Abstract
Fibrillin-1 is synthesized as a proprotein that undergoes proteolytic processing in the unique C-terminal domain by a member of the PACE/furin family of endoproteases. This family of endoproteases is active in the trans-Golgi network (TGN), but metabolic labeling studies have been controversial as to whether profibrillin-1 is processed intracellularly or after secretion. This report provides evidence that profibrillin-1 processing is not an intracellular event. Bafilomycin A1 and incubation of dermal fibroblasts at 22°C were used to block secretion in the TGN to confirm that profibrillin-1 processing did not occur in this compartment. Profibrillin-1 immunoprecipitation studies revealed that two endoplasmic reticulum-resident molecular chaperones, BiP and GRP94, interacted with profibrillin-1. To determine the proprotein convertase responsible for processing profibrillin-1, a specific inhibitor of furin, α-1-antitrypsin, Portland variant, was both expressed in the cells and added to cells exogenously. In both cases, the inhibitor blocked the processing of profibrillin-1, providing evidence that furin is the enzyme responsible for profibrillin-1 processing. These studies delineate the secretion and proteolytic processing of profibrillin-1, and identify the proteins that interact with profibrillin-1 in the secretory pathway.
Keywords: fibrillin-1, processing, chaperones, extracellular matrix, furin
Abbreviations used: BiP, immunoglobulin-binding protein; BME, β-mercaptoethanol; DMSO, dimethylsulfoxide; PMSF, phenylmethylsulfonyl fluoride; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; ER, endoplasmic reticulum; TGN, transGolgi network
Fibrillin-1 is a 350 kDa glycoprotein that is a major component of 10–12 nm microfibrils found in the extracellular matrix (ECM). Fibrillin-1 has a modular organization typical of ECM proteins with a repeated domain structure that has homology to epidermal growth factor (EGF), which is repeated 47 times. Another domain that is repeated has homology to motifs found in latent transforming growth factor β1 binding proteins (LTBPs). The carboxy-terminal domain (C-terminal) of fibrillin-1 and the fibulins shares homology [Giltay et al., 1999]. The single unique domains of fibrillin-1 include an amino-terminal (N-terminal) domain and a proline-rich domain [Pereira et al., 1993]. Mutations in fibrillin-1 cause Marfan syndrome, an autosomal dominant condition with skeletal, cardiovascular, and ocular complications.
Fibrillin-1 is synthesized as a proprotein, profibrillin-1, which undergoes proteolytic processing between amino acids 2731 and 2732 in the C-terminal domain, removing 140 of the 184 amino acids in this domain [Milewicz et al., 1995]. The processing site is immediately adjacent to a proprotein convertase consensus sequence for the paired amino acid cleavage enzymes (PACE) or furin family of endoproteases. The seven identified members of this family in mammalian cells are PACE/furin (furin), PC2, PC1/PC3 (PC3), PACE4, PC4, PC5/6B (PC6B), and LPC/PC7/8 (PC7) [Steiner, 1998]. Although cellular localization analyses have not been performed on all members of the enzyme family, many of these enzymes have been shown to be active in the trans-Golgi network (TGN) (reviewed in [Gensberg et al., 1998; Molloy et al., 1999]). Furin is the most extensively studied member of the PC family. Furin is a transmembrane protein that is synthesized as a 100 kDa protein that is rapidly converted into a 94 kDa protein by autocatalytic cleavage of the propeptide in the endoplasmic reticulum (ER). The propeptide remains bound and acts as an auto inhibitor of furin activity. The propeptide release occurs in the acidic pH of the TGN/endosomal compartment, where furin becomes active. Immunocytochemical studies have shown that furin is localized primarily to the TGN in steady state. However, a proportion of furin molecules can be found on the cell surface where furin cycles between the cell membrane and the TGN [Teuchert et al., 1999]. A truncated, active form of the enzyme is also secreted from cell lines over expressing furin [Rehemtulla et al., 1992].
It has been shown that many members of the PACE family can cleave at the consensus sequence in the C-terminal domain of profibrillin-1 [Raghunath et al., 1999]. Metabolic labeling studies showed no evidence of intracellular processing of endogenously synthesized profibrillin-1 in dermal fibroblasts [Milewicz et al., 1992]. In contrast, expression of a construct containing a portion of fibrillin-1, which included the C-terminal domain, was cleaved early in the secretory pathway in Cos-K1 cells [Ritty et al., 1999]. Therefore, the location in the secretory pathway in which profibrillin-1 is proteolytically processed has not been determined. If profibrillin-1 remains proteolytically unprocessed as it moves through the TGN, it would imply that cellular mechanisms are in place to prevent this processing.
In this report, we investigated the secretion and proteolytic processing of profibrillin-1, including the cellular location of profibrillin-1 processing, the PC family member responsible for proteolytic processing, and the proteins that interact with profibrillin-1 during secretion. Secretion blockers (lower temperature and bafilomycin A1) were used to confirm that profibrillin-1 processing did not occur in the TGN. We used a highly selective and potent inhibitor of furin termed the Portland variant of α1-antitrypsin (α1-PDX) [Anderson et al., 1997]. This furin-directed inhibitor contains an engineered consensus sequence for PC processing (reactive site sequence, -Arg355-Ile-Pro-Arg358↓-) that is not present in α1-antitrypsin (α1-NAT) (-Ala355-Ile-Pro-Met358-). The adenoviral constructs of the α1-PDX and α1-NAT were expressed in three human cell lines that synthesize and process profibrillin-1, dermal fibroblasts, an osteosarcoma cell line (MG63), and in aortic smooth muscle cells. In all cell lines studied, expression of the α1-PDX blocked the processing of profibrillin-1 to fibrillin-1 without disrupting protein synthesis or cell viability. Exogenous addition of the α1-PDX protein to dermal fibroblasts also resulted in inhibition of profibrillin-1 processing, providing further evidence that furin is responsible for the processing. Finally, we investigated the proteins associated with profibrillin-1 that may protect profibrillin-1 from premature processing and we report that two ER-resident molecular chaperones, BiP and GRP94, interact with profibrillin-1.
MATERIALS AND METHODS
Materials
Dermal fibroblasts were explanted from anonymously obtained neonatal foreskins using techniquespreviouslydescribed [Milewiczetal., 1992]. These experiments were performed with low passage (<P8) cells grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics (Invitrogen, Carlsbad, CA). MG63 and HA-VSMC cells were obtained from the American Type Culture Collection (ATCC).
For metabolic labeling, [35S]cysteine was used to label profibrillin-1 (Amersham Pharmacia Biotech, Piscataway, NJ) and Trans [35S]-label was used to label potential profibrillin-1 chaperones (ICN Pharmaceuticals, Inc., Irvine, CA) in DMEM lacking the appropriate amino acids. Reagents used during metabolic labeling included ammonium chloride, bafilomycin A1, brefeldin A (BFA), chloroquine, and methylamine (Sigma, St. Louis, MO). Protease inhibitors phenylmethylsulfonyl fluoride (PMSF) (Sigma), leupeptin, and pepstatin A (Calbiochem, San Diego, CA) were used in the lysis buffer at final concentrations of 0.2 mM, 0.5 μg/ml, and 0.5 μg/ml respectively.
