Abstract
The stimulation of non-specific immunity by lipopolysaccharides could not be correlated with the serum properdin level at the time of challenge. Zymosan was effective in stimulating an increase in properdin without raising resistance to infection. The development of a low properdin level during Esch. coli infection did not inevitably lead to death.
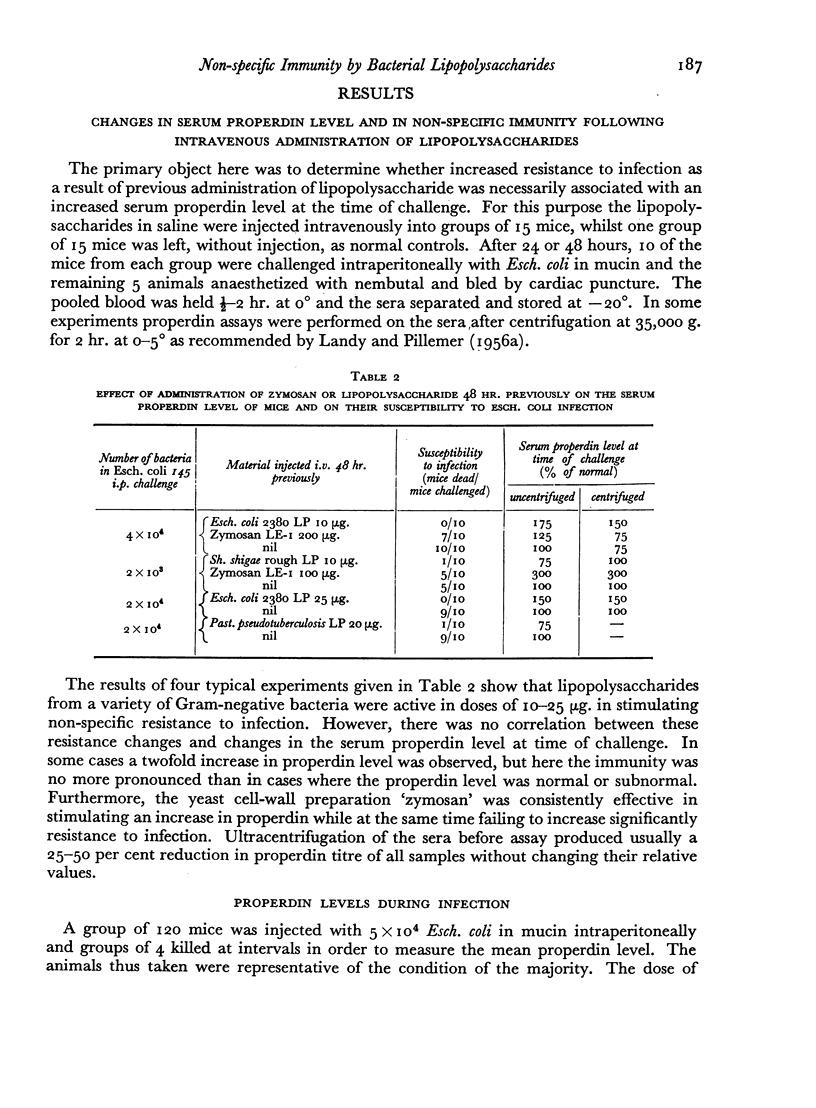
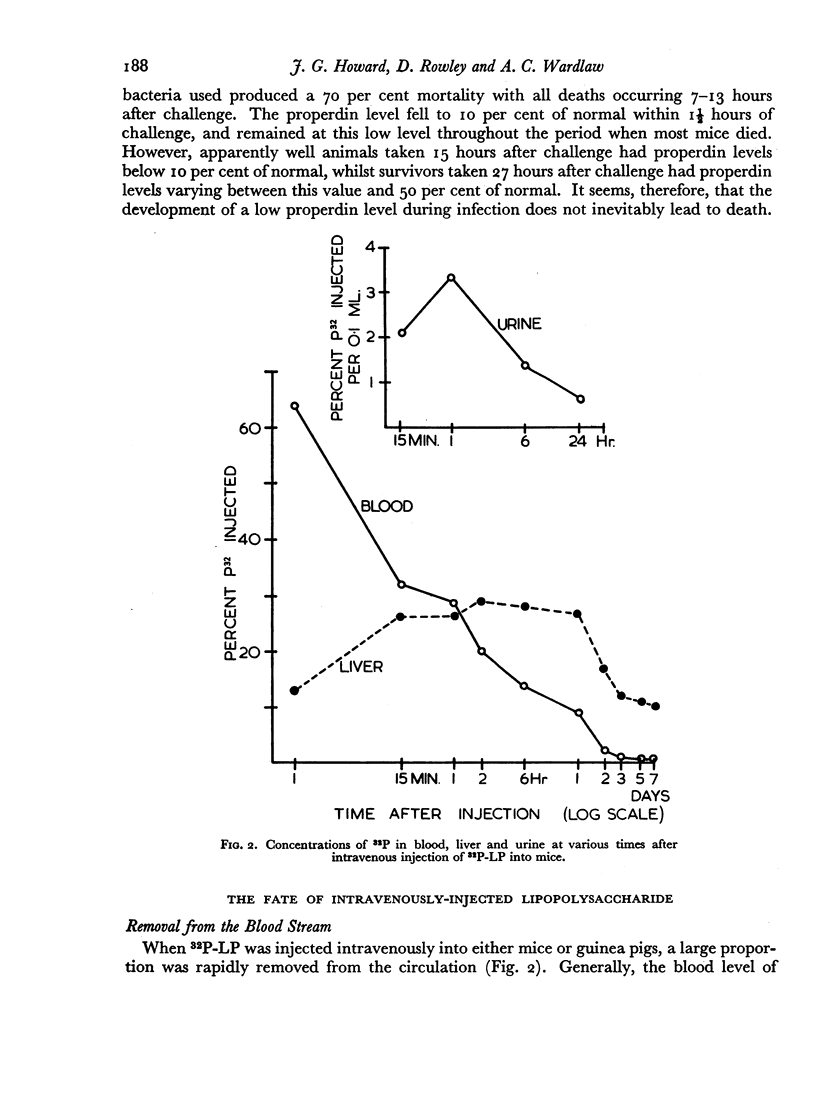
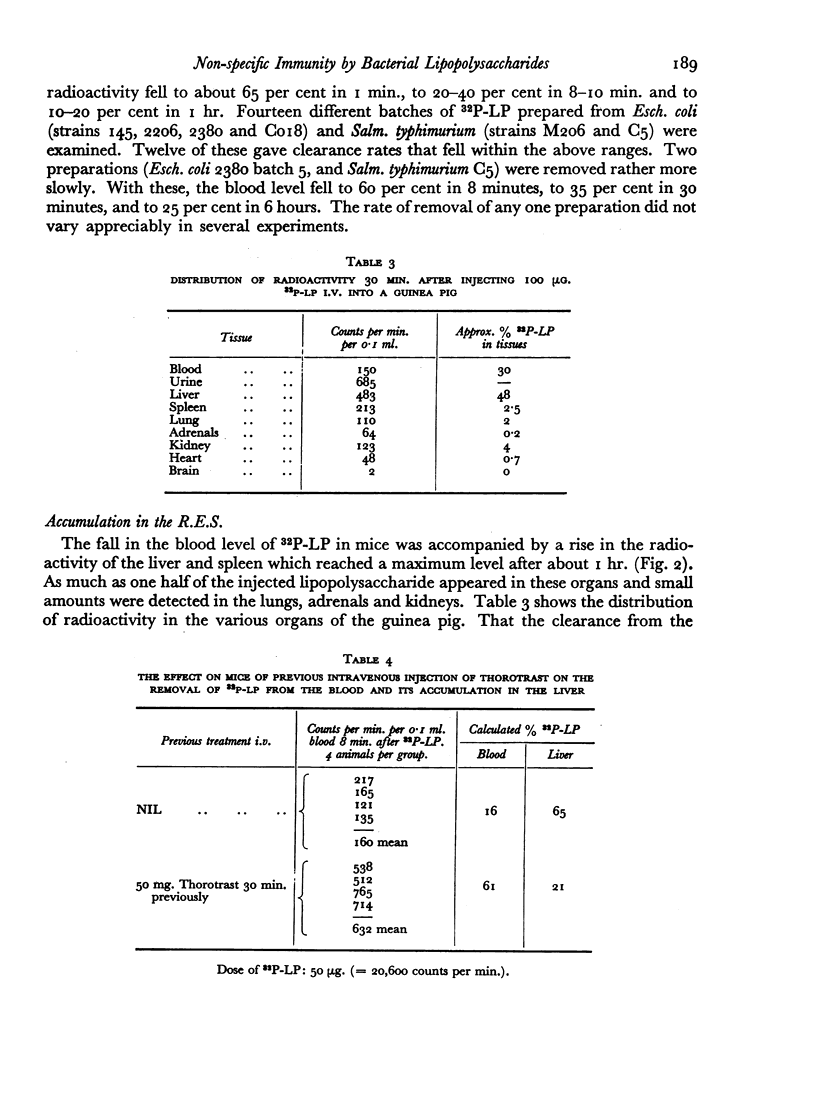
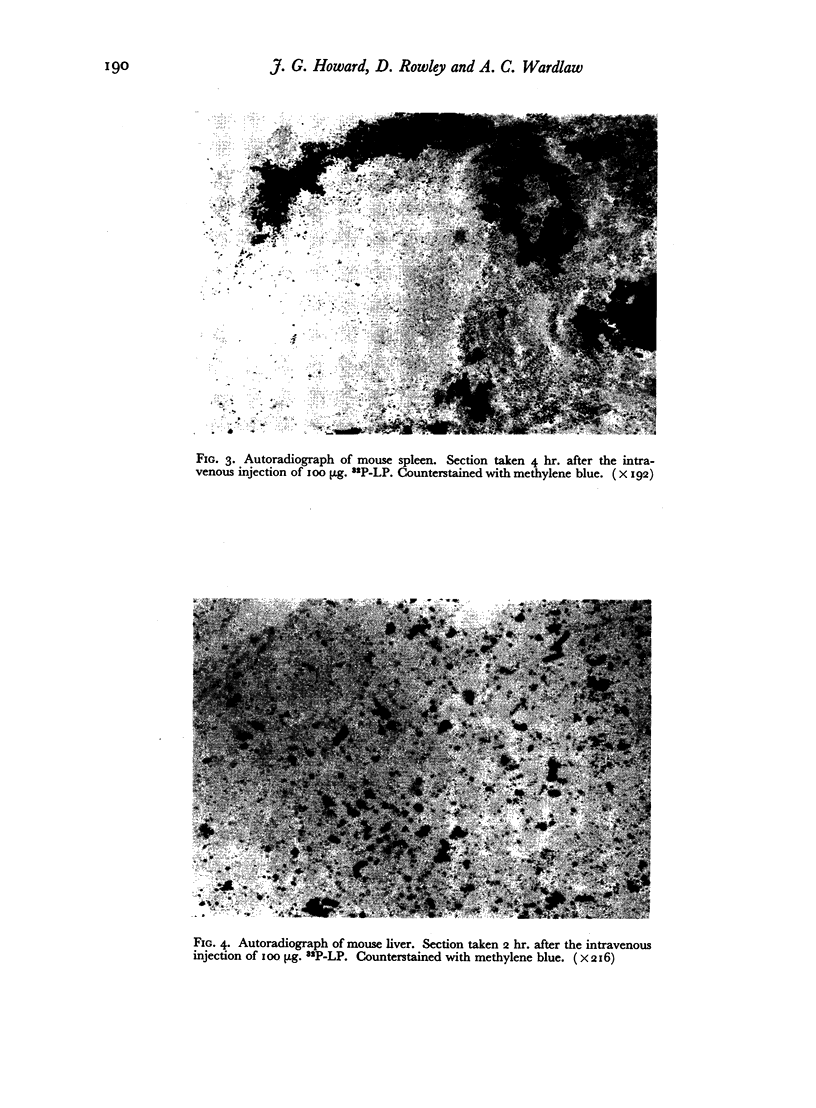
The fate of 32P-labelled lipopolysaccharide (32P-LP) after intravenous injection was followed in mice and guinea pigs. Most of it was accumulated rapidly in the