Abstract
Some antigens induce Ab responses without T lymphocyte help. Among these, many polysaccharide-based antigens cause marginal zone B cells to proliferate and differentiate into plasma cells. B1 cells also respond to some of these antigens. In this article, we report that antigen-specific B1b cells, in response to the T-independent antigen (4-hydroxy-3-nitrophenyl)-acetyl (NP)-Ficoll, develop into clones that sustain Ab production for months with continued production of plasma cells and the accumulation of antigen-specific B cells in follicles. The persistence of this T-independent plasmablast response contrasts with the short-term plasmablast growth associated with T-dependent extrafollicular responses. The nature of the cells responding to NP-Ficoll was probed by using chimeras that have B1 cells but lack primary B lymphopoietic capacity and have very few B2 cells or T cells. The chimeras were constructed by transferring 105 IgM+ IgD− peritoneal exudate cells into mice unable to produce their own T and B cells because of deficiency in recombinase-activating gene 1 (RAG-1). The chimeras mounted sustained IgM and IgG3 anti-NP Ab responses to NP-Ficoll. This finding was associated with continued NP-specific extrafollicular plasmablast growth and the accumulation of NP-specific B cells in follicles. B cells were not found in the marginal zones of chimeras, and they also lacked recirculating IgD+ cells and CD3+ cells. The absence of B2 and T cells confirms that hemopoietic cell chimerism leading to primary lymphopoiesis had not been established.
Keywords: B1 cells, memory, plasmablasts
A group of thymus-independent (TI) antigens, known as TI-2 antigens, are linked by their inability to induce responses in Bruton tyrosine kinase-deficient mice (1, 2). Many of the naturally occurring antigens in this group are bacterial capsular and cell-wall polysaccharides. Typically, responsiveness to TI-2 antigens is acquired later in ontogeny than that to other TI antigens or thymus-dependent (TD) antigens (3). This delay has massive consequences for infection from bacteria with dense polysaccharide capsules such as the pneumococci, which cause large numbers of infant deaths annually from meningitis and pneumonia (4, 5). Characteristically TI-2 antigens induce splenic marginal zone B cells to grow as plasmablasts in extrafollicular foci of the spleen (6–8). Kearney et al. (7) have studied responses to phosphorylcholine that forms part of bacterial cell-wall polysaccharide. Natural Ab exists that binds this determinant, which is recognized both in the marginal zone and B1 repertoires. Using transgenic B cells specific for phosphorylcholine with Ig variable-region idiotypes that are associated with entry to either the B1 or marginal zone repertoires, these authors have shown that both types of B cells can be involved in the response to this antigen.
Classically, B cell memory is associated with TD Ab responses, and these memory cells are derived from B cells that have proliferated and undergone affinity maturation and selection in germinal centers (9). Although in certain circumstances TI-2 antigens are also able to induce germinal center formation, these instances are abortive and generate neither plasma cells nor memory B cells (10, 11). Memory B cells can be defined as cells that have undergone antigen-driven proliferation and then become nonproliferating cells that can be induced, on reexposure to antigen, to proliferate and secrete Ab. Evidence for the production of cells that fulfill these criteria from B1 cells responding to a TI-2 antigen is presented in this article.
Multiple copies of haptens such as (4-hydroxy-3-nitrophenyl)-acetyl (NP) conjugated to the neutral polysaccharides, Ficoll or hydroxyethyl starch, are frequently used as test TI-2 antigens (8, 10, 12–16). NP-Ficoll induces extrafollicular responses from both naïve and memory marginal zone B cells (8, 17). By contrast, IgDhigh CD23+ recirculating B cells with specificity for NP do not mount an Ab response to this antigen in vivo (6, 17). Background IgM, but not IgG Ab, that binds NP is found in nonimmunized mice, indicating that these specificities are included in the B1 repertoire. Furthermore, Pyk-2-deficient mice, which are reported to lack marginal zone B cells but have B1 cells, produce Ab responses, albeit diminished, to the related hapten 2-4-trinitro phenyl conjugated to Ficoll (18).