For immunoprecipitation experiments, a polyclonal antibody specific for the proline-rich domain of fibrillin-1 was used (the kind gift of Dr. Robert Mecham, Washington University, St. Louis, MO). Antibodies used in immunoblot analysis were anti-GRP94 (Neomarkers, Union City, CA), anti-KDEL (StressGen Biotechnologies Corp., Victoria, BC, Canada), anti-ERP170 (the kind gift of Dr. J. Subjeck, Roswell Park Cancer Institute, Buffalo, NY), anti-ERP72 (Transduction Laboratories, Lexington, KY), and anti-protein disulfide isomerase, anti-calnexin, and anti-calreticulin (Affinity Bioreagents, Inc., Golden, CO). Hexokinase, apyrase, and ATP were purchased from Sigma.
Adenovirus constructs containing FLAG-tagged α1-antitrypsin (wild-type NAT), PDX variant, and the tet transactivating virus, have been previously described [Jean et al., 2000]. Anti-FLAGmAbM2waspurchasedfromSigma. Horseradish peroxidase-conjugated anti-mouse IgG was purchased from Amersham-Pharmacia (Piscataway, NJ).
Cell Culture and Metabolic Labeling
Metabolic labeling of newly synthesized proteins was also performed as previously described, with a few alterations [Milewicz et al., 1992]. Briefly, fibroblasts were seeded at 250,000 cells/35 mm dish and allowed to grow to confluence. After several washes with PBS, the cell layers were incubated in either cysteine-free or cysteine/methionine-free DMEM for 1 h. These media were replaced with media containing the appropriate [35S]cysteine or Tran35S-label (40 μCi/dish). After the pulse, the media were removed, and the cell sheets rinsed with PBS. The cell layers were lysed in either PBS–TDS (PBS with 0.1% Triton-X100, 12.1 mM sodium deoxycholate, 3.5 mM SDS), lysis buffer (50 mM Tris pH 8.0, 1% NP-40, 1 mM PMSF), or ATP-depleting buffer (ADB) (1% NP-40, 150 mM NaCl, 50 mM Tris pH 7.5, 11 mM glucose, 1 mM PMSF, 8 U/ml hexokinase, and 24 U/ml apyrase), depending on the subsequent experiment.
Secretion Blockers
To inhibit fibrillin-1 secretion in fibroblast cell strains, secretion-blocking reagents were included in the starvation and subsequent labeling of cell strains plated in 35 mm dishes as described above. Ammonium chloride was freshly made as a 2 M stock in water, and was used at a final concentration of 5 mM. Bafilomycin A1 was stored at −20°C as a 1 M stock in DMSO, and used at various concentrations. BFA was stored at −20°C as a 10 mg/ml stock in DMSO, and used at a final concentration of 10 μg/ml. Chloroquine and methylamine were both freshly made as 5 mM and 5 M stocks in water, and used at final concentrations of 50 μM and 10 mM respectively. To block secretion by lowering the temperature, cells plated in 35 mm dishes as above were pulsed with [35S]cysteine as described for 30 min. During the subsequent chase, cells were incubated at 22 or 37°C. Cultures incubated at 22°C were subsequently shifted to 37°C for further incubation.
Lysis Conditions, Cross-linking of Proteins, and Immunoprecipitation
For immunoprecipitation, cells were metabolically labeled with Tran [35S]-label and the cells lysed in situ in PBS–TDS. The lysates were collected and precleared by incubation with Protein G sepharose (Pharmacia) for 1 h at 4°C. The sepharose was pelleted by centrifugation, and the supernatants transferred to clean tubes containing either the anti-fibrillin-1 antibody or rabbit serum (RS). Protein G sepharose was added, and the samples were incubated overnight at 4°C with rotation. The immune complexes were pelleted and washed three times. After the final wash, the pellet was resuspended in Laemmli sample buffer containing BME and incubated at 100°C for 5 min. The samples were analyzed by SDS–PAGE using either 4 or 8% acrylamide gels. The gels were fixed, dried, and exposed to BIOMAX X-ray film (Eastman Kodak Co., Rochester, NY).
Crosslinking experiments were performed according to the method of Davis et al. [1998], using DSP, athiol-cleavablereagent,and DSS, a non-cleavable reagent. Crosslinked cell lysates (CL) were subsequently subjected to immunoprecipitation according to the procedure described above, and analyzed by SDS–PAGE after denaturation in Laemmli sample buffer containing BME and incubation at 100°C for 5 min.
For ATP-depletion studies, metabolically labeled cells were lysed with ADB and immunoprecipitated with an anti-fibrillin-1 antibody or RS [Chessler and Byers, 1993]. After overnight incubation at 4°C, immune complexes were pelleted and washed once. The pellets were resuspended in ADB without hexokinase and apyrase and divided into two separate aliquots. These parallel samples were incubated with and without ATP (4 mM) at room temperature for 30 min. The immune complexes were pelleted, and the pellets washed twice. The final pellet was resuspended in Laemmli sample buffer containing BME, incubated at 100°C, and analyzed by SDS–PAGE on an 8% gel. The gels were fixed, dried, and exposed to BIOMAX X-ray film (Kodak).
Immunoblot Analysis
Immunoblot analysis was performed on immunoprecipitated samples as described above, except that the cells were not metabolically labeled before lysis. After electrophoretic separation, the proteins were transferred to Hybond ECL nitrocellulose membranes according to the manufacturer’s directions (Amersham). Membranes were blocked overnight at 4°C in TBS-T (0.01 M Tris-HCl, pH 7.4, 0.15 M NaCl, 0.05% Tween-20) containing 5% milk solids. After two brief rinses with TBS-T, the membranes were incubated with the antibody of interest at 4°C overnight. The membranes were washed four times in TBS-T, and then incubated with the appropriate peroxidase-conjugated secondary antibody for 1 h at room temperature. After washing as above, the proteins were visualized by chemiluminescence (NEN Life Science Products, Boston, MA).
Adenovirus Infection and Metabolic Labeling
Fibroblasts were plated at 200,000 cells/35 mm dish and allowed to grow overnight. The next day, the cultures were coinfected with adenovirus recombinants expressing the tet transactivator (ta) together with adenovirus recombinants expressing either α1-NAT or α1-PDX. The cells were grown to confluence, followed by metabolic labeling of newly synthesized proteins as previously described. After several washes with PBS, the cell layers were incubated in cysteine-free DMEM for 1 h. These media were replaced with media containing [35S]cysteine (40 μCi/dish) for 30 min. After the pulse, the media were removed and the cell sheets rinsed with PBS. The cell layers were lysed in PBS–TDS, and aliquots analyzed by 4% SDS–PAGE. The gels were fixed, dried, and exposed to BIOMAX X-ray film (Eastman Kodak Co.).