reticulo-endothelial system. About a third was removed from the blood more slowly than the remainder, probably owing to a smaller particle size. Some of the 32P was excreted in the urine as inorganic phosphate. Very little radioactivity was found in the circulating leukocytes.
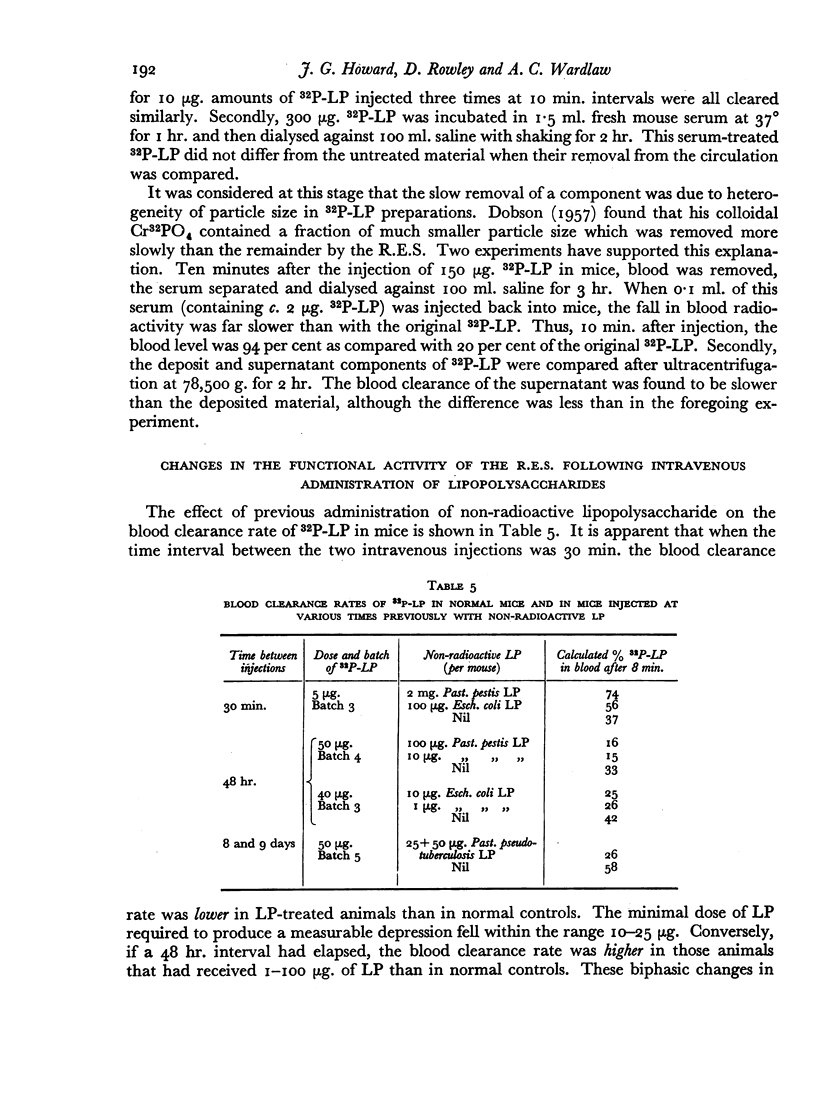
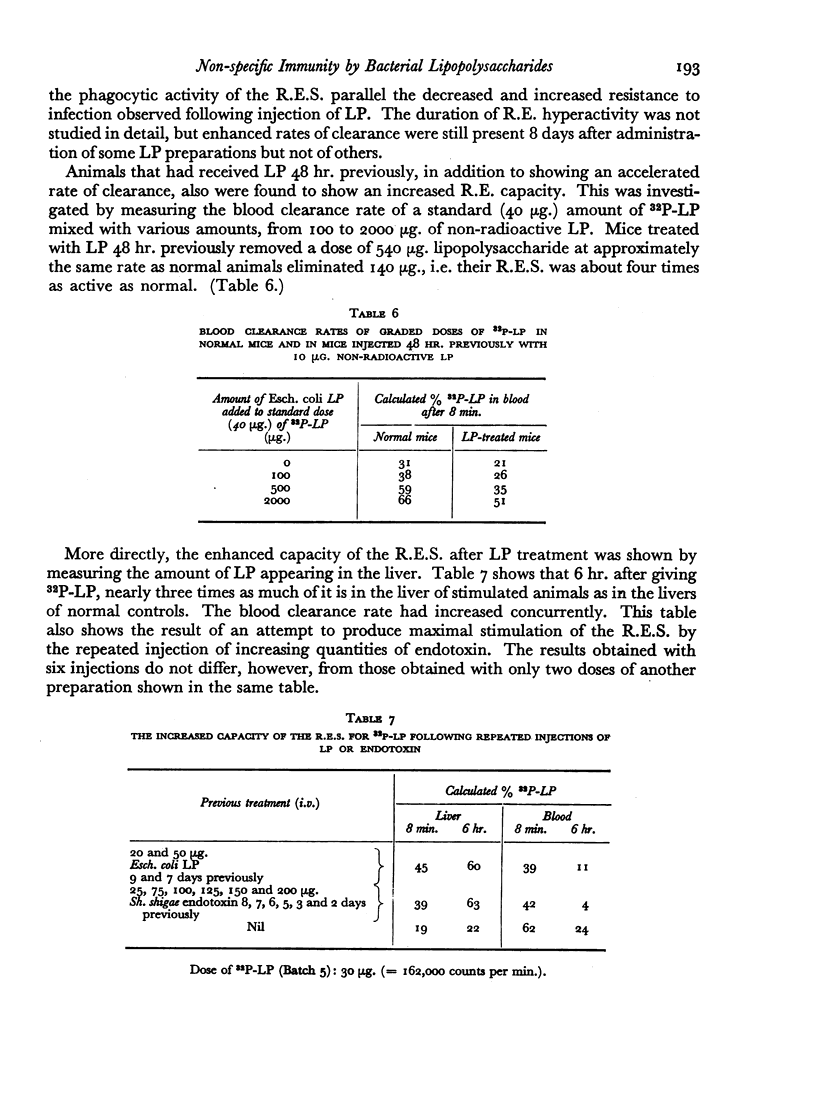