B cells recruited into extrafollicular Ab responses to the TD antigen NP-chicken γ-globulin grow as plasmablasts (proliferating Ab-secreting cells) for ≈4 days before terminally differentiating into plasma cells (nonproliferating Ab-secreting cells) (19). By contrast, plasmablasts can be found for months in extrafollicular Ab responses induced by immunization with NP-Ficoll (20). In this article, we address the basis for this persistence of plasmablasts in the response to NP-Ficoll. At first, the most obvious explanation seemed to be that naïve B cells recently produced in the marrow continue to be recruited into the response by persistent antigen. NP-Ficoll is resistant to degradation in the body and is taken up and retained by specialized macrophages in the splenic marginal zone (21); it can be held as an immune complex on the surface of follicular dendritic cells (20). Experiments reported here indicate that sustained responses to NP-Ficoll can be maintained without continued recruitment of naïve B cells. This article goes on to characterize mature B cell clones that can maintain a pool of NP-specific plasmablasts in response to NP-Ficoll.
Results
Self-Sustaining B Cell Clones Maintain Long-Term Plasmablast Responses to NP-Ficoll.
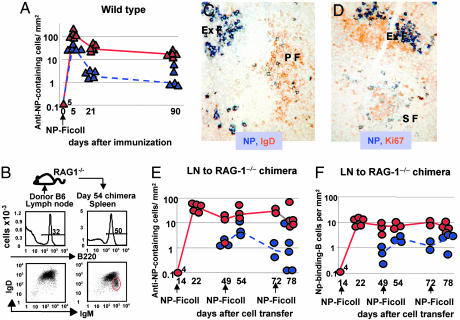
In response to i.p. immunization with NP-Ficoll, WT mice produced persistent NP-specific plasmacytoid cells (plasmablasts, plasma cells, or both) in splenic extrafollicular foci (Fig. 1A). Even 3 months after immunization, ≈10% of the plasmacytoid cells were in cell cycle, as assessed by Ki-67 nuclear staining. Comparable proportions of plasmacytoid cells were found to be in cell cycle in our previous studies of responses to NP-Ficoll in WT mice, as assessed by the uptake of the thymidine analogue 5-bromo-2′-deoxyuridine (20). To test whether this persistence of plasmablasts in responses to NP-Ficoll requires continued recruitment of naïve B cells, chimeric mice were constructed that had mature B cells but no capacity for primary B lymphopoiesis. The chimeras were created by transferring 107 lymph-node cells containing mature B and T cells into congenic recombinase-activating gene 1 (RAG-1)-deficient mice, which lack the capacity to produce T and B cells from hemopoietic progenitors (22) (Fig. 1B). Studies show that by 14 days after cell transfer such chimeras have reconstituted both follicular and marginal zone B cell subsets and produce Ab in response to immunization with NP-Ficoll (17). In this article, we tested whether the supply of mature NP-specific B cells would be exhausted through differentiation into short-lived Ab-producing cells. The chimeras were immunized i.p. with NP-Ficoll on days 14, 49, and 72 after cell transfer. Against expectation, plasmacytoid cells containing large amounts of anti-NP Ab were seen in splenic extrafollicular foci after each immunization (Fig. 1 C–E). As in WT mice, a proportion of these cells were plasmablasts, as assessed by expression of Ki-67 (Fig. 1 D and E). The additional immunizations had little obvious effect on the response apart from a possible trend for these immunizations to be followed by a temporary increase in the proportion of NP-specific plasmablasts (Fig. 1E). Ab titers achieved after the first immunization were maintained or somewhat increased after secondary and tertiary immunizations with NP-Ficoll. Typical Ab responses of chimeras are shown in the experiment described below and depicted in Fig. 2.
Fig. 1.