RESULTS
Pulse Chase Analysis Demonstrates a Lack of Intracellular Processing of Profibrillin-1
In previous metabolic fibroblast labeling, no intracellular processing of profibrillin-1 was observed, as assessed by the absence of a faster migrating fibrillin-1 on gel electrophoresis of the cell lysate [Milewicz et al., 1992; Raghunath et al., 1995]. In contrast, a construct of a portion of the profibrillin-1, including the C-terminal domain, underwent intracellular processing [Ritty et al., 1999]. To confirm the lack of intracellular processing of full-length profibrillin-1, pulse-chase analysis of profibrillin-1 secretion and processing by neonatal fibroblasts was performed at various times up to 6 h (Fig. 1). The large size of the profibrillin-1 molecule (350 kDa), along with the small fragment that is removed with processing (approximately 20 kDa), makes immunodetection difficult and unreliable. We have established a SDS–PAGE system to study profibrillin-1 to fibrillin-1 processing based on shift in migration of the protein with processing [Milewicz et al., 1992, 1995; Raghunath et al., 1999]. The pulse-chase analysis failed to identify any processed, faster migrating fibrillin-1 in the cell lysate (Fig. 1A). In contrast, both profibrillin-1 and fibrillin-1 were present simultaneously in the media by 1 h (Fig. 1A). The amount of fibrillin-1 secreted is greater than the amount of profibrillin-1, but this ratio varies among control cells and with different labeling of the same cell strain. The reason for this variability is not known. The lack of intracellular fibrillin-1 was confirmed by immunoprecipitation of the various time points using a fibrillin-1 specific polyclonal antibody directed against the proline-rich domain of the molecule (Fig. 1B). In contrast, immunoprecipitation of the media established that both profibrillin-1 and fibrillin-1 were present (Fig. 1B). These results demonstrate that full-length profibrillin-1 is not processed within the cell, but rather during or immediately after secretion.
Fig. 1.
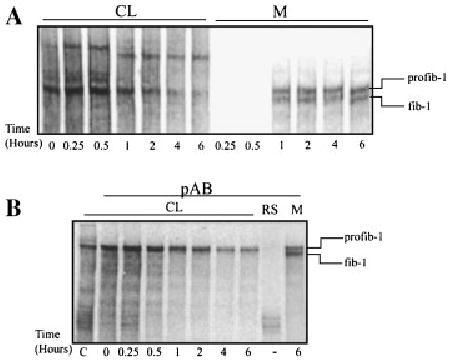
Pulse chase analysis of fibrillin-1 synthesis, secretion, and proteolytic processing indicates no intracellular processing of profibrillin-1. Neonatal fibroblasts were pulsed with [35S]cysteine for 15 min and then chased for various periods of time up to 6 h. The media and cell lysates (CL) were harvested and analyzed by 4% SDS–PAGE. A: Analysis of the cell lysate indicated that the higher migrating profibrillin-1 only was present in the CL. In the media, both profibrillin-1 and fibrillin-1 were present at 1 h. B: Immunoprecipitation of the CL using a fibrillin-1 polyclonal antibody directed against the proline-rich domain of the protein confirmed that only the profibrillin-1, the higher migrating form of the protein, was present in the CL. CL, cell lysate; M, media; profib-1, profibrillin-1; fib-1, fibrillin-1; RS, rabbit serum.
Profibrillin-1 Is not Proteolytically Processed When Secretion Is Blocked in the TGN
Agents were used to slow or block profibrillin-1 secretion to determine if slowing intracellular transit would induce processing. BFA blocks protein transport from the ER to the Golgi by disassembling the Golgi [Pelham, 1991]. The cis-, medial-, and trans-Golgi cisternae fuse with the ER, and secretory proteins accumulate [Misumi et al., 1986; Davis and Mecham, 1996]. Profibrillin-1 secretion inhibition by BFA did not result in intracellular profibrillin-1 processing (data not shown). Bafilomycin A1 was used to block the movement of profibrillin-1 from the TGN to the secretory vesicles later in the secretion pathway. Bafilomycin A1 is a specific inhibitor of vacuolar type H(+)-ATPases, and treatment with this drug inhibits the acidification in immature secretory granules necessary for intracellular transport [Henomatsu et al., 1993]. Increasing doses of bafilomycin A1 slowed secretion of profibrillin-1, evident by an increase in profibrillin-1 in the cell lysate, and completely blocked secretion at a dose of 5 μM (Fig. 2A). There was no evidence of intracellular processing of profibrillin-1 to fibrillin-1 based on the presence of a faster migrating form of fibrillin-1 in the cell lysate. Immunoprecipitation of intracellular profibrillin-1 using an antibody directed against the proline-rich domain in the amino terminal region of the protein confirmed that there was no intracellular fibrillin-1 processing (Fig. 2C).
Fig. 2.
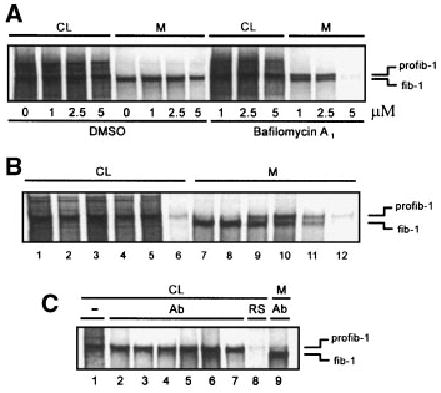
Effects of secretion inhibitors on profibrillin-1 processing. A: Neonatal dermal fibroblasts were treated with increasing concentrations of bafilomycin A1 during starvation and 6 h continuous metabolic labeling with [35S]cysteine. The CL and media were collected and analyzed by SDS–PAGE. Increasing concentrations of bafilomycin A1 inhibited secretion of profibrillin-1, with almost complete secretion inhibition seen at 5 μM. In addition, profibrillin-1 to fibrillin-1 processing was delayed in bafilomycin A1 treated cells. Reagent concentrations are labeled below the figure: bafilomycin A1 (μM); DMSO (the diluent) in corresponding amounts (5, 12, 25 μl). CL, cell lysate; M, media; profib-1, profibrillin-1; fib-1, fibrillin-1. B: To raise the intracellular pH, neonatal dermal fibroblasts were treated with various agents during starvation and metabolic labeling with [35S]cysteine. The media and CL were harvested and analyzed by SDS–PAGE. Cells treated with agents that raised intracellular pH demonstrated delayed profibrillin-1 processing in the media. Cells were left untreated (lanes 1 and 7), treated with 5 μl DMSO (diluent for bafilomycin A1) (lanes 2 and 8), 1 μM bafilomycin A1 (lanes 3 and 9), 5 mM ammonium chloride (lanes 4 and 10), 10 mM methylamine (lanes 5 and 11), and 50 μM chloroquine (lanes 6 and 12). CL, cell lysate; M, media. C: Immunoprecipita-tion of CL from treated fibroblast cultures using a fibrillin-1 polyclonal antibody (panel B, lanes 2–6) demonstrated no evidence of processed fibrillin-1 inside the cell. (−), non-precipitated CL control (lane 1); Ab, immunoprecipitated with anti-fibrillin-1 antibody (lane 2, control; lane 3, DMSO, lane 4, bafilomycin A1; lane 5, ammonium chloride; lane 6, methyla-mine; lane 7, chloroquine); RS, rabbit serum as negative im-munoprecipitation control (lane 8); media immunoprecipitated with anti-fibrillin-1 antibody as control for the migration of fibrillin-1 (lane 9). CL, cell lysate; M, media.
It was noted that with increasing concentrations of bafilomycin A1 the conversion of profibrillin-1 to fibrillin-1 was slowed (Fig. 2A). We sought to determine if other agents that raised the intracellular pH in the TGN/endosomal compartment would also slow the processing of profibrillin-1 to fibrillin-1. Ammonium chloride, methylamine, and chloroquine, as well as bafilomycin A1, were used to raise the pH of the TGN/endosomal compartment during the metabolic labeling of fibroblasts [Beers, 1996]. These agents all slowed the processing of profibrillin-1 to fibrillin-1 (Fig. 2B). Immunoprecipitation of treated CL using an antibody directed against the proline-rich domain in the amino terminal region of the protein failed to demonstrate the presence of processed fibrillin-1 within the cells (Fig. 2C).