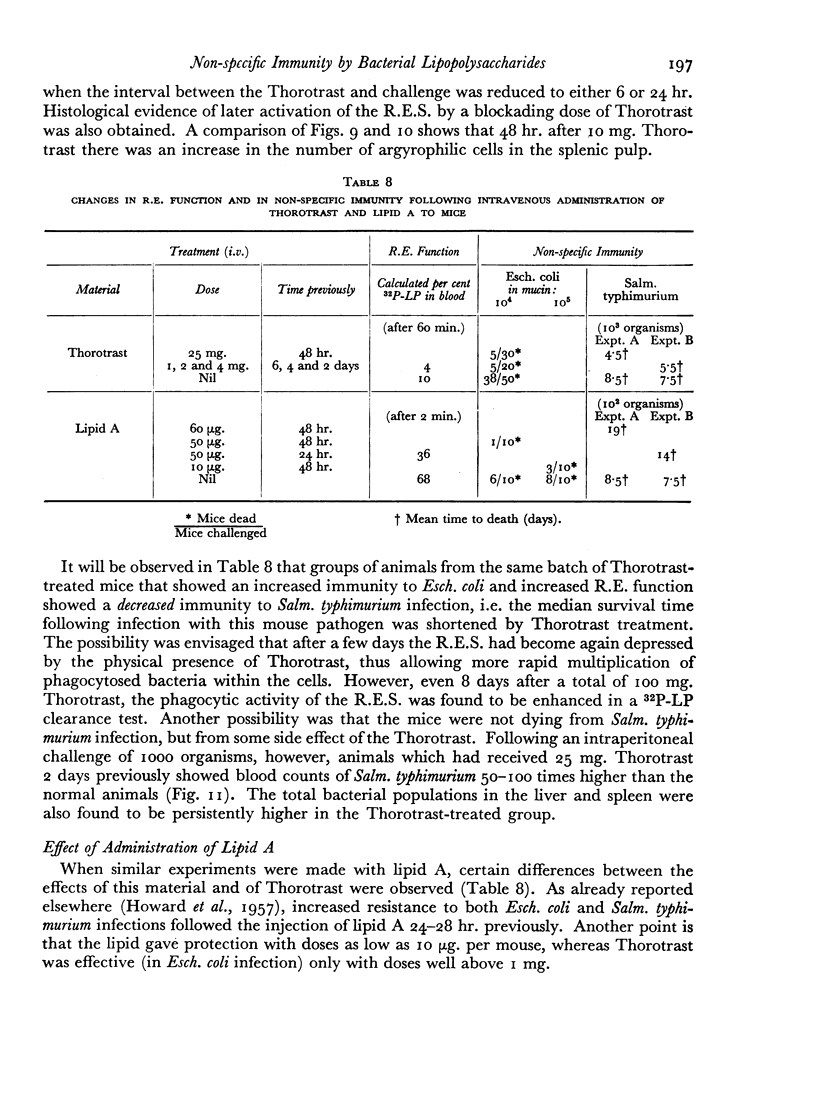
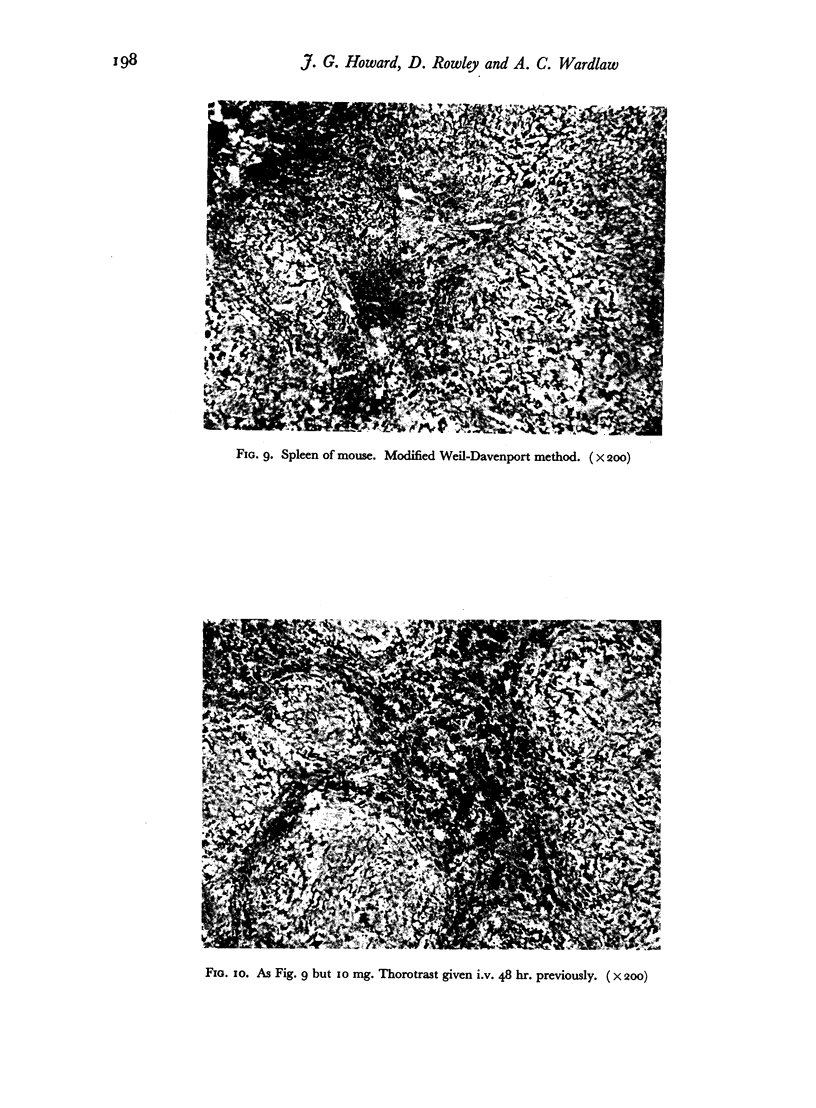
The blood clearance of 32P-LP was slowed by the administration of nonradioactive lipopolysaccharide 30 min. previously. When this interval was 48 hr. the reticulo-endothelial system was found to be hyperactive in terms of rate of clearance and phagocytic capacity. This was accompanied by an increase in the number of active macrophages in the liver and, to a lesser extent, in the spleen. These biphasic changes in the phagocytic activity of the R.E.S. parallel the decreased and increased resistance to infection following the injection of microgram amounts of lipopolysaccharide.
