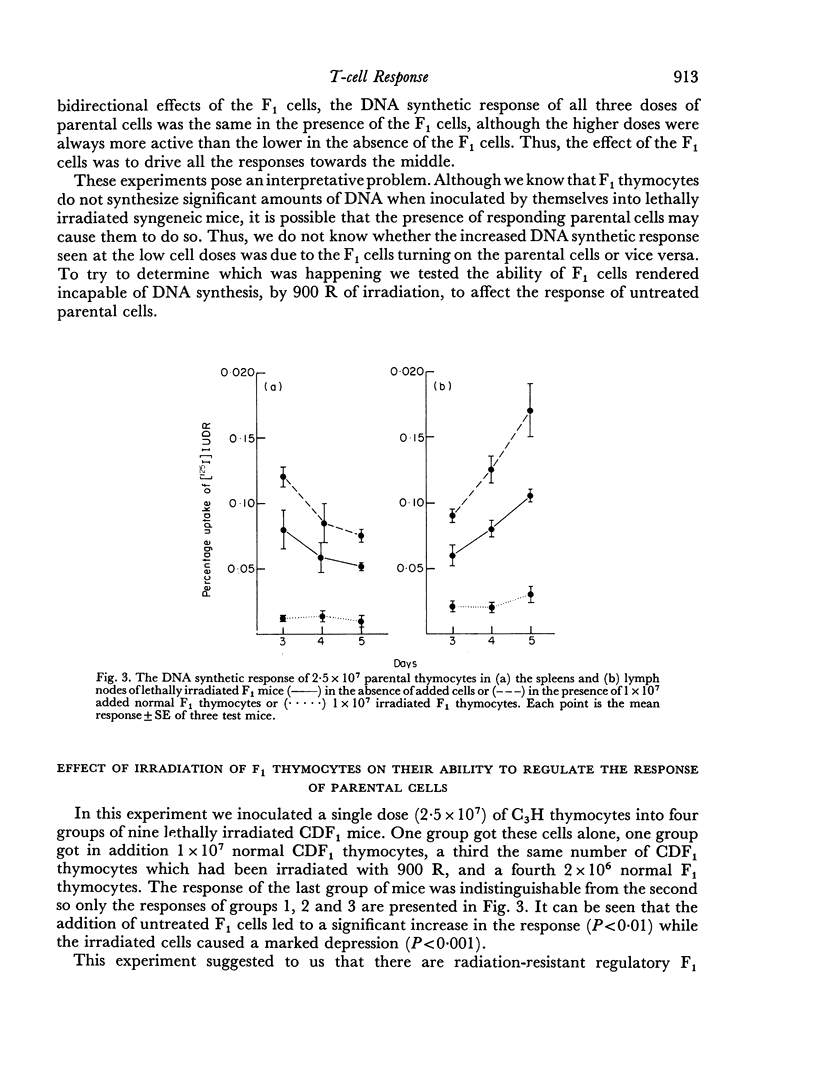
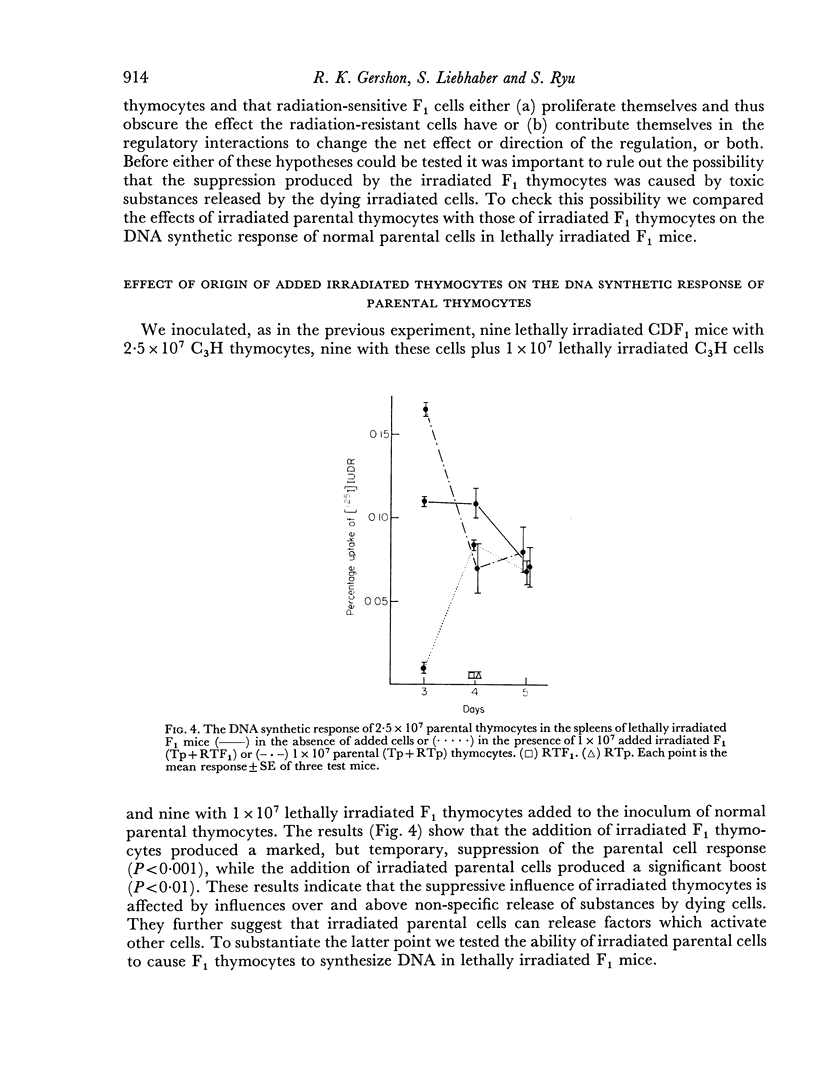
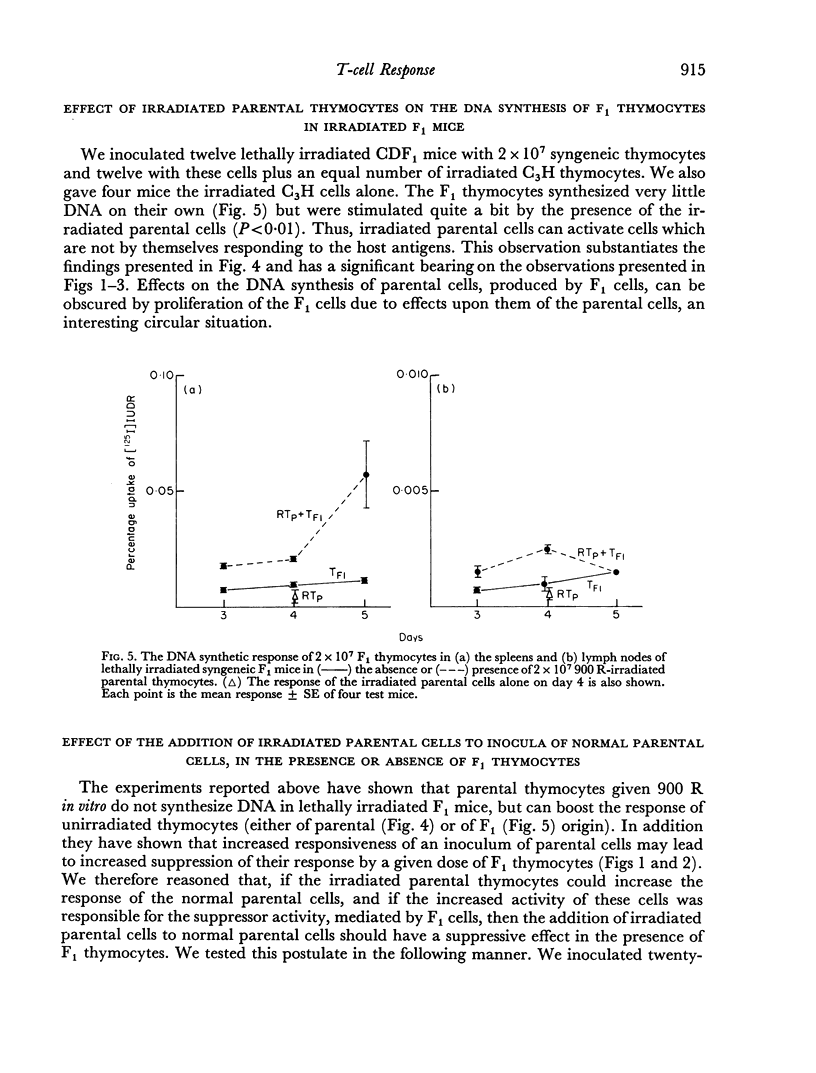
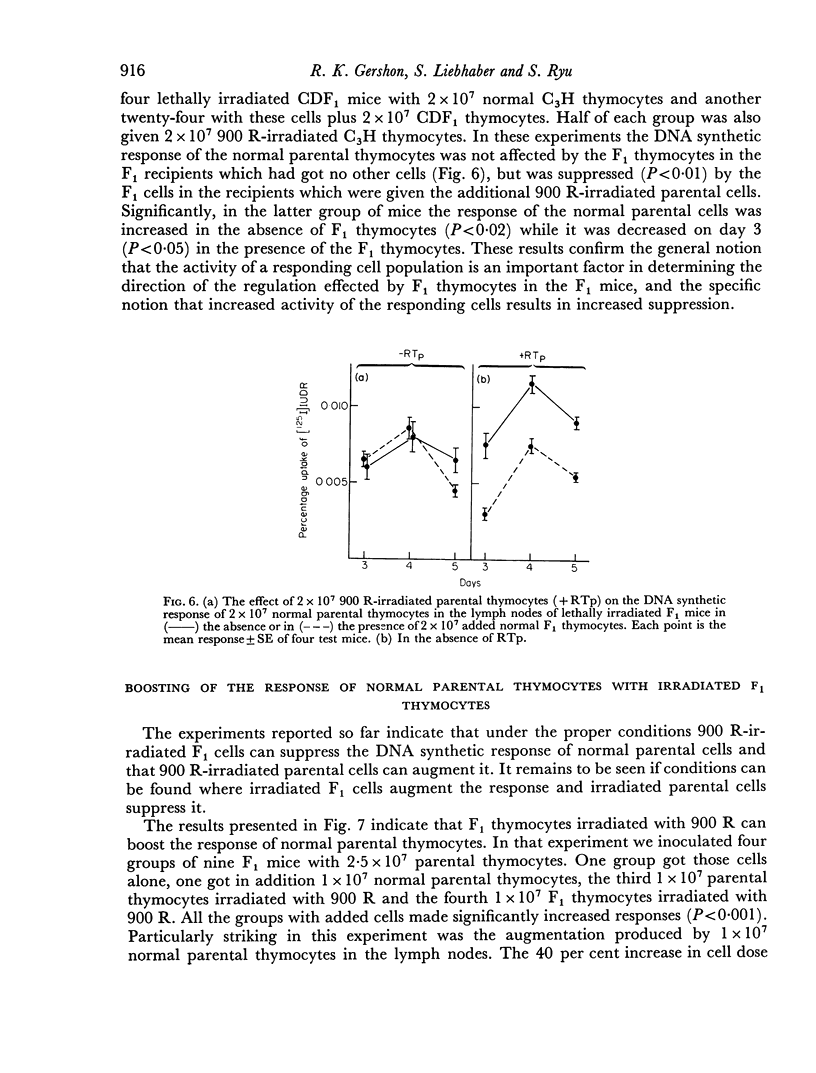
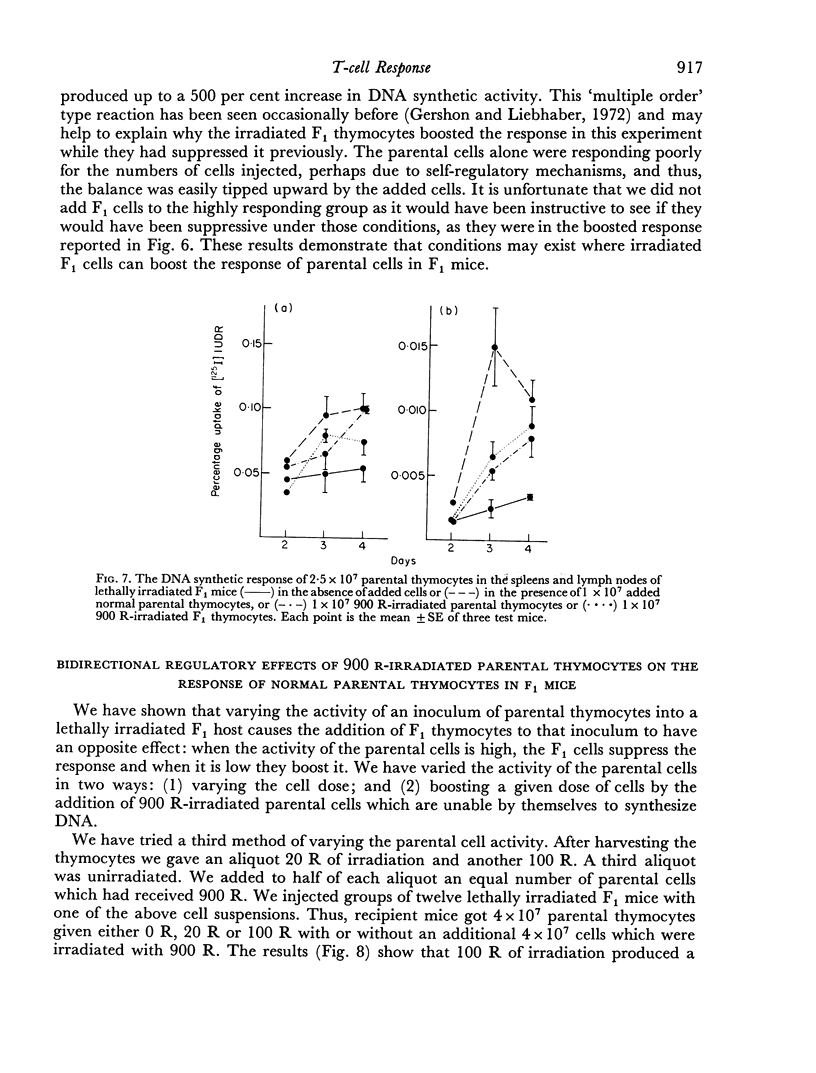
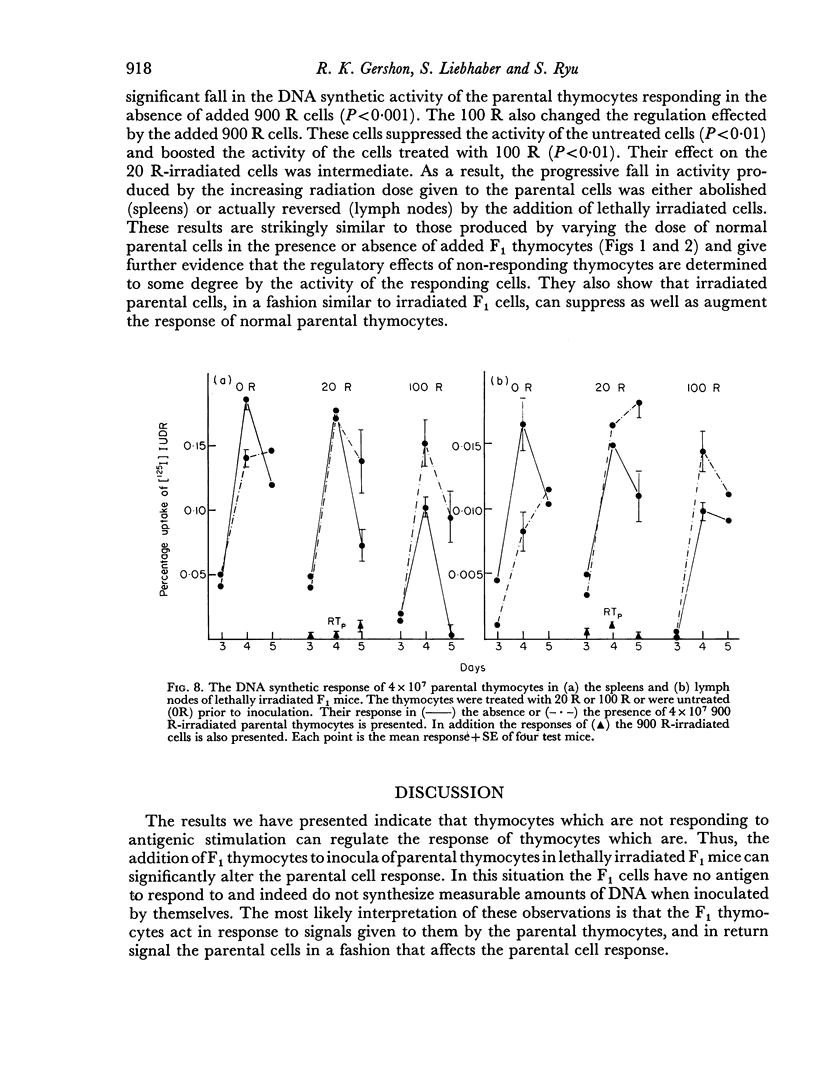
Parental thymocytes, inoculated into F1 mice which have been depleted of lymphoid cells by lethal irradiation, react to the host antigens which are contributed by the reciprocal parent in the F1 cross, by synthesizing DNA. The amount of DNA the parental thymocytes synthesize can be regulated by the addition of F1 thymocytes to the lethally irradiated F1 recipient. F1 thymocytes suppress the response of a highly responding inoculum of parental thymocytes and boost the response of an inoculum responding less well. These regulatory effects of F1 thymocytes are not abolished by 900 R of irradiation. 900 R-irradiated parental cells which are themselves incapable of DNA synthesis can also affect the response of DNA synthesizing cells. As with F1 thymocytes, the effect that 900 R-irradiated parental thymocytes has depends on the activity of the DNA synthesizing population; when it is high the effect is suppressive, when it is low there is augmentation. These results indicate that during the course of their response to antigen, T cells may transmit signals to other T cells which in turn transmit feedback signals to the primarily responding cells and in so doing alter their response. Such circular interactions between T cells may play an important role in immunological homeostasis.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler W. H., Takiguchi T., Marsh B., Smith R. T. Cellular recognition by mouse lymphocytes in vitro. II. Specific stimulation by histocompatibility antigens in mixed cell culture. J Immunol. 1970 Oct;105(4):984–1000. [PubMed] [Google Scholar]
