Abstract
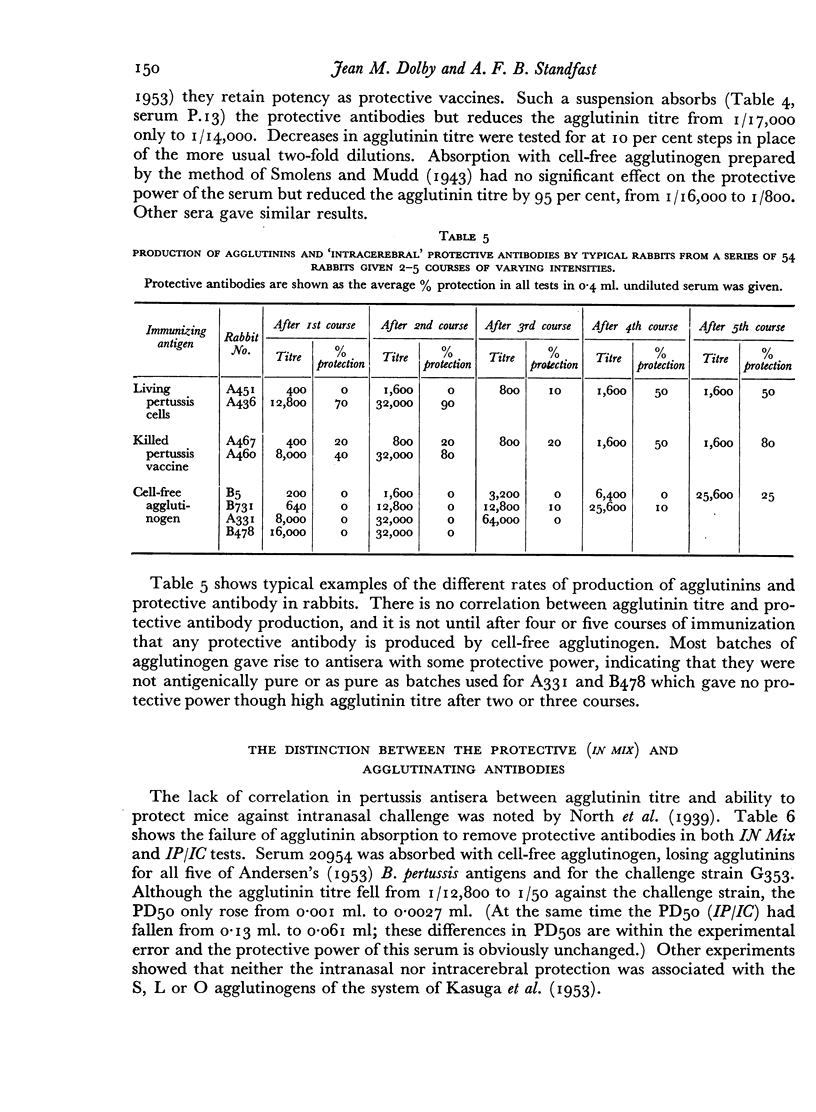
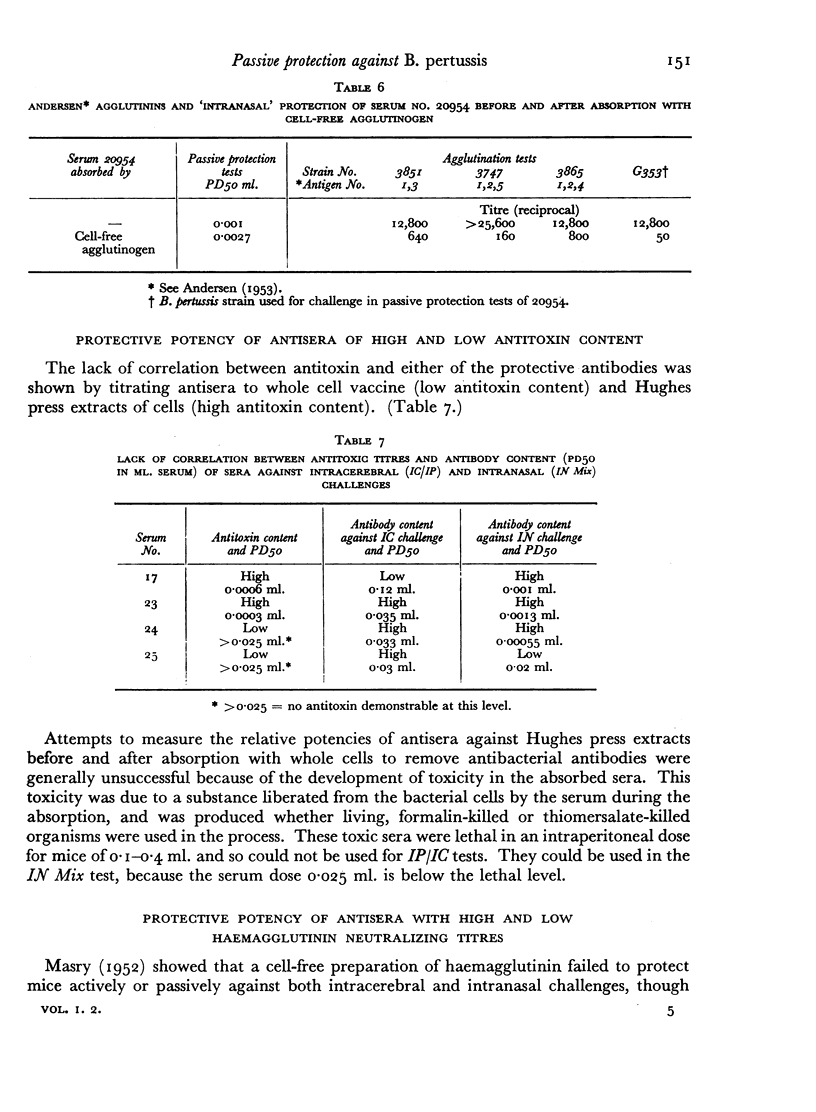
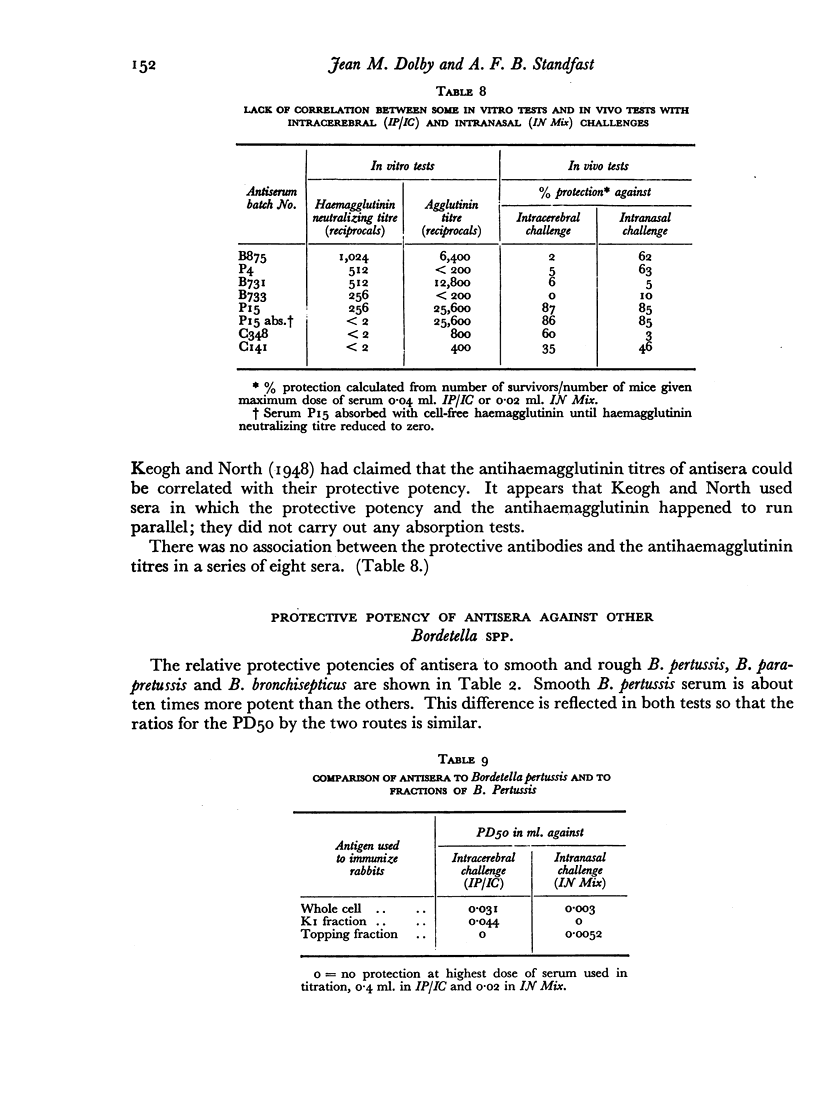
A survey of a number of rabbit antisera to Bordetella pertussis revealed the existence of two distinct antibodies, one passively protecting mice against lethal infection by the intracerebral route, the other passively protecting mice against lethal infection by the intranasal route. Neither is the antitoxin. Antisera against most, if not all, S forms of B. pertussis contain both types of protective antibody, and so to a lesser extent do B. parapertussis and B. bronchisepticus. Neither of the protective antigens is an agglutinogen, or the haemagglutinin. The two antigens can also be distinguished by active protection tests. Extensive investigations, however, had not led to an in vitro test for either of the protective antigens or their antibodies that would replace the mouse test.
