Abstract
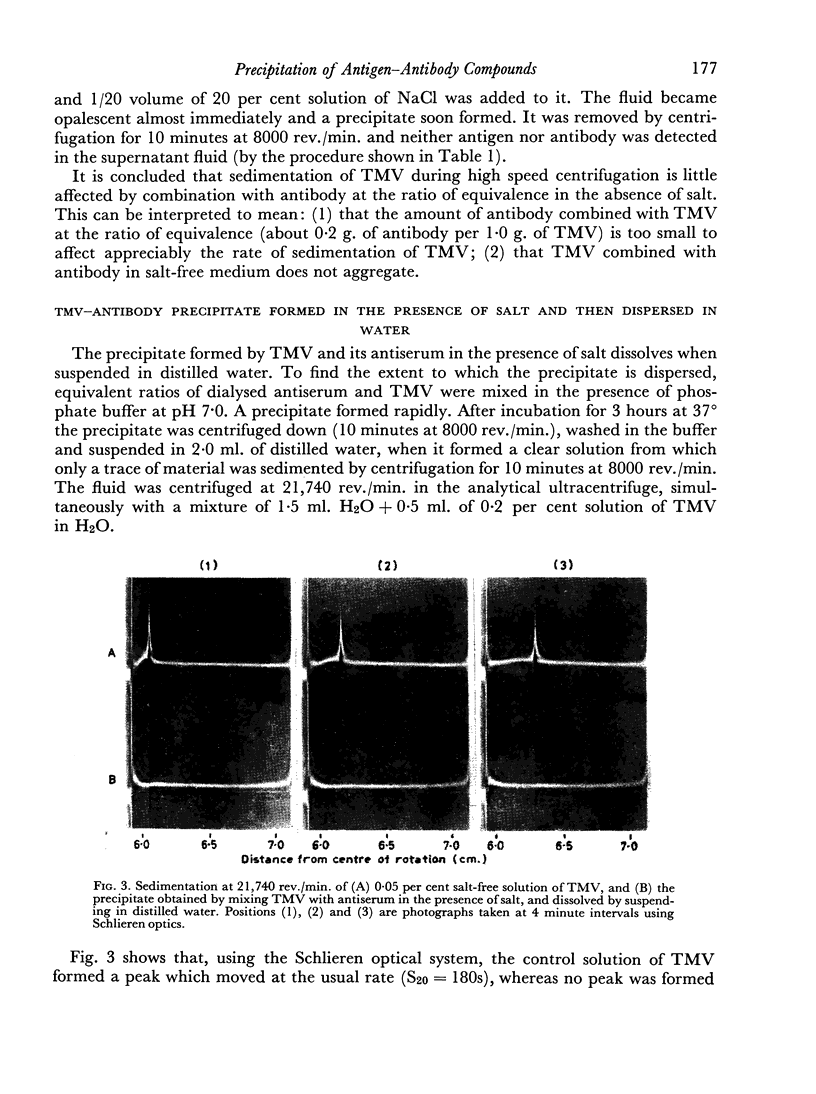
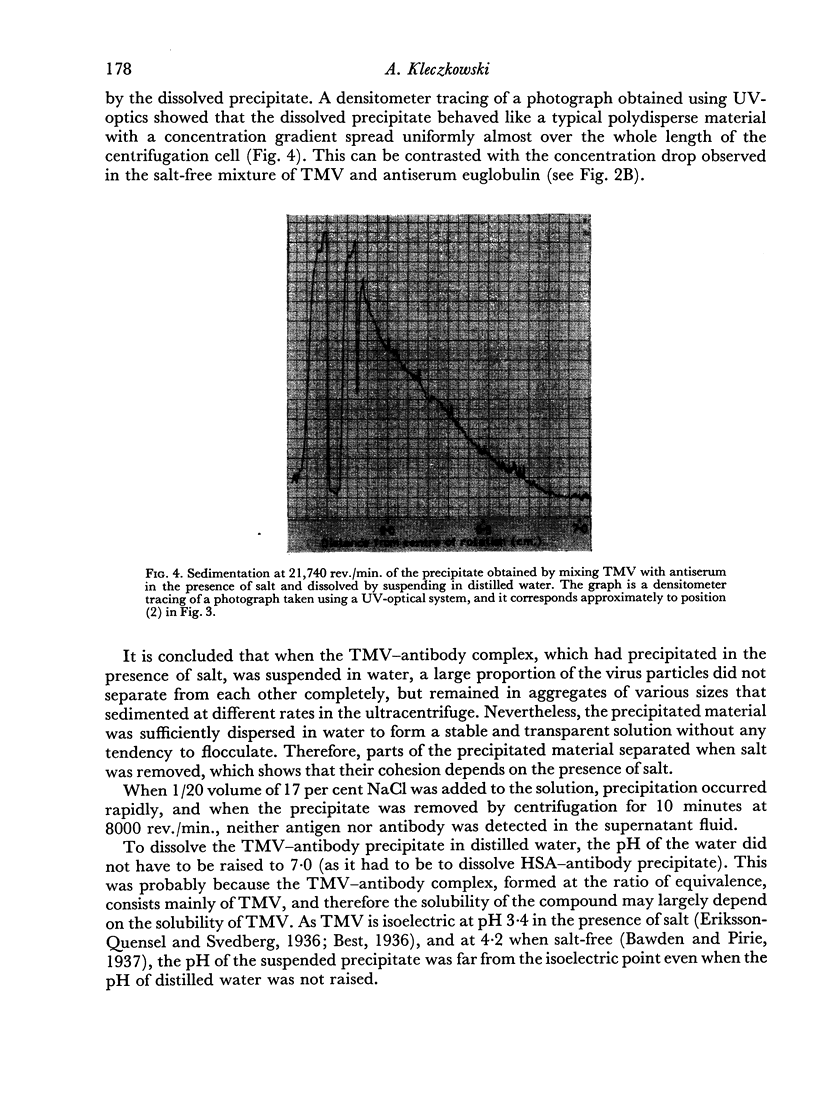
Tobacco mosaic virus (TMV) combines with its homologous antibody to much the same extent irrespective of whether or not salt is present, but without salt the complex not only fails to precipitate, but the virus particles do not aggregate. TMV—antibody precipitate formed in the presence of salt, like that formed between human serum albumin (HSA) and its homologous antibody, dissolves when suspended in distilled water to form stable and transparent solutions, although the precipitate may not disaggregate completely.
To dissolve HSA—antibody complex in distilled water, the pH of the water must be raised to about 7.0. At pH near 6.0, HSA—antibody complex precipitates even in the absence of salt, but the precipitate dissolves immediately when the pH is raised to 7.0.
All these facts are incompatible with the theory of precipitation based on the `lattice hypothesis', and argue strongly in favour of the theory that antigen—antibody complexes are hydrophobic and, as such, flocculate when sufficiently discharged either by salt or by suitably adjusting the pH of the medium.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALADJEM F., LIEBERMAN M. The antigen-antibody reaction. I. The influence of sodium chloride concentration on the quantitative precipitin reaction. J Immunol. 1952 Aug;69(2):117–130. [PubMed] [Google Scholar]