- Anderson R. E., Sprent J., Miller J. F. Cell-to-cell interaction in the immune response. 8. Radiosensitivity of thymus-derived lymphocytes. J Exp Med. 1972 Mar 1;135(3):711–717. doi: 10.1084/jem.135.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIGGS P. M., PAYNE L. N. Cytological identification of proliferating donor cells in chick embryos injected with adult chicken blood. Nature. 1959 Nov 14;184(Suppl 20):1594–1594. doi: 10.1038/1841594a0. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Alter B. J., Solliday S., Zoschke D. C., Janis M. Lymphocyte reactivity in vitro. II. Soluble reconstituting factor permitting response of purified lymphocyte. Cell Immunol. 1970 Jul;1(2):219–227. doi: 10.1016/0008-8749(70)90009-2. [DOI] [PubMed] [Google Scholar]
- Barchilon J., Liebhaber S. A., Gershon R. K. Significance of cell interactions in production of graft versus host splenomegaly. Yale J Biol Med. 1972 Oct;45(5):519–529. [PMC free article] [PubMed] [Google Scholar]
- Basten A., Beeson P. B. Mechanism of eosinophilia. II. Role of the lymphocyte. J Exp Med. 1970 Jun 1;131(6):1288–1305. doi: 10.1084/jem.131.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Asofsky R. Synergy among lymphoid cells mediating the graft-versus-host response. 3. Evidence for interaction between two types of thymus-derived cells. J Exp Med. 1972 Apr 1;135(4):764–779. doi: 10.1084/jem.135.4.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Asofsky R. Synergy among lymphoid cells mediating the graft-versus-host response. II. Synergy in graft-versus-host reactions produced by Balb-c lymphoid cells of differing anatomic origin. J Exp Med. 1970 Feb;131(2):235–246. doi: 10.1084/jem.131.