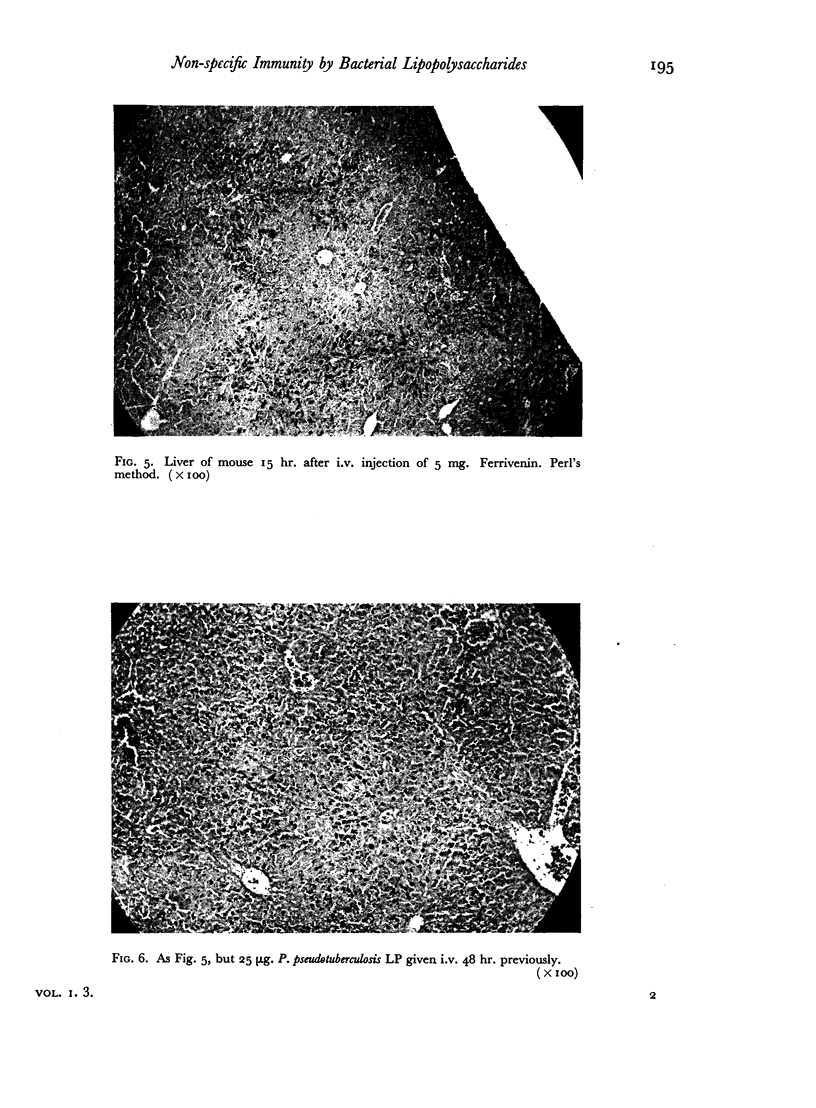
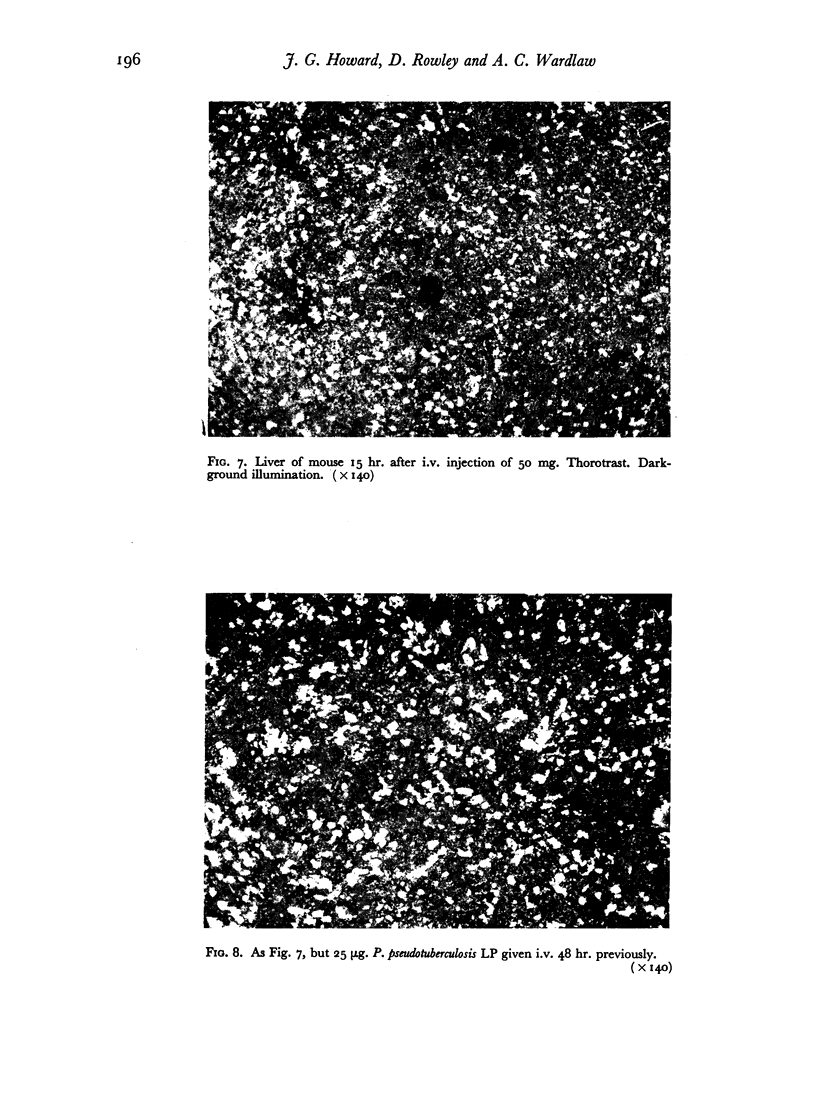
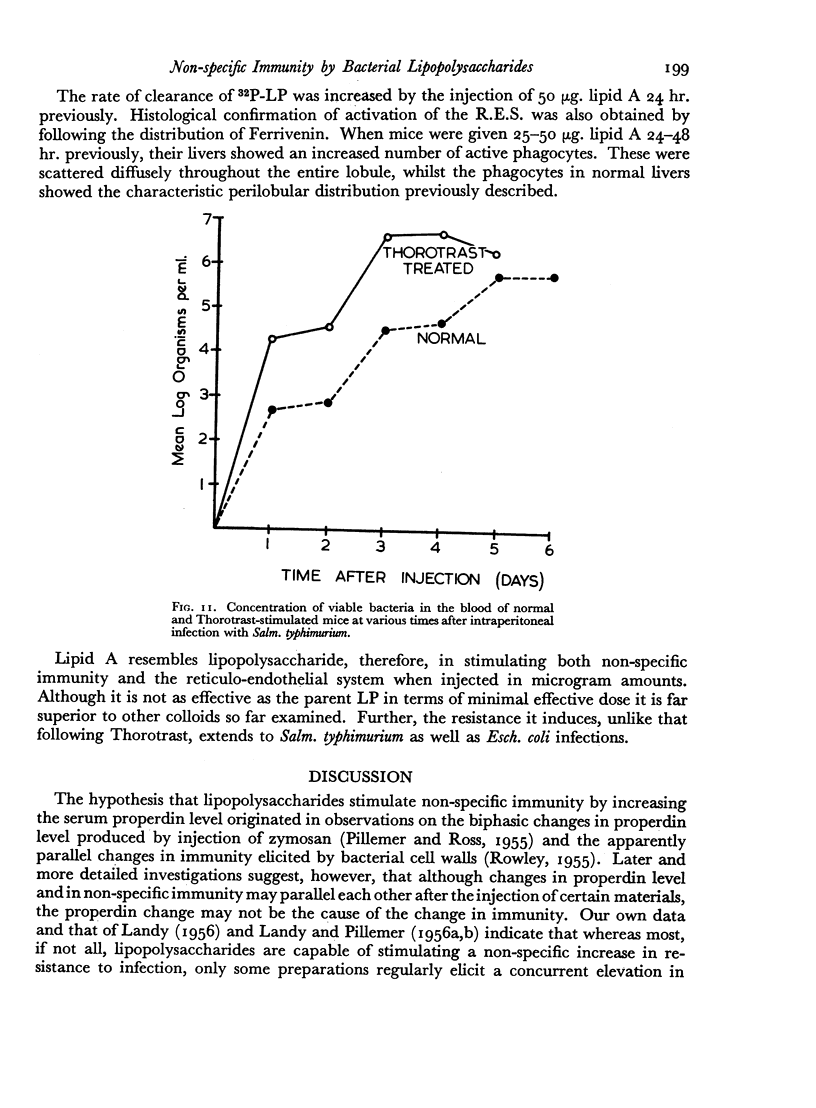
Successive depression and stimulation of the R.E.S. also followed the injection of milligram amounts of Thorotrast. The hyperactive phase was accompanied by increased resistance to Esch. coli infection and decreased resistance to Salm. typhimurium infection.
The lipid A component of lipopolysaccharide was also taken up by the R.E.S. which it stimulated. Resistance to both Esch. coli and Salm. typhimurium infections was enhanced.
The basis of lipopolysaccharide-induced non-specific immunity is discussed in relation to changes in the properdin and reticulo-endothelial systems. The superiority of lipopolysaccharide over other colloids in stimulating immunity is considered to be determined by lipid A.
