Abstract
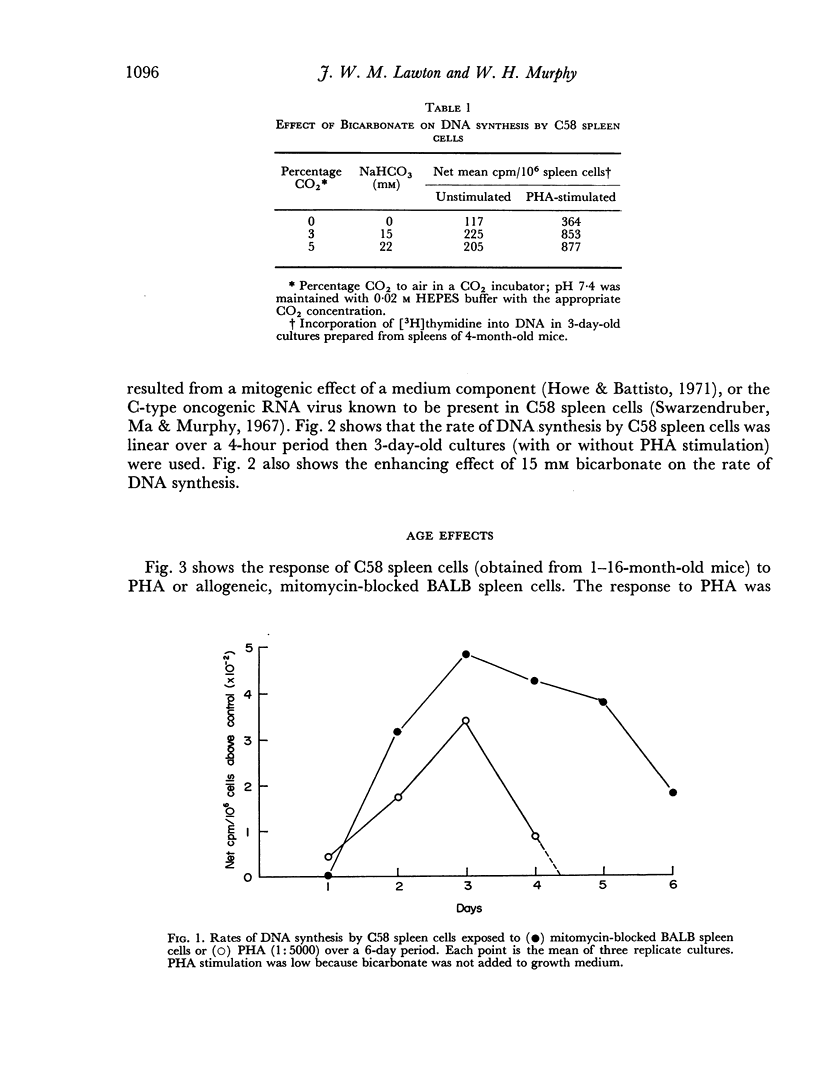
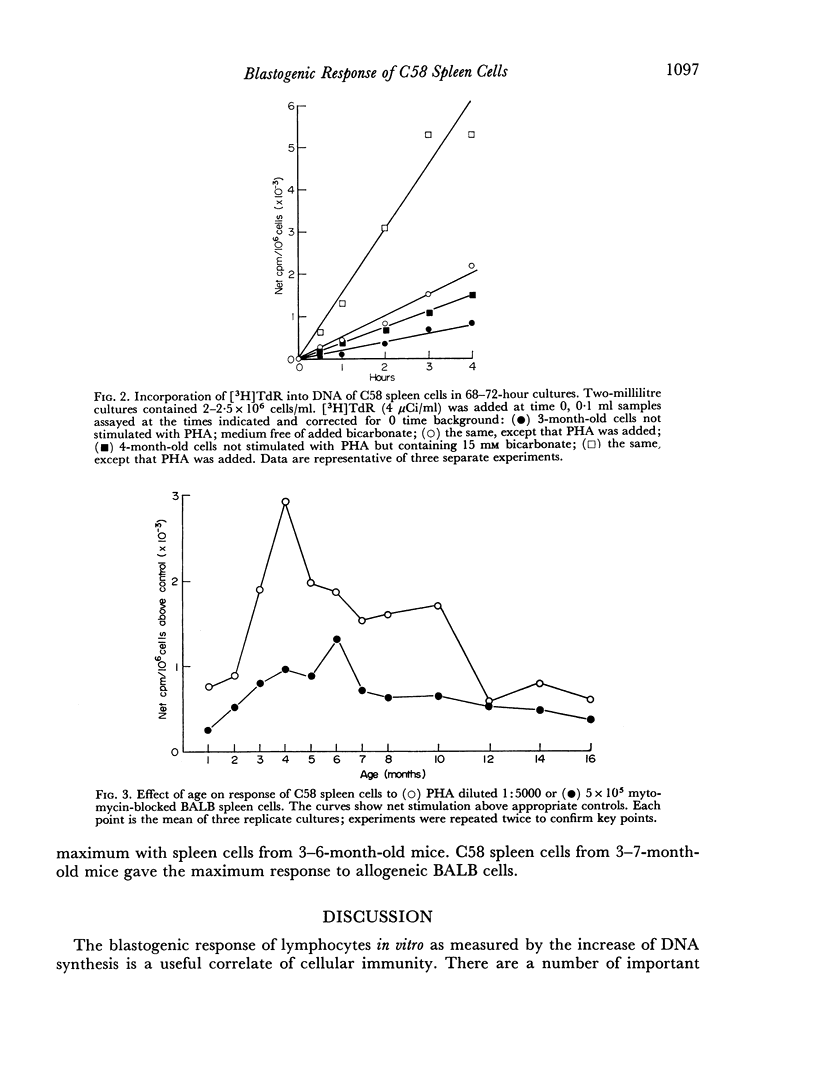
Optimum conditions were determined for the stimulation of DNA synthesis in C58 spleen cells by phytohaemagglutinin and allogeneic BALB spleen cells. Maximum stimulation occurred in 3 days in both cases. The rate of DNA synthesis was constant over a 4-hour labelling period with tritiated thymidine. DNA synthesis was significantly enhanced by 15 mM bicarbonate. The peak response of C58 spleen cells to PHA and allogeneic BALB spleen cells occurred when the mice were 3–7 months old; it decreased thereafter. This finding is discussed in relation to age-dependent immune polioencephalomyelitis in C58 mice.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler W. H., Takiguchi T., Smith R. T. Effect of age upon primary alloantigen recognition by mouse spleen cells. J Immunol. 1971 Nov;107(5):1357–1367. [PubMed] [Google Scholar]
- Alter B. J., Bach F. H. Lymphocyte reactivity in vitro. I. Cellular reconstitution of purified lymphocyte response. Cell Immunol. 1970 Jul;1(2):207–218. doi: 10.1016/0008-8749(70)90008-0. [DOI] [PubMed] [Google Scholar]
- Bain B. Tritiated-thymidine uptake in mixed leucocyte cultures: effect of specific activity and exposure time. Clin Exp Immunol. 1970 Feb;6(2):255–262. [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I. Phagocytosis by human monocytes. Blood. 1968 Sep;32(3):423–435. [PubMed] [Google Scholar]
- Coifman R. E., Good R. A., Meuwissen H. J. The function of irradiated blood elements. I. Limitations on the response to phytohemagglutinin as an indicator of immunocompetence in irradiated lymphocytes. Proc Soc Exp Biol Med. 1971 May;137(1):155–160. doi: 10.3181/00379727-137-35534. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Jacobson B. HEPES-buffered media in lymphocyte cultures. Proc Soc Exp Biol Med. 1971 Feb;136(2):387–393. doi: 10.3181/00379727-136-35271. [DOI] [PubMed] [Google Scholar]
- Eagle H. Buffer combinations for mammalian cell culture. Science. 1971 Oct 29;174(4008):500–503. doi: 10.1126/science.174.4008.500. [DOI] [PubMed] [Google Scholar]
- Fowler A. K., Hellman A., Steinman H. G., Quatrale A. C. Studies on the blastogenic response of murine lymphocytes. I. Quantitative measurement of stimulation by phytohemagglutinin. Proc Soc Exp Biol Med. 1971 Oct;138(1):345–349. doi: 10.3181/00379727-138-35893. [DOI] [PubMed] [Google Scholar]