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A. Immunologic complementation between thymus and marrow cells--a model for the two-cell theory of immunocompetence. Transplant Rev. 1969;1:92–113. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- DAVIES A. J., DOAK S. M. Fate of homologous adult spleen cells injected into new-born mice. Nature. 1960 Aug 13;187:610–611. doi: 10.1038/187610b0. [DOI] [PubMed] [Google Scholar]
- Davies A. J. The thymus and the cellular basis of immunity. Transplant Rev. 1969;1:43–91. doi: 10.1111/j.1600-065x.1969.tb00136.x. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Dvorak A. M., Simpson B. A., Richerson H. B., Leskowitz S., Karnovsky M. J. Cutaneous basophil hypersensitivity. II. A light and electron microscopic description. J Exp Med. 1970 Sep 1;132(3):558–582. doi: 10.1084/jem.132.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins W. L. Cellular immunology and the pathogenesis of graft versus host reactions. Prog Allergy. 1971;15:78–187. [PubMed] [Google Scholar]
- Elkins W. L. Specific and nonspecific lymphoid cell proliferation in the pathogenesis of graft-versus-host reactions. Transplantation. 1970 Mar;9(3):273–301. doi: 10.1097/00007890-197003000-00011. [DOI] [PubMed] [Google Scholar]
- FOX B. W., PRUSOFF W. H. THE COMPARATIVE UPTAKE OF I-125-LABELED 5-IODO-2'-DEOXYURIDINE AND THYMIDINE-H3 INTO TISSUES OF MICE BEARING HEPATOMA-129. Cancer Res. 1965 Feb;25:234–240. [PubMed] [Google Scholar]