To verify the lack of intracellular processing, the secretion pathway was also blocked in the TGN/endosomal compartment by decreasing the temperature [Sucic et al., 1999]. Cells were pulsed for 30 min with [35S]cysteine and then cooled to 22°C. The lower temperature effectively blocked secretion from the cells (Fig. 3A). After 2 h at 22°C, the cells were warmed to 37°C. Secretion of labeled profibrillin-1 was resumed when the cells were warmed, and the profibrillin-1 was efficiently processed upon secretion. Immunoprecipitation of the CL did not reveal any evidence of intracellular profibrillin-1 processing while the secretion was delayed, or after warming the cells and restoring secretion (Fig. 3B).
Fig. 3.
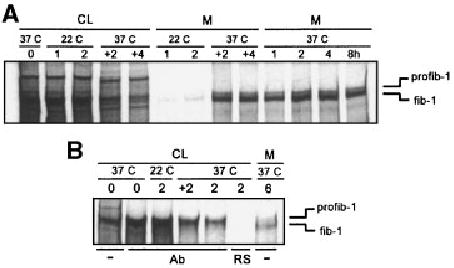
Effect of temperature shift on profibrillin-1 secretion. A: Neonatal dermal fibroblasts were metabolically labeled at 37°C with [35S]cysteine for 30 min, then chased with unlabeled media for 0, 1, 2, 4, and 8 h. Parallel cultures were incubated at 22°C after the pulse for 1 and 2 h, and then shifted to 37°C and the chase continued for an additional 2 and 4 h. Profibrillin-1 secretion was inhibited while the cultures were at 22°C, and resumed when the cultures were shifted to 37°C. CL, cell lysate; M, media. B: Immunoprecipitation of CL from specific time points shown in panel A using a fibrillin-1 specific polyclonal antibody demonstrate that intracellular fibrillin-1 was not present. (−), unprecipitated control; Ab, immunoprecipitated with anti-fibrillin-1 antibody; RS, pre-immune rabbit serum as negative control.
Inhibition of Profibrillin-1 Processing by α1-PDX in Human Dermal Fibroblasts, Aortic Smooth Muscle Cells, and MG63 Cells
We sought to identify which PC enzyme was responsible for profibrillin-1 processing. A highly potent inhibitor of furin, a variant of α1-antitrypsin called α1-PDX, was expressed in cell lines to determine if this inhibitor could prevent profibrillin-1 processing [Jean et al., 1998; Molloy et al., 1999]. Neonatal dermal fibroblasts were infected with viral stocks containing FLAG-tagged adenovirus constructs of either native α1-antitrypsin (α1-NAT), or α1-PDX, along with co-infection with the transactivating adenovirus construct (ta). Pulse-chase analysis of the cells 24 h after infection with the various viruses demonstrated that cells infected with α1-PDX showed delayed conversion of extracellular profibrillin-1 when compared with either uninfected control cells, cells infected with α1-NAT or cells infected with the trans-activating virus alone (Fig. 4A,B). Immunoblot analysis of the media from infected fibroblast confirmed the expression of the FLAG-tagged α1-PDX and α1-NAT proteins (Fig. 4C). These results indicate that α1-PDX inhibited profibrillin-1 to fibrillin-1 processing by neonatal dermal fibroblasts thereby indicating that furin is the PC responsible for profibrillin-1 maturation.
Fig. 4.
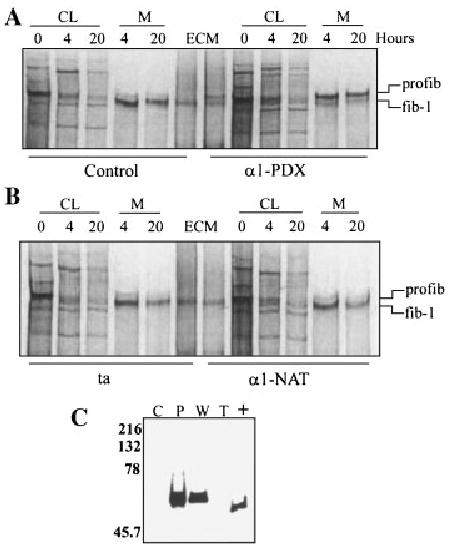
Fibroblast cell strains infected with adenovirus constructs expressing α1-PDX demonstrated delayed processing of profibrillin-1 to fibrillin-1. Fibroblasts were infected with transactivating virus (ta) either by itself, or co-infected with α1-PDX or α1-NAT. The cultures were radiolabeled with [35S]cysteine for 30 min, followed by both 4 and 20 h chases with non-radioactive DMEM. CL and media (M) fractions were harvested and separated by SDS–PAGE. A: Uninfected control cultures (control) and cultures co-infected with ta and α1-PDX (α1-PDX). B: Cultures infected with ta alone (ta) and cultures co-infected with ta and α1-NAT (α1-NAT). The positions of profibrillin-1 (profib-1) and fibrillin-1 (fib-1) are indicated. C: Immunoblot analysis of the media from infected cultures. Media was separated by SDS–PAGE through 10% gels and transferred to PVDF. Immunoblot analysis with mAb M2 detected positive signals in α1-PDX and α1-NAT infected CL and media. C, control; P, infected with α1-PDX and ta; W, infected with α1-NAT and ta; T, infected with ta.
We also sought to determine if α1-PDX could inhibit profibrillin-1 processing in other cell lines that convert profibrillin-1 to fibrillin-1. Human aortic smooth muscle cells and MG63 cells both synthesize and process profibrillin-1. These cells were infected with α1-NAT, α1-PDX, and the trans-activating virus, and pulse-chase analysis performed 48 h after infection. Similar to the results with the neonatal fibroblasts, infection with the α1-PDX constructs almost completely inhibited the processing of profibrillin-1 to fibrillin-1 in both cell types (Fig. 5A). Production of the α1-NAT and α1-PDX were confirmed through immunoblotting the media (Fig. 5B).
Fig. 5.
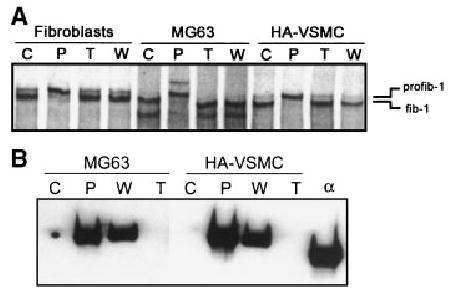
Delayed processing of profib-1 in cell strains infected with α1-PDX. Neonatal foreskin fibroblasts, MG63, and HA-VSMC were infected with transactivating virus (ta) either by itself, or co-infected with α1-PDX or α1-NAT. A: The cultures were radiolabeled with [35S]cysteine for 30 min, followed by a 20 h chase with non-radioactive DMEM. Cell strains infected with α1-PDX demonstrated delayed profibrillin-1 processing in the media. B: Immunoblot analysis of media from infected cultures. Media from MG63 and HA-VSMC cells infected with transactivating virus (ta) either by itself, or co-infected with α1-PDX or α1-NAT were separated by SDS–PAGE through 10% gels and transferred to PVDF. Immunoblot analysis with mAb M2 detected positive signals in α1-PDX and α1-NAT infected media. C, control; P, infected with α1-PDX and ta; W, infected with α1-NAT and ta; T, infected with ta.
