Abstract
The soluble antigens produced by twenty strains of Clostridium welchii Types B, C and D were investigated. The methods used were, in particular, the Ouchterlony double diffusion method in agar gels, besides the classical tests for lethal, haemolytic and enzymic factors, adapted where possible to give quantitative results.
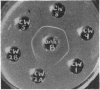
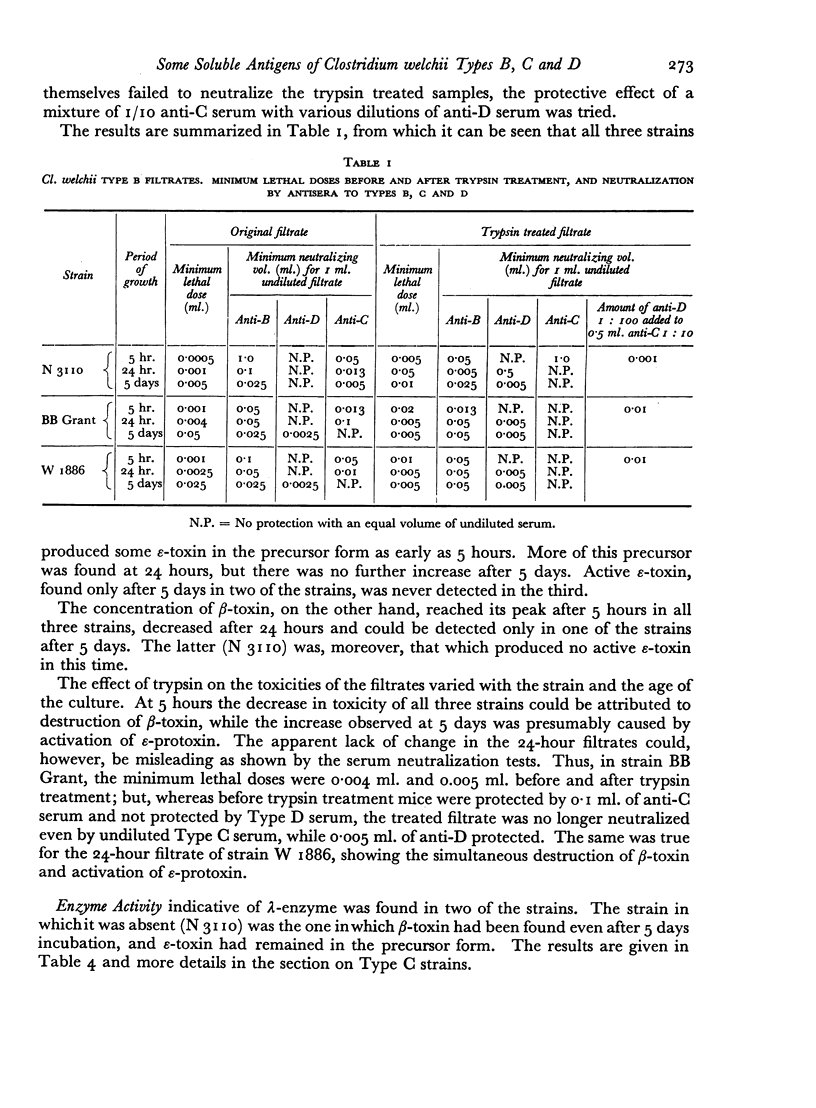
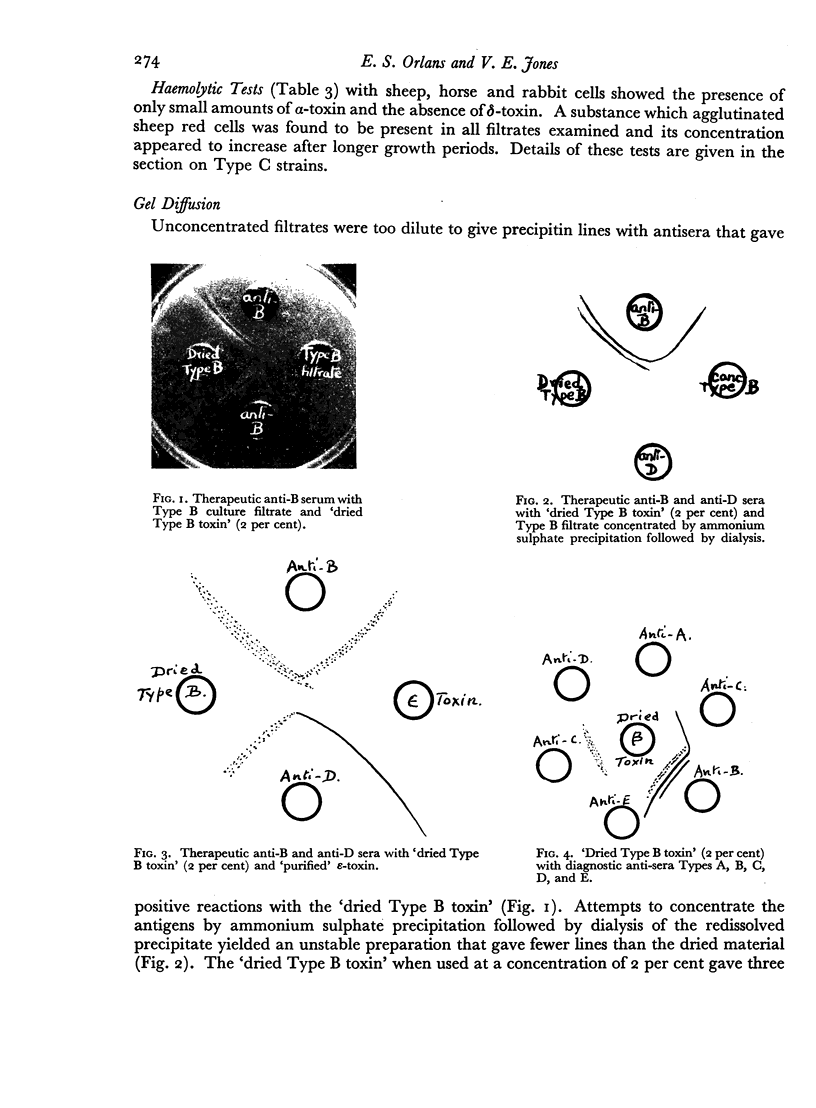
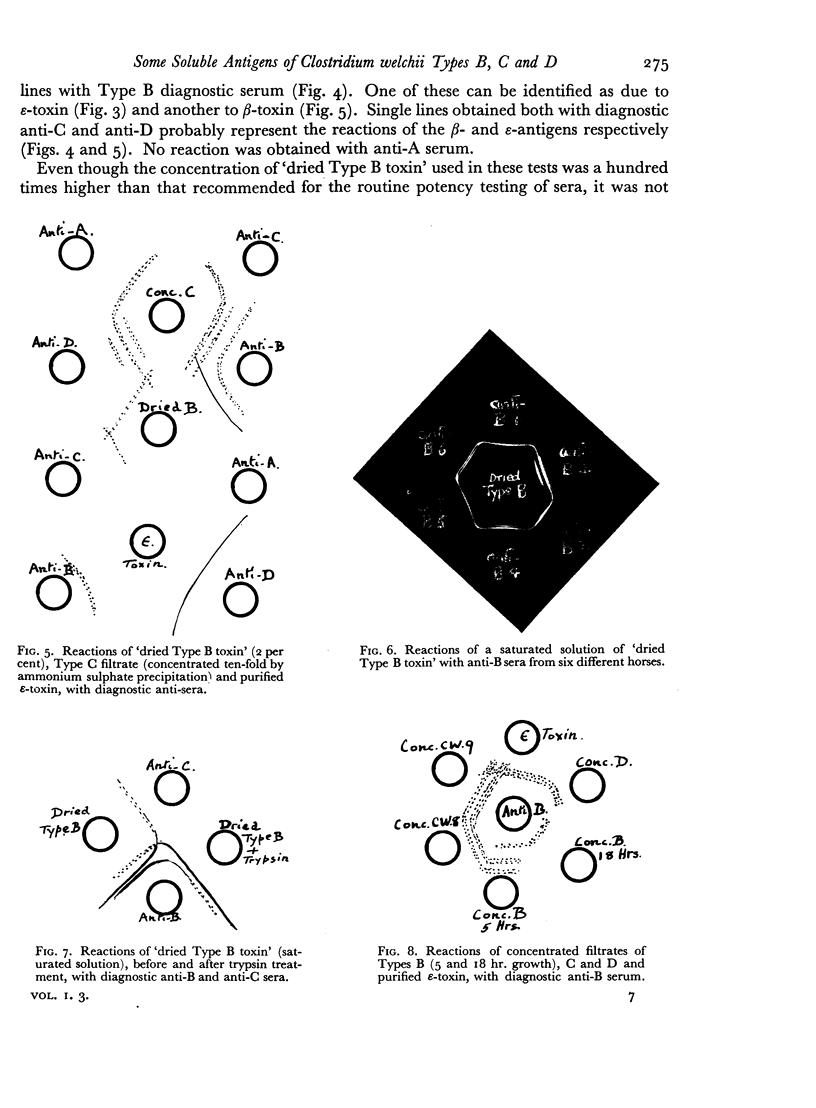