- FOX M. Cytological estimation of proliferating donor cells during graft-versus-host disease in F1 hybrid mice injected with parental spleen cells. Immunology. 1962 Jul;5:489–495. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Cohen P., Hencin R., Liebhaber S. A. Suppressor T cells. J Immunol. 1972 Mar;108(3):586–590. [PubMed] [Google Scholar]
- Gershon R. K., Hencin R. S. The DNA synthetic response of adoptively transferred thymocytes in the spleens of lethally irradiated mice. I. Effects of varying antigen and thymocyte doses. J Immunol. 1971 Dec;107(6):1723–1728. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Tolerance to sheep red cells: breakage with thymocytes and horse red cells. Science. 1972 Mar 3;175(4025):996–997. doi: 10.1126/science.175.4025.996. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Liebhaber S. A. The response of T cells to histocompatibility-2 antigens. Dose-response kinetics. J Exp Med. 1972 Jul 1;136(1):112–127. doi: 10.1084/jem.136.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgard H. R. Synergism of thymus and bone marrow in the production of gra a5hilgard HR: Synergism of thymus and bone marrow in the production of graft-versus-host splenomegaly in x-irradiated hosts. J Exp Med. 1970 Aug 1;132(2):317–328. doi: 10.1084/jem.132.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J. A., Dutton R. W. Cell components in the immune response. 3. Neonatal thymectomy: restoration in culture. Cell Immunol. 1970 Jul;1(2):190–195. doi: 10.1016/0008-8749(70)90006-7. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Dutton R. W. Immune response restoration with macrophage culture supernatants. Science. 1971 Jun 4;172(3987):1047–1048. doi: 10.1126/science.172.3987.1047. [DOI] [PubMed] [Google Scholar]
