Abstract
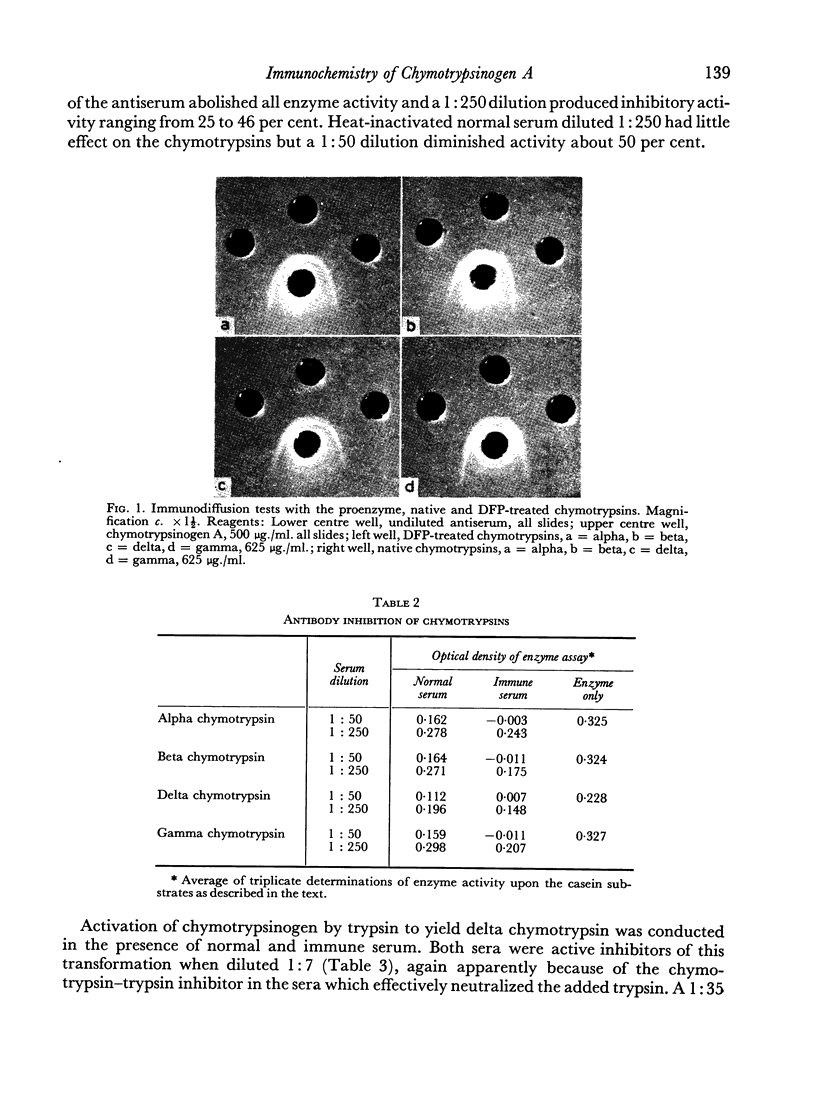
Antiserum prepared by immunization of rabbits with chymotrypsinogen A reacted in equal titre with the proenzyme and alpha, beta, delta and gamma chymotrypsin in serological tests. Inactivation of the four enzymes by diisopropyl-fluorophosphate did not diminish their precipitability. Immunodiffusion tests in gel plates with the proenzyme produced a single precipitin band which merged without spur formation with the bands produced by each of the native and inactivated enzymes. The antiserum partially inhibited activation of the zymogen by trypsin. The proteolytic activity of each of the four chymotrypsins was also inhibited by the antiserum.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLS A. K., JANSEN E. F. Stoichiometric inhibition of chymotrypsin. Adv Enzymol Relat Subj Biochem. 1952;13:321–343. doi: 10.1002/9780470122587.ch8. [DOI] [PubMed] [Google Scholar]
- BARRETT J. T. AN IMMUNOLOGICAL STUDY OF PROCARBOXYPEPTIDASE A AND ITS ENZYME. Immunology. 1965 Feb;8:129–135. [PMC free article] [PubMed] [Google Scholar]
- CINADER B. Antibodies against enzymes. Annu Rev Microbiol. 1957;11:371–390. doi: 10.1146/annurev.mi.11.100157.002103. [DOI] [PubMed] [Google Scholar]
- DREYER W. J., NEURATH H., RUPLEY J. A. Structural changes in the activation of chymotrypsinogen and trypsinogen; effect of urea on chymotrypsinogen and delta-chymotrypsin. Arch Biochem Biophys. 1956 Nov;65(1):243–259. doi: 10.1016/0003-9861(56)90191-6. [DOI] [PubMed] [Google Scholar]
- FLEMING T. C., RIDDEL G. H. Studies of the antigenic properties of alpha chymotrypsin. Am J Ophthalmol. 1961 May;51:1104–1107. doi: 10.1016/0002-9394(61)91799-8. [DOI] [PubMed] [Google Scholar]
- McCANN S. F., LASKOWSKI M. Determination of trypsin inhibitor in blood plasma. J Biol Chem. 1953 Sep;204(1):147–152. [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F., HARTLEY B. S., SHAW D. C. The amino acid sequence around the reactive serine residue of some proteolytic enzymes. Biochem J. 1960 Oct;77:149–163. doi: 10.1042/bj0770149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIECE J. L., BARRETT F. T. Non-immune gel precipitin tests with an enzyme antigen. Nature. 1963 Mar 9;197:1021–1021. doi: 10.1038/1971021a0. [DOI] [PubMed] [Google Scholar]
- PEANASKY R. J., LASKOWSKI M. Partial purification of the trypsin inhibitor from blood plasma. J Biol Chem. 1953 Sep;204(1):153–157. [PubMed] [Google Scholar]
- SCHAFFER N. K., MAY S. C., Jr, SUMMERSON W. H. Serine phosphoric acid from diisopropylphosphoryl chymotrypsin. J Biol Chem. 1953 May;202(1):67–76. [PubMed] [Google Scholar]
- SCHLAMOWITZ M., VARANDANI P. T., WISSLER F. C. Pepsinogen and pepsin: conformational relations, studied by iodination, immunochemical precipitation, and the influence of pepain inhibitor. Biochemistry. 1963 Mar-Apr;2:238–246. doi: 10.1021/bi00902a006. [DOI] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- VAN VUNAKIS H., LEHRER H. I., ALLISON W. S., LEVINE L. Immunochemical studies on the components of the pepsinogen system. J Gen Physiol. 1963 Jan;46:589–604. doi: 10.1085/jgp.46.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU F. C., LASKOWSKI M. Action of the naturally occurring trypsin inhibitors against chymotrypsins alpha and beta. J Biol Chem. 1955 Apr;213(2):609–619. [PubMed] [Google Scholar]