Purified α1-PDX was added exogenously to dermal fibroblast cultures. Increasing amounts of α1-PDX were added to the media of fibroblast cultures for 6 h while the cells were continuously exposed to [35S]cysteine, and the media, cell lysate, and ECM harvested separately (Fig. 6A). Increasing doses of α1-PDX resulted in increasing inhibition of profibrillin-1 to fibrillin-1, with complete inhibition at 10 μM. ECM deposition was diminished with increasing α1-PDX, as previously observed with other inhibitors of profibrillin-1 processing. As a control, the cells were exposed to a potent inhibitor of all PC enzymes, decanoyl-R-V-K-R-chloromethylketone, which also completely blocked profibrillin-1 processing and fibrillin-1 ECM deposition [Raghunath et al., 1999]. Similar results were obtained with MG-63 cells (Fig. 6A,B).
Fig. 6.
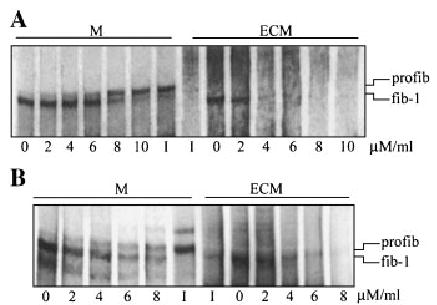
Addition of purified α1-PDX exogenously to dermal fibroblast cultures. Similar results were obtained with MG-63 cells A: Increasing amounts of α1-PDX were added to the media (M) of fibroblast cultures for 6 h while the cells were continuously exposed to [35S]cysteine, and the media, cell lysate, and ECM harvested separately (A). Increasing doses of α1-PDX resulted in increasing inhibition of profibrillin-1 to fibrillin-1 processing, with complete inhibition at 10 μM. ECM deposition was diminished with increasing α1-PDX concentration, as previously observed with profibrillin-1 processing. B: Increasing amounts of α1-PDX were also added to MG-63 cells, with similar results. As a control, the cells were exposed to a potent inhibitor (I) of all PC enzymes, decanoyl-R-V-K-R-chloromethylketone, which also completely blocked profibrillin-1 processing.
A secreted form of furin has been observed in cell lines over expressing furin. Since furin processes profibrillin-1 at the time of secretion, or immediately after secretion, Western analysis was done to confirm that dermal fibroblasts also produce a secreted form of furin. This analysis revealed a 60 kDa form of furin was secreted by dermal fibroblasts (Fig. 7). In the cell lysate, both the full length form (profurin) and the active form can be detected (104 and 94 kDa, respectively).
Fig. 7.
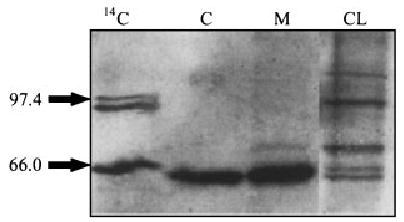
Immunoblot analysis of the secreted form of furin in the media and cell lysate of dermal fibroblasts. The cell lysate (CL) and media (M) were collected from dermal fibroblasts incubate serum-free media for 16 h, and analyzed on an 8% SDS–PAGE, followed by immunoblot analysis. This analysis confirmed that a 60 kDa form of furin was secreted by dermal fibroblasts. Full-length furin is present in the cell lysate, along with the secreted form. 14C, molecular size marker; C, furin control.
Identification of Profibrillin-1 Molecular Chaperones
The lack of intracellular profibrillin-1 processing by furin, which is known to be active in the TGN, implied that cellular mechanisms were in place to prevent premature processing. The possibility existed that an intracellular protein became associated with profibrillin-1 in the ER and remained bound through the TGN to prevent the premature processing of profibrillin-1 within the cell. Therefore, we sought to characterize the proteins that associated with profibrillin-1 within the secretory pathway.
Chaperones have been shown to co-immunoprecipitate differentially with their substrates following non-denaturing solubilization of cells in buffers containing detergents such as Triton-X100 and NP-40. Therefore, immunoprecipitation analyses of profibrillin-1 from CL were performed using a polyclonal antibody that recognized both profibrillin-1 and fibrillin-1 in the presence of both Triton-X100 and NP-40 to maximize the possible protein interactions. Because ATP binding was known to disrupt some interactions between proteins and chaperones, the dermal fibroblasts were solubilized in NP-40 under ATP-depleting conditions. To identify ATP-dependent chaperones, the immunoprecipitates were subsequently incubated with ATP. Finally, cross-linking of the intracellular proteins was done with both DSP and DSS, followed by immunoprecipitation.
Immunoprecipitation of profibrillin-1 after metabolic labeling demonstrated that proteins migrating at molecular weights of approximately 90 and 70 kDa were consistently associated with profibrillin-1 (Fig. 8A). The binding of the 70 kDa protein was shown to be ATP-dependent since this protein was present when the immunoprecipitate was incubated with ATP. The 70 kDa protein was also present with DSP cross-linking but not with DSS (data not shown). Based on the size of the protein and its ATP-dependent binding, the protein was tentatively identified as BiP, a well-characterized molecular chaperone known to interact with nascent polypeptides within the ER and previously described to interact with fibrillin-1 [Ashworth et al., 1999]. The identity of the protein was confirmed by immunoblotting using both a BiP-specific polyclonal antibody (data not shown) and a KDEL-specific monoclonal antibody known to recognize BiP and GRP94 (Fig. 8B). It was noted that the KDEL-specific monoclonal antibody also detected the 90 kDa protein (Fig. 8B). Subsequent immunoblotting with a polyclonal antibody specific for GRP94 confirmed this observation (Fig. 8C).
Fig. 8.
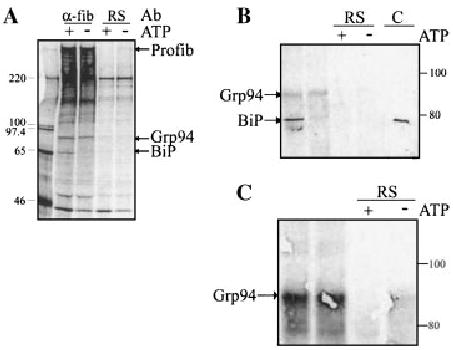
Identification of proteins associated with profibrillin-1 in the secretory pathway. A: Neonatal dermal fibroblasts were metabolically labeled with [35S]cysteine for 4 h, lysed in PBS–TDS, and immunoprecipitated with either anti-fibrillin-1 antibody (α-fib) or rabbit serum (RS) as a negative control. The migrations of proteins consistently found associated with profibrillin-1 are indicated. B: Neonatal dermal fibroblasts were lysed in ATP-depleting buffer, and immunoprecipitated with either anti-fibrillin-1 antibody or pre-immune RS as a negative control. Lysates were then subjected to incubation with or without ATP (+, −). After electrophoresis, immunoblot analysis of immunoprecipitated CL confirmed the identification of BiP using a KDEL-specific monoclonal antibody, which recognized a protein of the correct migration in both the fibroblast cell lysate and the positive control (C). This antibody also recognized a protein at the expected migration for Grp94. C: Immunoblot of immunoprecipitated CL after incubation with or without ATP (+, −). The identity of Grp94 was confirmed using an anti-Grp94-specific antibody.