- Ito T., Cudkowicz G. Sensitivity to radiation and insensitivity to vinblastine of the inducer function of thymus-derived cells. Cell Immunol. 1971 Dec;2(6):595–601. doi: 10.1016/0008-8749(71)90007-4. [DOI] [PubMed] [Google Scholar]
- Jones G. Lymphocyte activation. I. Expression of theta, H-2 and immunoglobulin determinants on lymphocytes stimulated by phytohaemagglutinin, pokeweed mitogen, concanavalin A or histocompatibility antigen. Clin Exp Immunol. 1972 Nov;12(3):391–402. [PMC free article] [PubMed] [Google Scholar]
- Kasakura S., Lowenstein L. A factor stimulating DNA synthesis derived from the medium of leukocyte cultures. Nature. 1965 Nov 20;208(5012):794–795. doi: 10.1038/208794a0. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Radioresistance of cooperative function of carrier-specific lymphocytes in antihapten antibody responses. Science. 1970 Oct 23;170(3956):462–464. doi: 10.1126/science.170.3956.462. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Enhanced immune responsiveness to a thymus-independent antigen early after adult thymectomy: evidence for short-lived inhibitory thymus-derived cells. Eur J Immunol. 1972 Apr;2(2):114–118. doi: 10.1002/eji.1830020204. [DOI] [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. Radioresistance of the enhancing effect of cells from carrier-immunized mice in an in vitro primary immune response. Proc Natl Acad Sci U S A. 1971 Apr;68(4):699–703. doi: 10.1073/pnas.68.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhaber S. A., Barchilon J., Gershon R. K. Bidirectional effects of interaction between parental thymocytes and F1 spleen cells in the production of graft vs host splenomegaly. J Immunol. 