Abstract
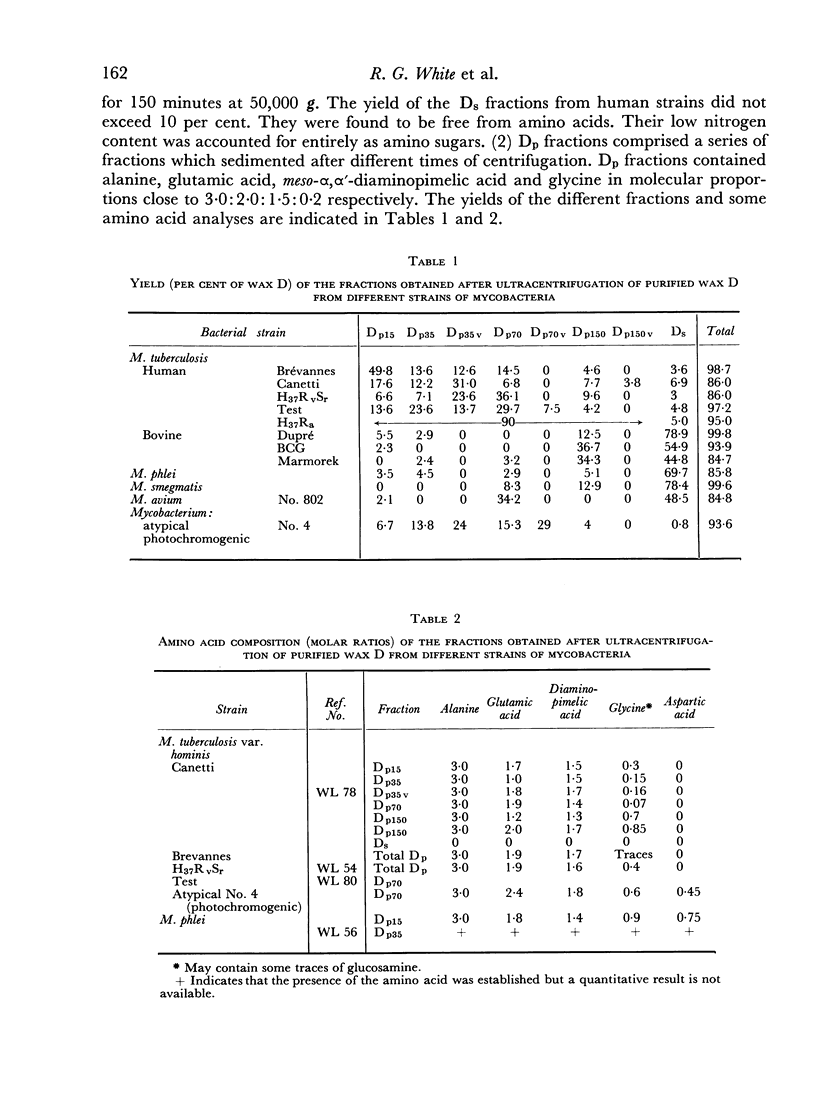
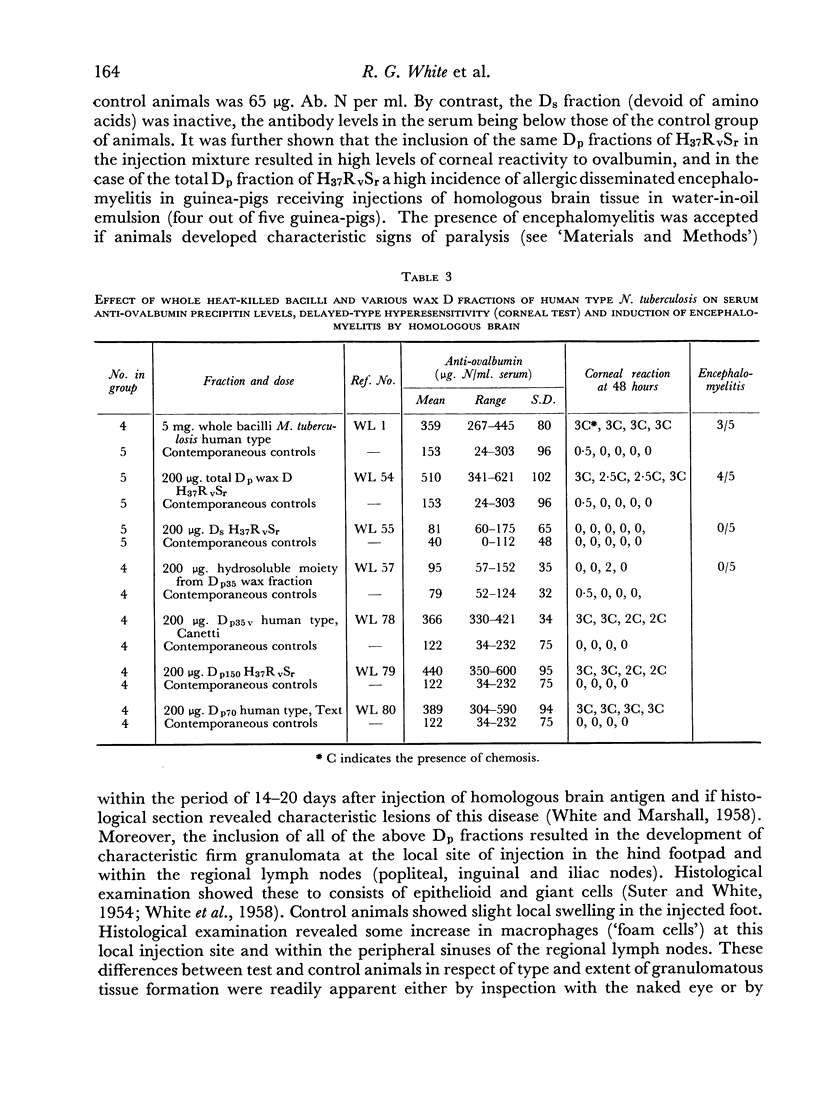
By fractionation of wax D from mycobacteria by ultracentrifugation in ether, it is possible to prepare from wax D of human strains peptide-containing and non-peptide-containing compounds. The peptide-containing fractions, like the parent wax D, were able to act as adjuvants by increasing serum anti-ovalbumin levels, by increasing corneal hypersensitivity to ovalbumin, and by inducing