- Howe M. L., Battisto J. R. In vitro lymphocyte transformation induced by pre-existing hypersensitivity to foetal calf serum. Clin Exp Immunol. 1971 Apr;8(4):617–623. [PMC free article] [PubMed] [Google Scholar]
- Kishimoto S., Yamamura Y. Immune responses in aged mice: changes of antibody-forming cell precursors and antigen-reactive cells with ageing. Clin Exp Immunol. 1971 Jun;8(6):957–962. [PMC free article] [PubMed] [Google Scholar]
- Lawton J. W., Murphy W. H. Histopathology in immune polioencephalomyelitis in C58 mice. Arch Neurol. 1973 Jun;28(6):367–370. doi: 10.1001/archneur.1973.00490240027002. [DOI] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon inhibits DNA synthesis induced in mouse lymphocyte suspensions by phytohaemagglutinin or by allogeneic cells. Nat New Biol. 1972 May 24;237(73):120–121. doi: 10.1038/newbio237120a0. [DOI] [PubMed] [Google Scholar]
- Murphy W. H., Tam M. R., Lanzi R. L., Abell M. R., Kauffman C. Age dependence of immunologically induced central nervous system disease in C58 mice. Cancer Res. 1970 Jun;30(6):1612–1622. [PubMed] [Google Scholar]
- Phillips S. M., Carpenter C. B., Merrill J. P. Cellular immunity in the mouse. I. In vitro lymphocyte reactivity. Cell Immunol. 1972 Oct;5(2):235–248. doi: 10.1016/0008-8749(72)90050-0. [DOI] [PubMed] [Google Scholar]
- Phillips S. M., Carpenter C. B., Merrill J. P. Cellular immunity in the mouse. II. Correlation of in vivo and in vitro phenomena. Cell Immunol. 1972 Oct;5(2):249–263. doi: 10.1016/0008-8749(72)90051-2. [DOI] [PubMed] [Google Scholar]
- Rodey G. E., Good R. A. The in vitro response to phytohemagglutinin of lyphoid cells from normal and neonatally thymectomized adult mice. Int Arch Allergy Appl Immunol. 1969;36(4):399–407. doi: 10.1159/000230760. [DOI] [PubMed] [Google Scholar]
- Sager M. A., Lawton J. W., Murphy W. H. Serum transmissibility of immune polioencephalomyelitis in C58 mice. J Immunol. 1973 Jan;110(1):219–226. [PubMed] [Google Scholar]
- Shipman C., Jr Evaluation of 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES) as a tissue culture buffer. Proc Soc Exp Biol Med. 1969 Jan;130(1):305–310. doi: 10.3181/00379727-130-33543. [DOI] [PubMed] [Google Scholar]