1972 Aug;109(2):238–243. [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P. J. The abrogation of sheep erythrocyte tolerance in rats by means of the transfer of allogeneic lymphocytes. J Exp Med. 1970 Nov;132(5):916–925. doi: 10.1084/jem.132.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Mosier D., Cantor H. Functional maturation of mouse thymic lymphocytes. Eur J Immunol. 1971 Dec;1(6):459–461. doi: 10.1002/eji.1830010610. [DOI] [PubMed] [Google Scholar]
- Munro A., Hunter P. In vitro reconstitution of the immune response of thymus-deprived mice to sheep red blood cells. Nature. 1970 Jan 17;225(5229):277–278. doi: 10.1038/225277a0. [DOI] [PubMed] [Google Scholar]
- NOWELL P. C., DEFENDI V. DISTRIBUTION OF PROLIFERATING DONOR CELLS IN RUNT DISEASE IN RATS. Transplantation. 1964 May;2:375–382. doi: 10.1097/00007890-196405000-00006. [DOI] [PubMed] [Google Scholar]
- Nakić B., Kastelan A., Mikuska J., Bunarević A. Quantitative analysis of the chimaeric state in mice. II. Cytological examination of the proportion of proliferating donor and host cells in runt disease in mice. Immunology. 1967 Jun;12(6):615–627. [PMC free article] [PubMed] [Google Scholar]
- Nisbet N. W., Simonsen M. Primary immune response in grafted cells. Dissociation between the proliferation of activity and the proliferation of cells. J Exp Med. 1967 Jun 1;125(6):967–981. doi: 10.1084/jem.125.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J. J., Moore M. A., Harrison G. A. Chromosome marker studies in the graft-versus-host reaction in the chick embryo. Nature. 1965 Jul 17;207(994):313–315. doi: 10.1038/207313b0. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., Marshall-Clarke S. Cross-reactions between erythrocytes at the T-cell level. Immunology. 1973 Mar;24(3):579–588. [PMC free article] [PubMed] [Google Scholar]