We sought to determine if other known chaperones such as grp170, ERp72, protein disulfide isomerase, calnexin, or calreticulin interacted with profibrillin-1 during secretion. Immunoprecipitation using both sets of conditions, followed by subsequent immunoblotting with antibodies specific for these proteins, did not demonstrate any interaction of these ER proteins with profibrillin-1 (data not shown). In all these experiments, a protein of the correct molecular weight was identified when fibroblast CL were immunoblotted directly as a control.
DISCUSSION
Based on previous pulse-chase studies of full-length profibrillin-1 synthesized and secreted by dermal fibroblasts, we concluded that profibrillin-1 was processed to fibrillin-1 at the time of secretion or immediately after secretion [Milewicz et al., 1992]. Subsequently, it was shown that a member of the furin protease family cleaved profibrillin-1 in the C-terminal domain [Lonnqvist et al., 1998; Raghunath et al., 1999]. Since members of the furin family of enzymes are known to be active in the TGN, further examination was done to confirm that profibrillin-1 is not processed intracellularly. Pulse chase analysis, followed by immunoprecipitation of intracellular profibrillin-1, supported the lack of intracellular processing of profibrillin-1. When inhibitors were used to block secretion in the TGN (bafilomycin A1 and 22°C), there was no evidence of intracellular processing, despite the fact that furin is fully active at 22°C [Sucic et al., 1999]. These results were in contrast to the results obtained using a partial profibrillin-1 construct containing the C- and N-terminal domains along with a central region of fibrillin-1. This miniprofibrillin-1 protein was cleaved early in the secretory pathway in Cos-K1 cells when exposed to the secretion blocker BFA [Ritty et al., 1999]. A number of factors may have caused the intracellular processing of the miniprofibrillin-1 protein. Cos-K1 cells do not express endogenous profibrillin-1, and therefore may not have had the cellular mechanisms in place to prevent premature processing during secretion. Alternatively, the miniprofibrillin-1 construct may have had an altered conformation that exposed the C-terminal domain to proteases inappropriately early in the secretory pathway. The experiments in this report utilized dermal fibroblasts that constitutively synthesize and secrete full-length profibrillin-1.
Bafilomycin A1, ammonium chloride, chloroquine, and methylamine are reagents that increase intracellular pH. Incubation of fibroblasts with these reagents resulted in delayed profibrillin-1 processing, implying that the acidic environment of the secretory pathway was important for profibrillin-1 processing. It has also been shown that activation of the furin endoprotease required both autocatalytic propeptide cleavage in the ER and subsequent acidification in the Golgi, and these agents may have disrupted this activation [Anderson et al., 1997]. Alternatively, the progressively lower pH encountered in the secretory pathway may play a role in controlling profibrillin-1 processing. In the case of the furin propeptide cleavage required for activation, the cleavage consensus sequence containing an Arg in the P6 position (-Arg-X-X-X-Lys/Arg-Arg↓-motif) confers cleavage at an acidic pH of approximately 6.0 [Molloy et al., 1999].
Previous studies indicated that profibrillin-1 was not incorporated into the ECM [Milewicz et al., 1995]. Furthermore, if profibrillin-1 processing was inhibited, diminished amounts of microfibrils were assembled by dermal fibroblasts and no profibrillin-1 could be demonstrated in the ECM [Raghunath et al., 1999]. These results suggested that profibrillin-1 processing might occur in concert with the assembly of fibrillin-1 monomers into microfibrils. If this were the case, then this processing should take place upon secretion to prevent macroaggregates from forming within the secretory pathway. Similar proteolytic processing controls collagen fiber assembly. Type I collagen is a heterotrimer composed of two alpha 1 chains and one alpha 2 chain. These alpha chains fold as proproteins within the ER to form the procollagen triple helix. After secretion, procollagen is proteolytically processed by peptide removal from both the N- and C-terminal ends of the molecule. Once cleaved, collagen molecules rapidly aggregate into ordered structures. It has been shown that this proteolytic processing occurs very close to the cell membrane within invaginations of the cell surface in concert with aggregation [Kadler et al., 1996]. A similar cellular process may be in place for fibrillin-1, with profibrillin-1 processing as one of the signals for microfibril assembly. In fact, one of the fibrillar collagens, collagen type V, has been shown to undergo propeptide removal by furin in the C-terminal domain, and cleavage by bone morphogenetic protein (BMP-1) in the N-terminal propeptide domain to produce mature triple helical monomers capable of forming fibrils [Unsold et al., 2002].
The redundancy in the functional characterization and expression patterns of the PC enzymes excluded using these approaches to identify the PC enzyme responsible for profibrillin-1 C-terminal processing. An inhibitor of furin was engineered by construction of a variant of α1-antitrypsin, termed α1-PDX, which contained the minimal furin consensus sequence in the reactive site loop (-Arg-X-X-Arg↓-). Analysis of the specificity of α1-PDX showed it was selective for furin (Ki = 0.6 nM), and to a lesser extent PC6B (Ki = 2.3 nM), but not to other PCs [Anderson et al., 1993; Jean et al., 2000]. The efficiency of the SDS-stable complex formation for furin and α1-PDX was also much greater than that for PC6B. Localization studies have found furin to be stable when treated with BFA, whereas PC6B was dispersed by BFA treatment, indicating that PC6B does not remain localized to the TGN [Xiang et al., 2000]. Finally, the selectivity of α1-PDX for furin was further demonstrated when α1-PDX is added exogenously to cells [Jean et al., 1998]. Although furin is localized to the TGN, it cycles between this compartment and the cell surface. Furin is required for cellular uptake of α1-PDX for which it is a specific inhibitor of cellular furin. Based on the selectivity of α1-PDX for furin, we tested the ability of α1-PDX to block profibrillin-1 proteolytic processing. Using an adenoviral expression vector, both α1-PDX and α1-NAT were expressed in three human cell lines that process profibrillin-1. In every case, expression of α1-PDX effectively blocked profibrillin-1 processing. Furthermore, exogenous addition of the α1-PDX protein to fibroblast and MG-63 cells inhibited profibrillin-1 processing, providing further evidence that the PC responsible for profibrillin-1 processing is furin.
Our data indicate that profibrillin-1 moves through the TGN without undergoing proteolytic processing by active furin. These results provide evidence that some proproteins that are processed by furin escape proteolytic processing in the TGN, and are processed later in the secretory pathway or after secretion. Previous studies have identified the calcium dependent nature of profibrillin-1 processing by using low concentrations of EGTA [Raghunath et al., 1994]. Gelsolin has been shown to escape furin processing in the TGN in a similar manner. It is stabilized by Ca2+ in the slightly acidic conditions found in the Golgi and Golgi-derived transport containers, which prevents endoproteolytic cleavage by furin in these compartments and is secreted as a full length protein [Chen et al., 2001]. Another proprotein, the Semliki Forest virus glycoprotein precursor p62, is processed during transport from the TGN to the cell surface by furin [Band et al., 2001].