- FUDENBERG H. H., DREWS G., NISONOFF A. SEROLOGIC DEMONSTRATION OF DUAL SPECIFICITY OF RABBIT BIVALENT HYBRID ANTIBODY. J Exp Med. 1964 Jan 1;119:151–166. doi: 10.1084/jem.119.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLECZKOWSKI A. An electrophoretic study of the mechanism of precipitin reactions: variation in reversibility. Immunology. 1959 Apr;2(2):97–103. [PMC free article] [PubMed] [Google Scholar]
- KLECZKOWSKI A. Serological behaviour of tobacco mosaic virus and of its protein fragments. Immunology. 1961 Apr;4:130–141. [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski A. A quantitative study of complex formation in heated protein mixtures. Biochem J. 1949;44(5):573–581. doi: 10.1042/bj0440573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski A. Combination between different proteins and between proteins and yeast nucleic acid. Biochem J. 1946;40(5-6):677–687. doi: 10.1042/bj0400677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleczkowski A. The effect of salts on the formation of protein complexes during heat denaturation. Biochem J. 1943 Apr;37(1):30–36. doi: 10.1042/bj0370030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKHAM R. A graphical method for the rapid determination of sedimentation coefficients. Biochem J. 1960 Dec;77:516–519. doi: 10.1042/bj0770516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A., RIVERS M. M. Recombination of a mixture of univalent antibody fragments of different specificity. Arch Biochem Biophys. 1961 May;93:460–462. doi: 10.1016/0003-9861(61)90296-x. [DOI] [PubMed] [Google Scholar]