- SIMONSEN M. Graft versus host reactions. Their natural history, and applicability as tools of research. Prog Allergy. 1962;6:349–467. [PubMed] [Google Scholar]
- Simpson E., Beverley P. C. Cell-mediated responses to tumour xenografts in mice. Int J Cancer. 1972 Mar 15;9(2):299–304. doi: 10.1002/ijc.2910090207. [DOI] [PubMed] [Google Scholar]
- Sjöberg O. Effect of allogeneic cell interaction on the primary immune response in vitro. Cell types involved in suppression and stimulation of antibody synthesis. Clin Exp Immunol. 1972 Nov;12(3):365–375. [PMC free article] [PubMed] [Google Scholar]
- Tigelaar R. E., Asofsky R. Graft-vs-host reactivity of mouse thymocytes: effect of cortisone pretreatment of donors. J Immunol. 1973 Feb;110(2):567–574. [PubMed] [Google Scholar]
- Tigelaar R. E., Asofsky R. Synergy among lymphoid cells mediating the graft-versus-host response. IV. Synergy in the GVH reaction quantitated by a mortality assay in sublethally irradiated recipients. J Exp Med. 1972 May 1;135(5):1059–1070. doi: 10.1084/jem.135.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Silvers W. K., Nowell P. C. Quantitative studies on the mixed lymphocyte interaction in rats. II. Relationship of the proliferative response to the immunologic status of the donors. J Exp Med. 1967 Oct 1;126(4):655–665. doi: 10.1084/jem.126.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEISS I. M., FOX M. Donor and host contribution to splenomegaly in homologous mouse chimaeras. Nature. 1963 Feb 16;197:673–675. doi: 10.1038/197673a0. [DOI] [PubMed] [Google Scholar]