To evaluate the forms of furin being synthesized by dermal fibroblast cell strains, immunoblot analysis was performed. This assay revealed a novel secreted 60 kDa form in the media. Previous reports have delineated a 90 kDa secreted form of furin in COS-1 cells [Rehemtulla et al., 1992].
The lack of intracellular processing by furin, which is active in the TGN led us to investigate the intracellular proteins that bind to profibrillin-1. To study the intracellular proteins that interact with profibrillin-1, two different buffers were used for immunoprecipitation, along with protein cross-linking using DSP and DSS. Immunoblotting of the immunoprecipitated proteins was used to confirm the identity of proteins associated with profibrillin-1. These studies demonstrated that profibrillin-1 interacted with two ER-resident molecular chaperones, BiP and GRP94. BiP interacts with many secretory and membrane proteins within the ER during the course of their maturation [Kuznetsov and Nigam, 1998]. GRP94 is another molecular chaperone present in the ER that binds to proteins at different stages of maturation from BiP, distinguished by their oxidation state [Melnick et al., 1994]. In contrast to other studies, GRP94 binding was not reduced in the presence of ATP [Melnick et al., 1994; Linnik and Herscovitz, 1998]. These studies have indicated that the binding and hydrolysis of ATP by GRP94 was not an inherent property of GRP94, and it had been suggested that GRP94 release with ATP was due to interactions with other ATP-dependent chaperones [Wearsch and Nicchitta, 1997]. The binding of GRP94 to profibrillin-1 in the presence of ATP may be attributed to differences in the chaperone composition present in the GRP94–profibrillin-1 complex.
A number of ER-resident proteins were not found to be associated with profibrillin-1, specifically ERp72, grp170, protein disulfide isomerase, calnexin, and calreticulin. Calnexin and calreticulin are two homologous protein chaperones that prevent transport of misfolded glycoproteins by associating transiently and selectively with newly synthesized glycoprotein folding intermediates [Kim and Arvan, 1995]. Prolonged association with either protein was observed when proteins were misfolded or unable to oligomerize. Previous studies using expression of fibrillin-1 peptides in a transcription–translation system, followed by exposure of these peptides to semipermeabilized cells, indicated that calreticulin but not calnexin was bound to the fibrillin-1 protein fragment [Ashworth et al., 1999]. The binding demonstrated in this system may have been secondary to misfolding of the partial fibrillin-1 protein in the in vitro translation system. We cannot exclude the possibility that calreticulin interacted with profibrillin-1 transiently and was not detected by our methodologies. Both ERp72 and grp170 have been shown to interact with thyroglobulin and thrombospondin, and both of these large glycoproteins oligomerize within the ER but do not appear to associate with profibrillin-1 [Kuznetsov et al., 1997].
Based on the data presented here and previously published data, the following regulation of profibrillin-1 secretion is proposed. Fibrillin-1 is synthesized as a preproprotein and directed into the ER, where the signal peptide is removed. Profibrillin-1 interacts with BiP and GRP94 in the ER, presumably to facilitate folding. Profibrillin-1 moves through the TGN, where C-terminal processing by furin is prevented by an identified mechanism. Our data indicates that furin is the enzyme responsible for the proteolytic processing of profibrillin-1. Profibrillin-1 is proteolytically processed to fibrillin-1 by furin as it is secreted, or immediately after secretion. This proteolytic processing is dependent on the pH gradient between the TGN and secretory vesicles. If profibrillin-1 retains part of the C-terminal domain, it cannot be incorporated into the ECM. Previous data indicate that a furin-like enzyme may also process the N-terminal domain, although the location of this processing and its functional consequences has not been investigated [Reinhardt et al., 2000].
Acknowledgments
DMM is a Doris Duke Distinguished Clinical Scientist. The authors acknowledge Dr. Robert Mecham and Dr. J. Subjeck for the kind gifts of antibodies and Sean Molloy for his excellent technical assistance. The authors also thank Elaine Davis for her scientific contributions.
Footnotes
Grant sponsor: NIH; Grant numbers: R01 AR43626 (to DMM), NIH R01 AR46718 (to DMM), R01 DK37274 (to GT); Grant sponsor: March of Dimes Clinical Scientist Award (to DMM)
Elizabeth A. Putnam’s present address is Department of Pharmaceutical Sciences, The University of Montana, Missoula, MT 59812.
References
- Anderson ED, Thomas L, Hayflick JS, Thomas G. Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed alpha 1-antitrypsin variant. J Biol Chem. 1993;268:24887–24891. [PubMed] [Google Scholar]
- Anderson ED, VanSlyke JK, Thulin CD, Jean F, Thomas G. Activation of the furin endoprotease is a multiple-step process: Requirements for acidification and internal propeptide cleavage. EMBO J. 1997;16:1508–1518. doi: 10.1093/emboj/16.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth JL, Kelly V, Rock MJ, Shuttleworth CA, Kielty CM. Regulation of fibrillin carboxy-terminal furin processing by N-glycosylation, and association of amino-and carboxy terminal sequence. J Cell Sci. 1999;112(Pt 22):4163–4171. doi: 10.1242/jcs.112.22.4163. [DOI] [PubMed] [Google Scholar]
- Band AM, Maatta J, Kaariainen L, Kuismanen E. Inhibition of the membrane fusion machinery prevents exit from the TGN and proteolytic processing by furin. FEBS Lett. 2001;505:118–124. doi: 10.1016/s0014-5793(01)02798-3. [DOI] [PubMed] [Google Scholar]
- Beers MF. Inhibition of cellular processing of surfactant protein C by drugs affecting intracellular pH gradients. J Biol Chem. 1996;271:14361–14370. doi: 10.1074/jbc.271.24.14361. [DOI] [PubMed] [Google Scholar]
- Chen CD, Huff ME, Matteson J, Page L, Phillips R, Kelly JW, Balch WE. Furin initiates gelsolin familial amyloidosis in the Golgi through a defect in Ca(2+) stabilization. EMBO J. 2001;20:6277–6287. doi: 10.1093/emboj/20.22.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chessler SD, Byers PH. BiP binds type I procollagen pro alpha chains with mutations in the carboxyl-terminal propeptide synthesized by cells from patients with osteogenesis imperfecta. J Biol Chem. 1993;268:18226–18233. [PubMed] [Google Scholar]
- Davis EC, Mecham RP. Selective degradation of accumulated secretory proteins in the endoplasmic reticulum. A possible clearance pathway for abnormal tropoelastin. J Biol Chem. 1996;271:3787–3794. [PubMed] [Google Scholar]
- Davis EC, Broekelmann TJ, Ozawa Y, Mecham RP. Identification of tropoelastin as a ligand for the 65-kDa FK506-binding protein, FKBP65, in the secretory pathway. J Cell Biol. 1998;140:295–303. doi: 10.1083/jcb.140.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensberg K, Jan S, Matthews GM. Subtilisin-related serine proteases in the mammalian constitutive secretory pathway. Semin Cell Dev Biol. 1998;9:11–17. doi: 10.1006/scdb.1997.0196. [DOI] [PubMed] [Google Scholar]