Full text
PDF



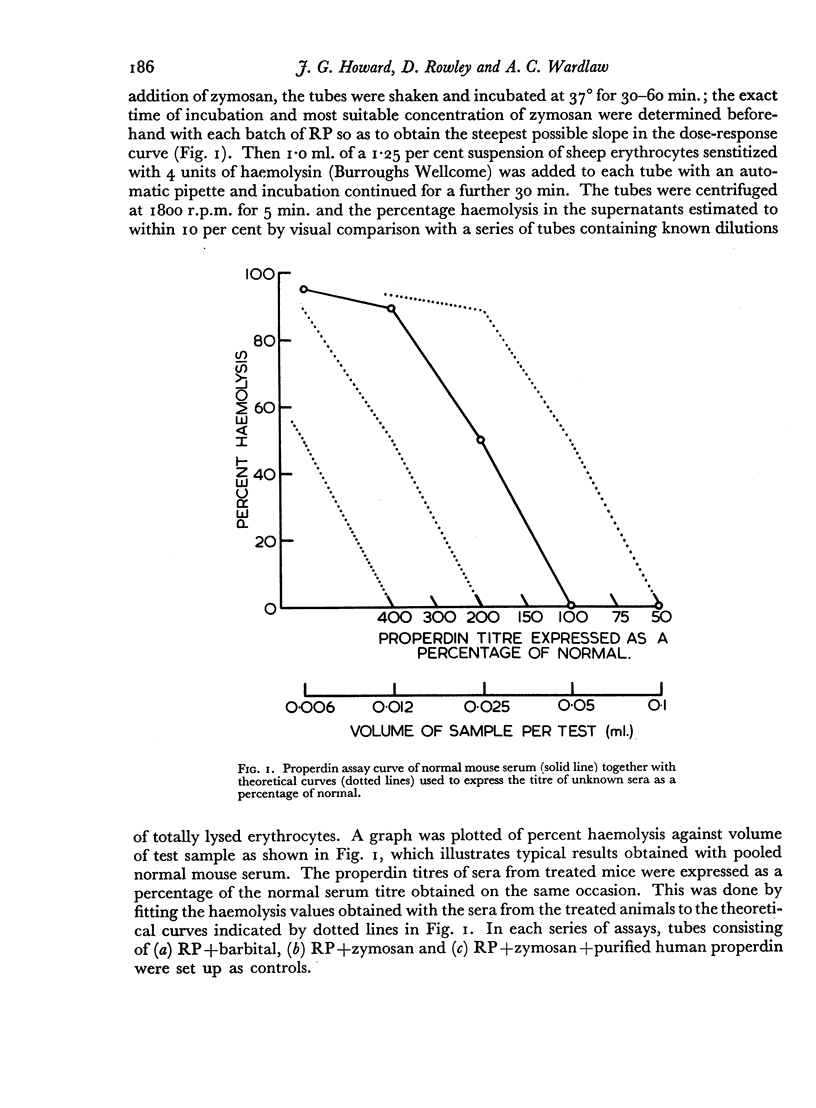






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., BENACERRAF B., HALPERN B. N. The effect of Salm. typhi and its endotoxin on the phagocytic activity of the reticuloendothelial system in mice. Br J Exp Pathol. 1955 Jun;36(3):226–235. [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEDLER R. W. Reversible changes in the susceptibility of mice to bacterial infections. I. Changes brought about by injection of pertussis vaccine or of bacterial endotoxins. J Exp Med. 1956 Jul 1;104(1):53–65. doi: 10.1084/jem.104.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD J. G., ROWLEY D., WARDLAW A. C. Stimulation of non-specific immunity by the lipid A component of bacterial lipopolysaccharide. Nature. 1957 Feb 9;179(4554):314–315. doi: 10.1038/179314a0. [DOI] [PubMed] [Google Scholar]
- LANDY M., PILLEMER L. Elevation of properdin levels in mice following administration of bacterial lipopolysaccharides. J Exp Med. 1956 Jun 1;103(6):823–833. doi: 10.1084/jem.103.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDY M., PILLEMER L. Increased resistance to infection and accompanying alteration in properidin levels following administration of bacterial lipopolysaccharides. J Exp Med. 1956 Sep 1;104(3):383–409. doi: 10.1084/jem.104.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS S., ESPLIN D. W., DONALDSON D. M. Lack of bactericidal effect of mouse serum on a number of common microorganisms. Science. 1954 Jun 18;119(3103):877–877. doi: 10.1126/science.119.3103.877. [DOI] [PubMed] [Google Scholar]
- PILLEMER L., BLUM L., LEPOW I. H., WURZ L., TODD E. W. The properdin system and immunity. III. The zymosan assay of properdin. J Exp Med. 1956 Jan 1;103(1):1–13. doi: 10.1084/jem.103.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PILLEMER L., ROSS O. A. Alterations in serum properdin levels following injection of Zymosan. Science. 1955 May 20;121(3151):732–733. doi: 10.1126/science.121.3151.732. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. Rapidly induced changes in the level of non-specific immunity in laboratory animals. Br J Exp Pathol. 1956 Jun;37(3):223–234. [PMC free article] [PubMed] [Google Scholar]
- SMULDERS J. Recherches sur l'athrocytose discriminante dans le système réticulo-endothélial. Etude qualitative et quantitative. Arch Biol (Liege) 1951;62(2):133–193. [PubMed] [Google Scholar]
- WARDLAW A. C., PILLEMER L. The properdin system and immunity. V. The bactericidal activity of the properdin system. J Exp Med. 1956 May 1;103(5):553–575. doi: 10.1084/jem.103.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]