encephalomyelitis after homologous guinea-pig brain injection. The peptide—carbohydrate moiety resulting from hydrolysis of the whole wax D was found inactive in all these biological effects.
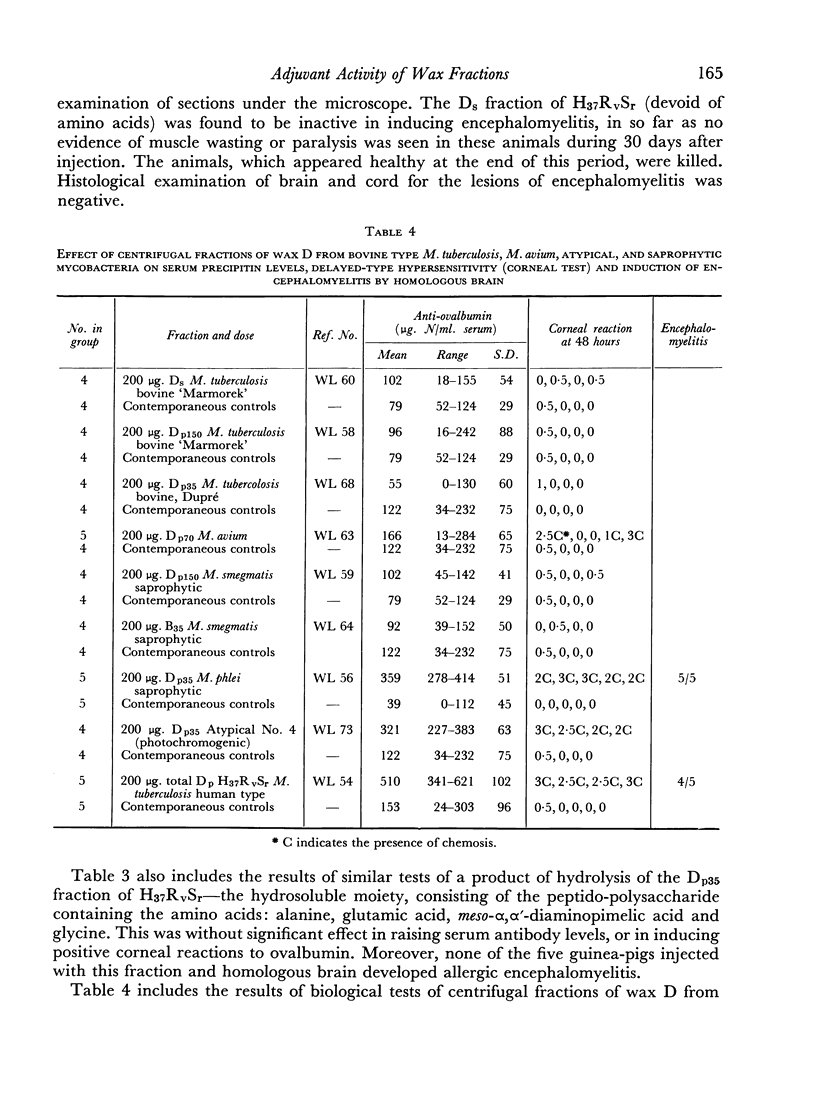
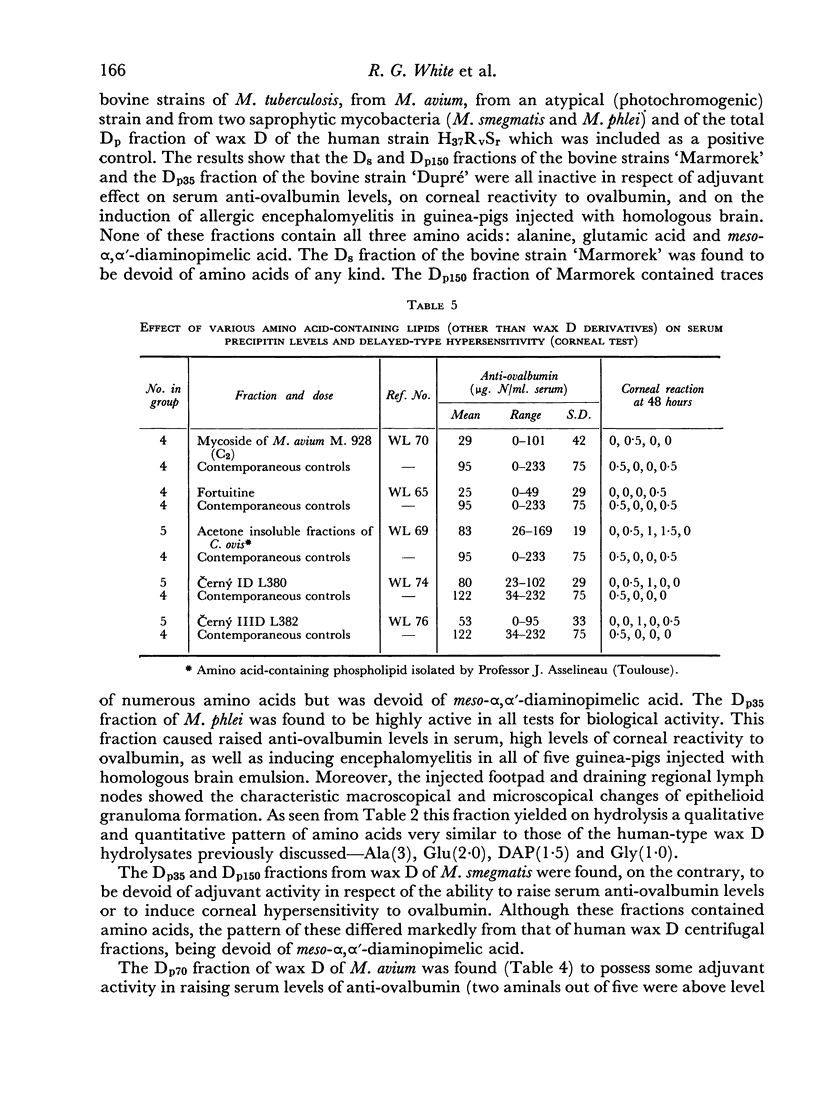
When the same centrifugal technique was applied to several bovine types of Mycobacterium tuberculosis, M. avium and to atypical and saprophytic mycobacteria, analogous peptide and non-peptide-containing fractions were obtained. The amino acid patterns of these were of great variety, and in most cases differed from those present in human type wax D. In three instances a small proportion of a peptide-containing fraction was obtained (from M. phlei, M. avium and an atypical mycobacterium), which closely resembled a human type wax D. These fractions were found to have adjuvant activity. All other fractions of wax D of bovine, avian and saprophytic strains were inactive.