- Giltay R, Timpl R, Kostka G. Sequence, recombinant expression, and tissue localization of two novel extracellular matrix proteins, fibulin-3 and fibulin-4. Matrix Biol. 1999;18:469–480. doi: 10.1016/s0945-053x(99)00038-4. [DOI] [PubMed] [Google Scholar]
- Henomatsu N, Yoshimori T, Yamamoto A, Moriyama Y, Tashiro Y. Inhibition of intracellular transport of newly synthesized prolactin by bafilomycin A1 in a pituitary tumor cell line, GH3 cells. Eur J Cell Biol. 1993;62:127–139. [PubMed] [Google Scholar]
- Jean F, Stella K, Thomas L, Liu G, Xiang Y, Reason AJ, Thomas G. alpha1-antitrypsin portland, a bioengineered serpin highly selective for furin: Application as an antipathogenic agent. Proc Natl Acad Sci USA. 1998;95:7293–7298. doi: 10.1073/pnas.95.13.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean F, Thomas L, Molloy SS, Liu G, Jarvis MA, Nelson JA, Thomas G. A protein-based therapeutic for human cytomegalovirus infection. Proc Natl Acad Sci USA. 2000;97:2864–2869. doi: 10.1073/pnas.050504297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J. 1996;316(Pt 1):1–11. doi: 10.1042/bj3160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PS, Arvan P. Calnexin and BiP act as sequential molecular chaperones during thyroglobulin folding in the endoplasmic reticulum. J Cell Biol. 1995;128:29–38. doi: 10.1083/jcb.128.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov G, Nigam SK. Folding of secretory and membrane proteins. N Engl J Med. 1998;339:1688–1695. doi: 10.1056/NEJM199812033392307. [DOI] [PubMed] [Google Scholar]
- Kuznetsov G, Chen LB, Nigam SK. Multiple molecular chaperones complex with misfolded large oligomeric glycoproteins in the endoplasmic reticulum. J Biol Chem. 1997;272:3057–3063. doi: 10.1074/jbc.272.5.3057. [DOI] [PubMed] [Google Scholar]
- Linnik KM, Herscovitz H. Multiple molecular chaperones interact with apolipoprotein B during its maturation. The network of endoplasmic reticulum-resident chaperones (ERp72, GRP94, calreticulin, and BiP) interacts with apolipoprotein b regardless of its lipidation state. J Biol Chem. 1998;273:21368–21373. doi: 10.1074/jbc.273.33.21368. [DOI] [PubMed] [Google Scholar]
- Lonnqvist L, Reinhardt D, Sakai L, Peltonen L. Evidence for furin-type activity-mediated C-terminal processing of profibrillin-1 and interference in the processing by certain mutations. Hum Mol Genet. 1998;7:2039–2044. doi: 10.1093/hmg/7.13.2039. [DOI] [PubMed] [Google Scholar]
- Melnick J, Dul JL, Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Pyeritz RE, Crawford ES, Byers PH. Marfan syndrome: Defective synthesis, secretion, and extracellular matrix formation of fibrillin by cultured dermal fibroblasts. J Clin Invest. 1992;89:79–86. doi: 10.1172/JCI115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewicz DM, Grossfield J, Cao SN, Kielty C, Covitz W, Jewett T. A mutation in FBN1 disrupts profibrillin processing and results in isolated skeletal features of the Marfan syndrome. J Clin Invest. 1995;95:2373–2378. doi: 10.1172/JCI117930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi Y, Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986;261:11398–11403. [PubMed] [Google Scholar]
- Molloy SS, Anderson ED, Jean F, Thomas G. Bi-cycling the furin pathway: From TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- Pelham HR. Multiple targets for brefeldin A. Cell. 1991;67:449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- Pereira L, D’Alessio M, Ramirez F, Lynch JR, Sykes B, Pangilinan T, Bonadio J. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome [published erratum appears in Hum Mol Genet 1993 Oct;2(10):1762] Hum Mol Genet. 1993;2:961–968. doi: 10.1093/hmg/2.10.1762. [DOI] [PubMed] [Google Scholar]
- Raghunath M, Kielty CM, Kainulainen K, Child A, Peltonen L, Steinmann B. Analyses of truncated fibrillin caused by a 366 bp deletion in the FBN1 gene resulting in Marfan syndrome. Biochem J. 1994;302(Pt 3):889–896. doi: 10.1042/bj3020889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunath M, Kielty CM, Steinmann B. Truncated profibrillin of a Marfan patient is of apparent similar size as fibrillin: Intracellular retention leads to over-N-glycosylation. J Mol Biol. 1995;248:901–909. doi: 10.1006/jmbi.1995.0270. [DOI] [PubMed] [Google Scholar]
- Raghunath M, Putnam EA, Ritty T, Hamstra D, Park ES, Tschodrich-Rotter M, Peters R, Rehemtulla A, Milewicz DM. Carboxy-terminal conversion of profibrillin to fibrillin at a basic site by PACE/furin-like activity required for incorporation in the matrix. J Cell Sci. 1999;112(Pt 7):1093–1100. doi: 10.1242/jcs.112.7.1093. [DOI] [PubMed] [Google Scholar]
- Rehemtulla A, Dorner AJ, Kaufman RJ. Regulation of PACE propeptide-processing activity: Requirement for a post-endoplasmic reticulum compartment and autoproteolytic activation. Proc Natl Acad Sci USA. 1992;89:8235–8239. doi: 10.1073/pnas.89.17.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt DP, Gambee JE, Ono RN, Bachinger HP, Sakai LY. Initial steps in assembly of microfibrils. Formation of disulfide-cross-linked multimers containing fibrillin-1. J Biol Chem. 2000;275:2205–2210. doi: 10.1074/jbc.275.3.2205. [DOI] [PubMed] [Google Scholar]
- Ritty TM, Broekelmann T, Tisdale C, Milewicz DM, Mecham RP. Processing of the fibrillin-1 carboxyl-terminal domain. J Biol Chem. 1999;274:8933–8940. doi: 10.1074/jbc.274.13.8933. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Sucic JF, Moehring JM, Inocencio NM, Luchini JW, Moehring TJ. Endoprotease PACE4 is Ca2+-dependent and temperature-sensitive and can partly rescue the phenotype of a furin-deficient cell strain. Biochem J. 1999;339(Pt 3):639–647. [PMC free article] [PubMed] [Google Scholar]
- Teuchert M, Berghofer S, Klenk HD, Garten W. Recycling of furin from the plasma membrane. Functional importance of the cytoplasmic tail sorting signals and interaction with the AP-2 adaptor medium chain subunit. J Biol Chem. 1999;274:36781–36789. doi: 10.1074/jbc.274.51.36781. [DOI] [PubMed] [Google Scholar]
- Unsold C, Pappano WN, Imamura Y, Steiglitz BM, Greenspan DS. Biosynthetic processing of the pro-alpha 1(V)2pro-alpha 2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases. J Biol Chem. 2002;277:5596–5602. doi: 10.1074/jbc.M110003200. [DOI] [PubMed] [Google Scholar]
- Wearsch PA, Nicchitta CV. Interaction of endoplasmic reticulum chaperone GRP94 with peptide substrates is adenine nucleotide-independent. J Biol Chem. 1997;272:5152–5156. doi: 10.1074/jbc.272.8.5152. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Molloy SS, Thomas L, Thomas G. The PC6B cytoplasmic domain contains two acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments. Mol Biol Cell. 2000;11:1257–1273. doi: 10.1091/mbc.11.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]