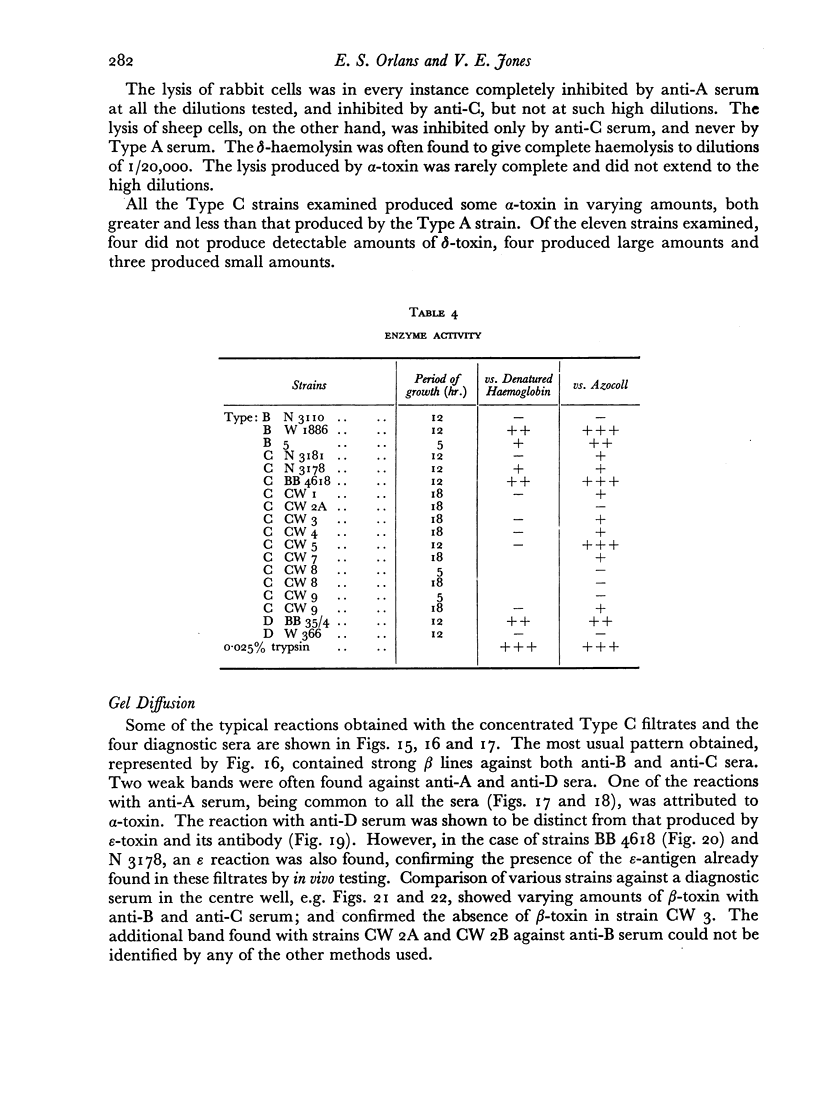
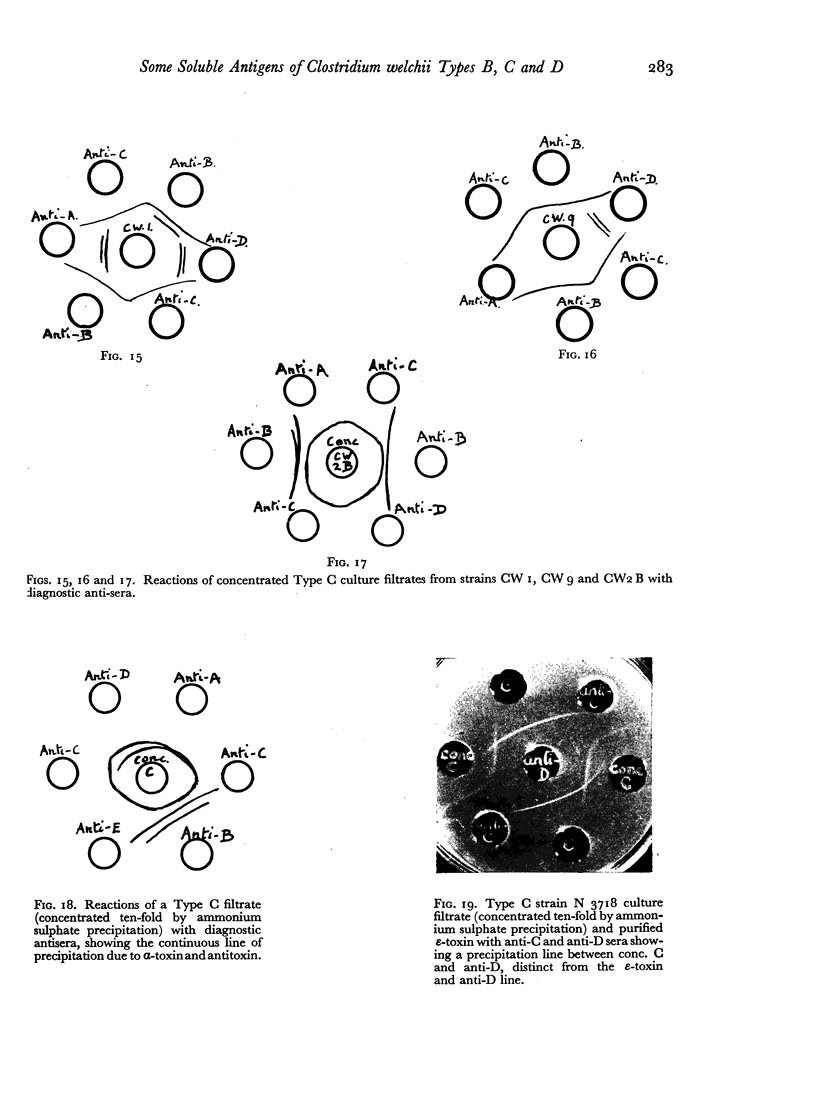
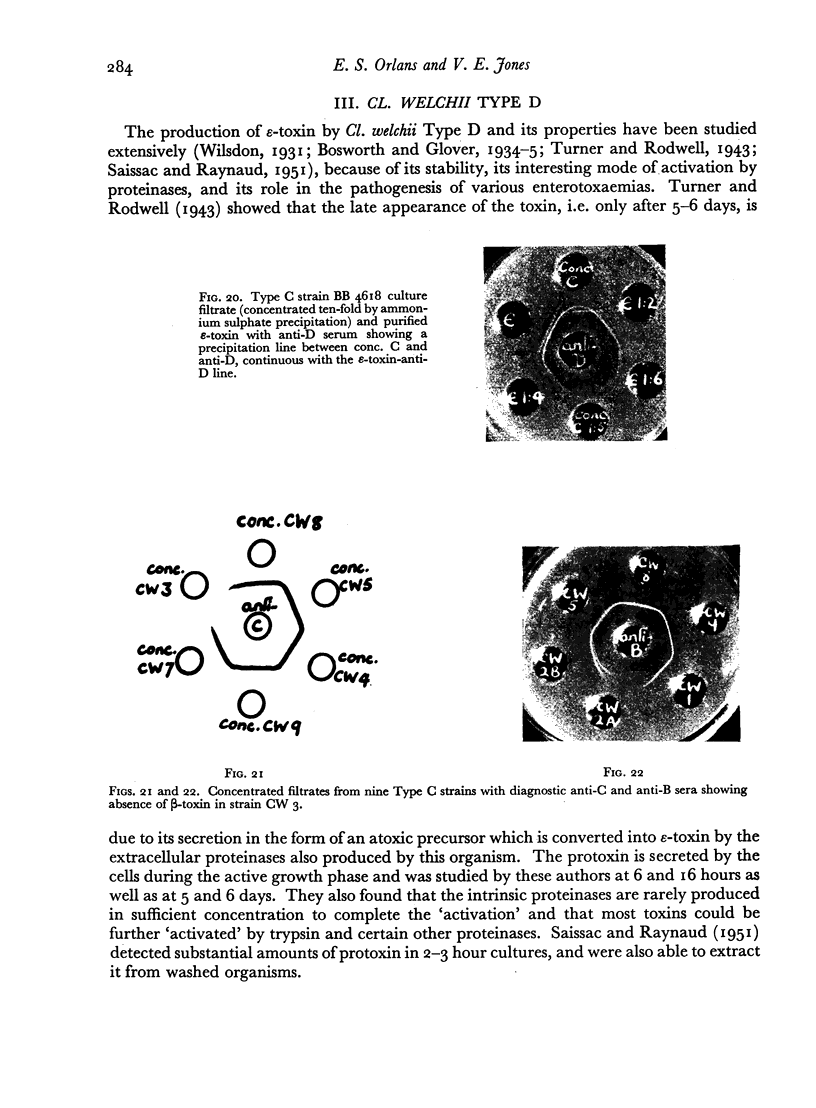
Some concentration of the culture filtrates was found necessary to demonstrate the antigens by gel diffusion, and this