The practical importance of the two distinct antigens and their antibodies is shown by the fact that assay by the intranasal and intracerebral routes of challenge will not arrange vaccines in the same order of potency either in active or in passive protection tests.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EVANS D. G., PERKINS F. T. An agglutinin-production test in the study of pertussis vaccines. J Pathol Bacteriol. 1953 Oct;66(2):479–488. doi: 10.1002/path.1700660218. [DOI] [PubMed] [Google Scholar]
- EVANS D. G., PERKINS F. T. An improved method for testing the ability of pertussis vaccines to produce agglutinin. J Pathol Bacteriol. 1954 Jul;68(1):251–257. doi: 10.1002/path.1700680131. [DOI] [PubMed] [Google Scholar]
- FISHER S. The hemagglutinin of Haemophilus pertussis. II. Observations on the structure of the hemagglutinating complex of culture supernatants. Aust J Exp Biol Med Sci. 1950 Sep;28(5):509–516. [PubMed] [Google Scholar]
- JESAITIS M. A., GOEBEL W. F. The chemical and antiviral properties of the somatic antigen of Phase II Shigella sonnei. J Exp Med. 1952 Nov;96(5):409–424. doi: 10.1084/jem.96.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASUGA T., NAKASE Y., UKISHIMA K., TAKATSU K. Studies on Haemophilus pertussis. I. Antigen structure of H. pertussis and its phases. Kitasato Arch Exp Med. 1953 Nov;26(2-3):121–133. [PubMed] [Google Scholar]
- MacPHERSON C. F. C., MAURER P. H., ALEXANDER H. E., REDMAN W. A method for the quantitative measurement of agglutinin nitrogen in antisera to Hemophilus pertussis, phase I. Can J Med Sci. 1952 Jun;30(3):284–293. doi: 10.1139/cjms52-036. [DOI] [PubMed] [Google Scholar]
- McGOVERN J. P. Passive intraperitoneal mouse protection test in a study of immune response to H. pertussis vaccination. Pediatrics. 1950 Jan;5(1):38–44. [PubMed] [Google Scholar]
- TAYLOR E. M., MOLONEY P. J., REID D. B. Comparison of methods for assessing the antigenic response to pertussis vaccine. Can J Microbiol. 1956 Apr;2(2):94–101. doi: 10.1139/m56-014. [DOI] [PubMed] [Google Scholar]
- WINTER J. L. Development of antibodies in children convalescent from whooping cough. Proc Soc Exp Biol Med. 1953 Aug-Sep;83(4):866–870. doi: 10.3181/00379727-83-20517. [DOI] [PubMed] [Google Scholar]