These facts support the role of a peptide of D- and L-alanine, D-glutamic acid and meso-α,α'-diaminopimelic acid in determining the adjuvant action of wax D fractions and whole mycobacteria. The structure of the peptidoglycolipid of an adjuvant-active mycobacterial wax corresponds closely in amino acid, amino sugar and hexose composition with the mucopeptide of the bacterial cell wall, and evidence is discussed for the concept of wax D fractions as partial replicas of a fundamental cell wall polymer.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASSELINEAU J., BUC H., JOLLES P., LEDERER E. Sur la structure chimique d'une fraction peptido-glycolipidique (cire D) isolée de Mycobacterium tuberculosis var. hominis. Bull Soc Chim Biol (Paris) 1958;40(12):1953–1964. [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- HOLDSWORTH E. S. The nature of the cell-wall of Corynebacterium diphtheriae. Biochim Biophys Acta. 1952 Jan;8(1):110–110. doi: 10.1016/0006-3002(52)90016-4. [DOI] [PubMed] [Google Scholar]
- IKAWA M., SNELL E. E. Cell wall composition of lactic acid bacteria. J Biol Chem. 1960 May;235:1376–1382. [PubMed] [Google Scholar]
- IKAWA M., SNELL E. E. D-glutamic acid and amino sugars as cell wall constituents in lactic acid bacteria. Biochim Biophys Acta. 1956 Mar;19(3):576–578. doi: 10.1016/0006-3002(56)90499-1. [DOI] [PubMed] [Google Scholar]
- JOLLES P., SMOUR D., LEDERER E. Analytical studies on wax D, a macromolecular peptidoglycoplied fraction from humn strains of Mycobacterium tuberculosos. Arch Biochem Biophys. 1962 Sep;Suppl 1:283–289. [PubMed] [Google Scholar]
- Kekwick R. A., Cannan R. K. The hydrogen ion dissociation curve of the crystalline albumin of the hen's egg. Biochem J. 1936 Feb;30(2):227–234. doi: 10.1042/bj0300227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERER E. Glycolipids of acid-fast bacteria. Adv Carbohydr Chem. 1961;16:207–238. doi: 10.1016/s0096-5332(08)60263-5. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., MORRIS L. A modified procedure for the automatic analysis of amino acids. Anal Biochem. 1960 Nov;1:187–201. doi: 10.1016/0003-2697(60)90045-2. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Cell-wall amino-acids and amino-sugars. Nature. 1957 Aug 17;180(4581):338–339. doi: 10.1038/180338a0. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. IV. The composition of the cell walls of some Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1953 Apr;10(4):512–523. doi: 10.1016/0006-3002(53)90296-0. [DOI] [PubMed] [Google Scholar]
- SMITH D. W., RANDALL H. M., MACLENNAN A. P., LEDERER E. Mycosides: a new class of type-specific glycolipids of Mycobacteria. Nature. 1960 Jun 11;186:887–888. doi: 10.1038/186887a0. [DOI] [PubMed] [Google Scholar]
- SNELL E. E., RADIN N. S., IKAWA M. The nature of D-alanine in lactic acid bacteria. J Biol Chem. 1955 Dec;217(2):803–818. [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. The composition of the spore coats of Bacillus megatherium, B. subtilis and B. cereus. Biochem J. 1956 Mar;62(3):459–465. doi: 10.1042/bj0620459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., WHITE R. G. The response of the reticulo-endothelial system to the injection of the purified wax and the lipopolysaccharide of tubercle bacilli; a histologic and an immunologic study. Am Rev Tuberc. 1954 Nov;70(5):793–805. doi: 10.1164/art.1954.70.5.793. [DOI] [PubMed] [Google Scholar]
- VILKAS E., MIQUEL A. M., LEDERER E. [On the isolation and structure of fortuitine, peptidolipid from Mycobacterium fortuitum]. Biochim Biophys Acta. 1963 Apr 23;70:217–218. doi: 10.1016/0006-3002(63)90745-5. [DOI] [PubMed] [Google Scholar]
- WHITE R. G., BERNSTOCK L., JOHNS R. G., LEDERER E. The influence of components of M. tuberculosis and other Mycobacteria upon antibody production to ovalbumin. Immunology. 1958 Jan;1(1):54–66. [PMC free article] [PubMed] [Google Scholar]
- WHITE R. G., COONS A. H., CONNOLLY J. M. Studies on antibody production. IV. The role of a wax fraction of Mycobacterium tuberculosis in adjuvant emulsions on the production of antibody to egg albumin. J Exp Med. 1955 Jul 1;102(1):83–104. doi: 10.1084/jem.102.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORK E. Biochemistry of the bacterial cell wall. Nature. 1957 Apr 27;179(4565):841–847. doi: 10.1038/179841a0. [DOI] [PubMed] [Google Scholar]