was effected satisfactorily only by freeze-drying. Precipitin bands, due to α-, β- and ε-toxins and their corresponding antibodies, were identified using these concentrated filtrates. Additional precipitin bands were due to antigens that have not yet been identified.
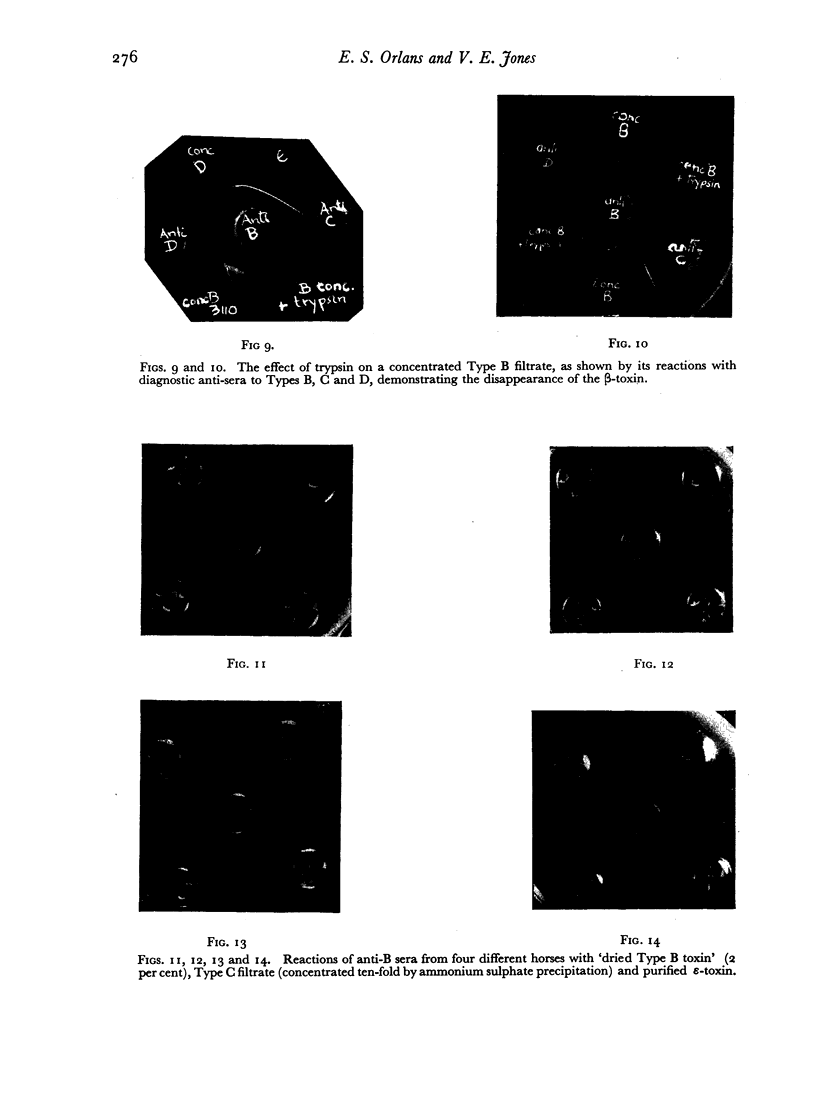
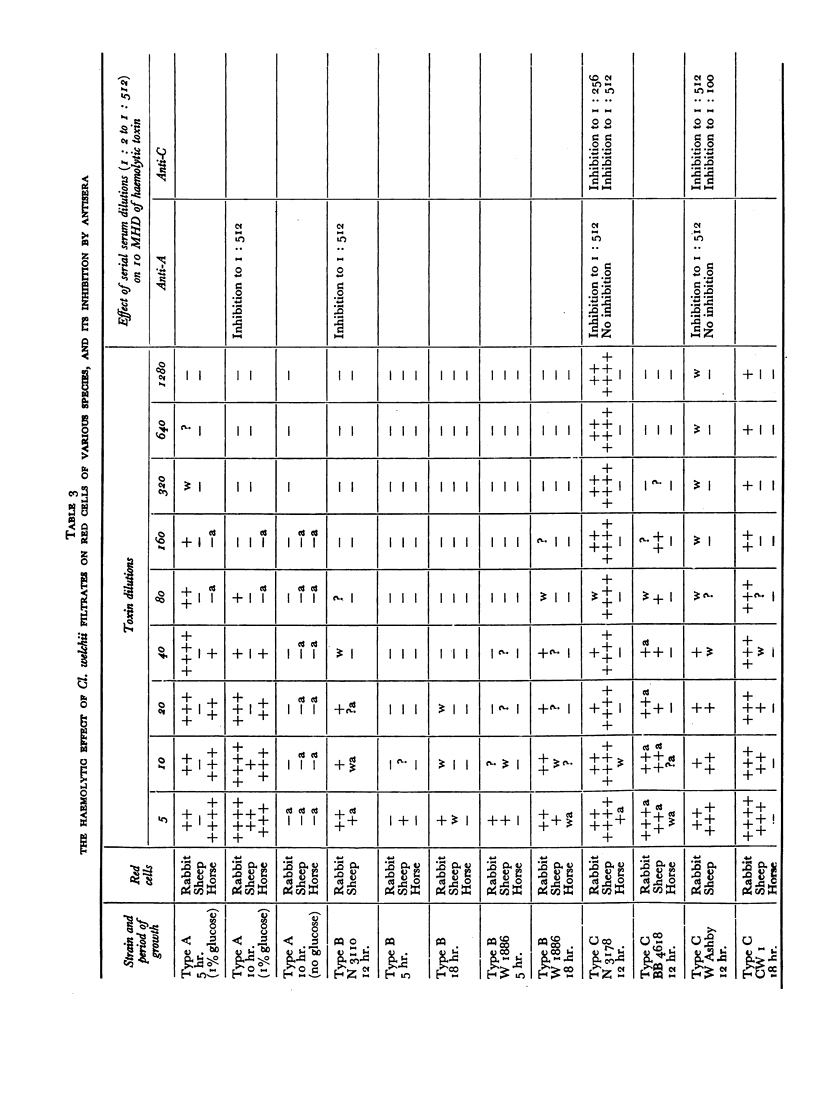
All the toxins investigated appeared during the active growth phase; some, e.g. β- and δ-toxins, were found to decline rapidly with longer incubation. This decline, together with the conversion of the ε-precursor to active toxin, could be attributed to the action of the enzymes which were also present in the filtrates, and the same effect could be produced by treatment with trypsin.
