Abstract
The phagocytic activity of human polymorphonuclear leucocyte preparations, which were free from plasma, has been estimated by direct determination under phase contrast of the number of living cells containing test particles. Spores of Aspergillus fumigatus were phagocytosed in the absence of added serum but phagocytosis of paraffin wax particles occurred only in the presence of serum containing the heat-labile and C′4 components of complement. In view of the unreactive nature of the paraffin hydrocarbons, it was considered unlikely that natural antibody played any part in the phenomenon.
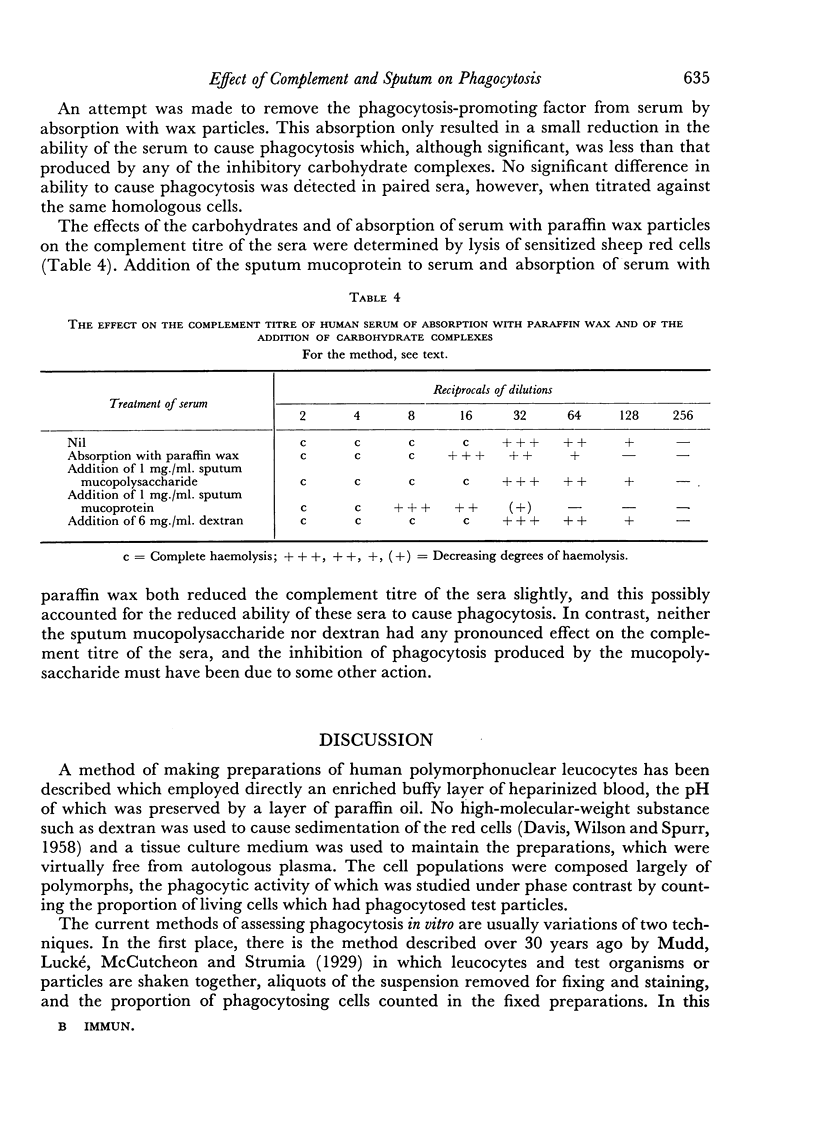
Although no phagocytosis of wax particles occurred in the absence of serum, almost 100 per cent of cells were phagocytic in preparations containing adequate concentrations of serum. It was therefore possible to determine the serum concentration necessary for 50 per cent of the polymorphs to phagocytose wax particles. By this means it was demonstrated that the addition of the carbohydrate components of sputum had a small but significant inhibitory effect on phagocytosis and that dextran had no such effect. The sputum mucoprotein depressed the complement titre of serum and this might have accounted for the reduction in the ability of a serum to promote phagocytosis when this complex was added. The sputum mucopolysaccharide had no such effect on the complement titre of serum and must have exerted its inhibitory action in some other way.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D., MORGAN W. T. J., WATKINS W. M. The isolation and properties of the human blood-group a substance. Biochem J. 1950 Apr;46(4):426–438. doi: 10.1042/bj0460426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROGAN T. D. The carbohydrate complexes of bronchial secretion. Biochem J. 1959 Jan;71(1):125–131. doi: 10.1042/bj0710125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS V. E., WILSON W. L., SPURR C. L. The efficiency of oxidative phosphorylation by normal and leukemic human leukocytes. Blood. 1958 Apr;13(4):367–375. [PubMed] [Google Scholar]
- KEKWICK R. A. Addendum; physico-chemical examination of blood-group a substance. Biochem J. 1950 Apr;46(4):438–439. [PubMed] [Google Scholar]
- MICHAEL J. G., WHITBY J. L., LANDY M. Studies on natural antibodies to gram-negative bacteria. J Exp Med. 1962 Jan 1;115:131–146. doi: 10.1084/jem.115.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- NUNGESTER W. J., BOSCH J. K., ALONSO D. Resistance lowering effect of human respiratory tract mucin. Proc Soc Exp Biol Med. 1951 Apr;76(4):777–780. doi: 10.3181/00379727-76-18630. [DOI] [PubMed] [Google Scholar]
- POLLEY M. J., MOLLISON P. L. The role of complement in the detection of blood group antibodies: special reference to the antiglobulin test. Transfusion. 1961 Jan-Feb;1:9–22. doi: 10.1111/j.1537-2995.1961.tb00006.x. [DOI] [PubMed] [Google Scholar]
- ROWLEY D., WHITBY J. L. The bactericidal activity of mouse macrophages in vitro. Br J Exp Pathol. 1959 Oct;40:507–515. [PMC free article] [PubMed] [Google Scholar]
- RYTEL M. W., STOLLERMAN G. H. THE OPSONIC EFFECT OF COMPLEMENT ON THE PHAGOCYTOSIS OF GAMMA-GLOBULIN-COATED BENTONITE PARTICLES. J Immunol. 1963 Apr;90:607–611. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SMITH H., GALLOP R. C., STANLEY J. L. The virulence-enhancing factor of mucins. V. The different components of the third factor involved in virulence enhancement. Biochem J. 1952 Sep;52(1):15–22. doi: 10.1042/bj0520015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM I., BUGHER J. C., HORSFALL F. L., Jr Ultracentrifugation studies of a urinary mucoprotein which reacts with various viruses. J Biol Chem. 1955 Jan;212(1):125–133. [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr A mucoprotein derived from human urine which reacts with influenza, mumps, and Newcastle disease viruses. J Exp Med. 1952 Jan;95(1):71–97. doi: 10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]