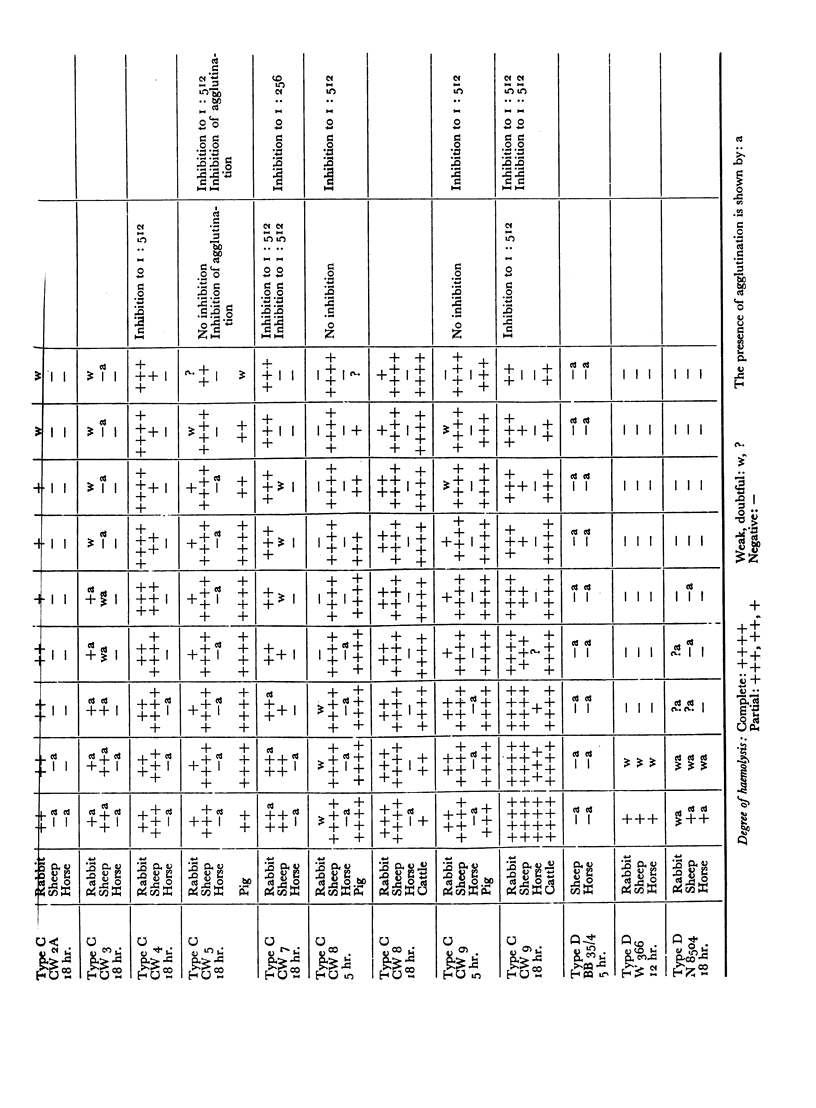
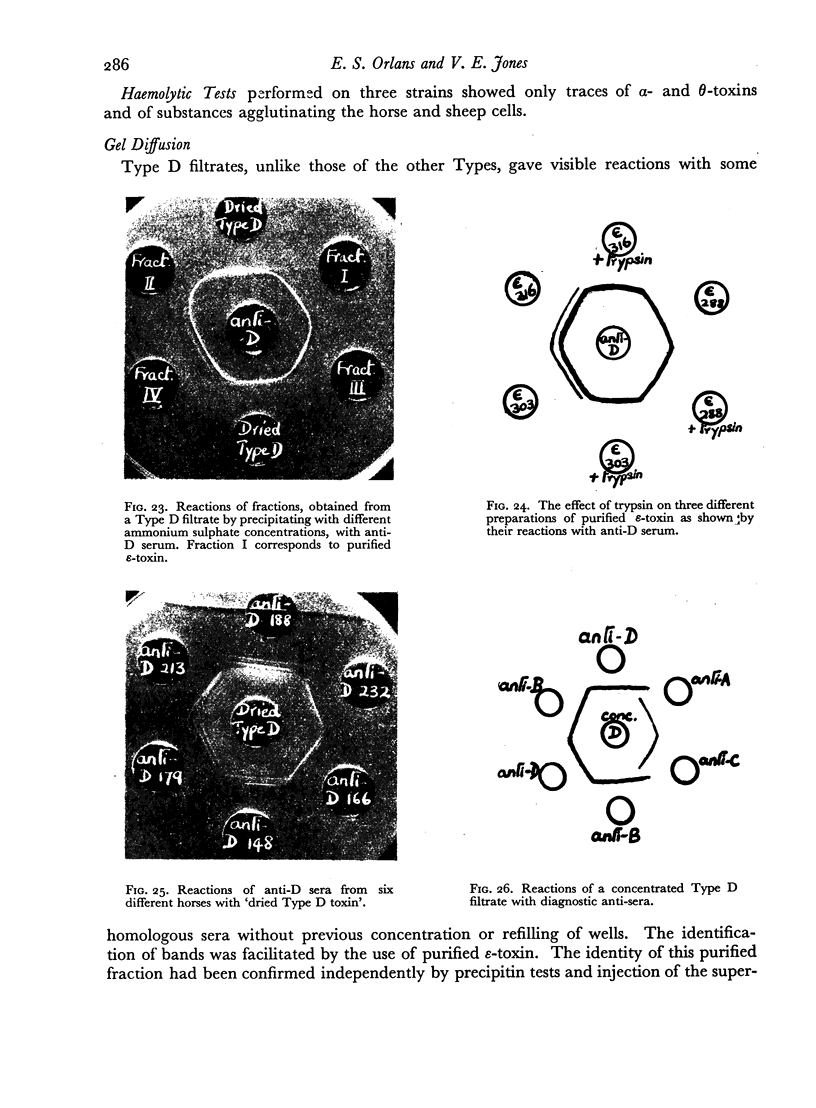
The antigens of the Type D strains conformed to the expected pattern. Those of the Type B and C strains, however, exhibited wide variation in distribution and quantity, and were occasionally found in combinations that fitted neither of the accepted Types.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIDWELL E. Proteolytic enzymes of Clostridium welchii. Biochem J. 1950 May;46(5):589–598. doi: 10.1042/bj0460589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Van Heyningen W. E. The effect of the pH and the presence of glucose during growth on the production of alpha and theta toxins and hyaluronidase by Clostridium welchii. Biochem J. 1942 Sep;36(7-9):624–630. doi: 10.1042/bj0360624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYWARD B. J., AUGUSTIN R. Quantitative gel diffusion methods for assay of antigens and antibodies. Int Arch Allergy Appl Immunol. 1957;11(3-4):192–205. doi: 10.1159/000228415. [DOI] [PubMed] [Google Scholar]
- JACOTOT H., VALLEE A., VIRAT B. Etude sur la transmission expérimentale de la myxomatose au lièvre. Ann Inst Pasteur (Paris) 1955 Jan;88(1):1–10. [PubMed] [Google Scholar]
- MACFARLANE M. G. The biochemistry of bacterial toxins; variation in haemolytic activity of immunologically distinct lecithinases towards erythrocytes from different species. Biochem J. 1950 Sep;47(3):270–279. doi: 10.1042/bj0470270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OAKLEY C. L. Bacterial toxins: demonstration of antigenic components in bacterial filtrates. Annu Rev Microbiol. 1954;8:411–428. doi: 10.1146/annurev.mi.08.100154.002211. [DOI] [PubMed] [Google Scholar]
- OAKLEY C. L., FULTHORPE A. J. Antigenic analysis by diffusion. J Pathol Bacteriol. 1953 Jan;65(1):49–60. doi: 10.1002/path.1700650105. [DOI] [PubMed] [Google Scholar]
- OAKLEY C. L., WARRACK G. H. Routine typing of Clostridium welchii. J Hyg (Lond) 1953 Mar;51(1):102–107. doi: 10.1017/s0022172400015527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUDIN J. B. Specific precipitation in gels and its application to immunochemical analysis. Methods Med Res. 1952;5:335–378. [PubMed] [Google Scholar]
- SAISSAC R., RAYNAUD M. Extraction de la toxin de Welchia agni var. wilsdoni (perfringens D) a partir des corps microbiens. Ann Inst Pasteur (Paris) 1951 Apr;80(4):434–437. [PubMed] [Google Scholar]
- STACK M. V., MORGAN W. T. J. The preparation and properties of enzymes from Clostridium welchii (type B) filtrates which destroy blood group substances. Br J Exp Pathol. 1949 Oct;30(5):470–483. [PMC free article] [PubMed] [Google Scholar]