Evidence for persistent B cell clones that sustain extrafollicular responses to NP-Ficoll. (A) Immunohistological analysis of the splenic-specific Ab response of WT mice to NP-Ficoll. Blue triangles represent the number of NP-specific plasmablasts per mm2 of section at intervals after immunization; red triangles depict the total number of NP-specific plasmacytoid cells per mm2 (the sum of the numbers of NP-specific plasmablasts and NP-specific plasma cells). Triangles represent values from individual mice; continuous red and dashed blue lines connect median values. The number next to the day-0 red triangle indicates the number of superimposed points. (B) Construction of chimeras that have mature B cells but no capacity for B lymphopoiesis. Flow cytometry shows IgM and IgD expression of donor B220+ peripheral lymph-node cells (Left); 107 lymph-node cells were transferred into each RAG-1-deficient recipient. IgM and IgD expression of splenic B cells 54 days after lymph-node cell transfer is shown (Right); the red circle highlights an emergent IgMhigh IgDlow population not obvious in the donor population. (C) A spleen section from a chimera immunized on days 14 and 49 after cell transfer with NP-Ficoll and analyzed 6 days after the second immunization. NP-specific plasmacytoid cells (with strong blue cytoplasmic staining) are seen in an extrafollicular focus (ExF), whereas NP-specific B cells (with less strong blue surface staining) are seen among the orange-stained IgD+ recirculating B cells in a primary follicle (PF). (D) A similar section, this time identifying plasmablasts with cytoplasmic NP-binding (blue) and nuclear Ki-67 expression (brown) in an ExF. Some NP-specific B cells are seen in a secondary follicle (SF), and a proportion of these are Ki-67+; the other Ki-67+ cells in the follicle are germinal center B cells. (E) Plots similar to those in A, except that the responses of chimeras are followed. Groups of chimeras were immunized 14, 14 and 49, or 14, 49, and 72 days after construction by lymph-node transfer. Red symbols show the total numbers of NP-specific plasmacytoid cells per mm2, and blue symbols show the numbers of NP-specific plasmablasts per mm2. Red lines and dashed blue lines connect median values. The number next to the day-0 red circle indicates the number of superimposed points. (F) Similar plots to those in E, depicting data from the same chimeras but showing the numbers, in follicles and the T zone, of NP-specific B cells (red circles) and B blasts (blue circles) per mm2 of section. Red lines and dashed blue lines connect median values.
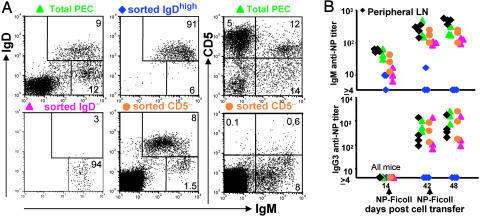
Fig. 2.
The relative ability of different B cell subsets to render RAG-1-defficient mice responsive to NP-Ficoll. (A) Flow cytometry plots of the unsorted and sorted donor populations used to construct chimeras in RAG-1−/− mice (a plot of donor peripheral lymph-node cells is shown in Fig. 1B). (B) NP-specific serum IgM and IgG3 Ab titers of chimeras immunized with NP-Ficoll on days 14 and 42 after cell transfer. The titers were determined on blood samples taken immediately before the first immunization, just before the second immunization, and 6 days after the second immunization. The symbols correspond to the donor cells used to construct the chimeras shown in A.
NP-Specific B Cells Accumulate in B Cell Follicles but Not Marginal Zones During Responses to NP-Ficoll.
Immunohistology of the spleens of chimeras that had received two or three immunizations with NP-Ficoll show that NP-specific B cells as well as plasmablasts and plasma cells were present (Fig. 1 C and D). The NP-specific B cells induced by NP-Ficoll were located mainly in follicles, both primary follicles, which lack germinal centers (Fig. 1C), and secondary follicles, which have germinal centers (Fig. 1D). Some NP-specific B cells were also located in the outer T zone, where their size and frequent expression of Ki-67 indicates these cells were mostly B blasts. The combined numbers of NP-specific B cells in the follicles and T zone and the proportion of these in cell cycle, as assessed by Ki-67 expression, are shown in Fig. 1F. The presence of B cells in follicles is consistent with our earlier finding of persistent and mainly nonproliferating NP-specific B cells in the follicles of WT mice after primary immunization with NP-Ficoll (20). Critically, there was a virtual absence of NP-specific follicular B cells before immunization in both WT mice and chimeras (Fig. 1F). This finding implies that the NP-specific B cells in the follicles of immunized chimeras have arisen after immunization by proliferation and that many have now come out of cell cycle. Despite the follicular location of the NP-specific B cells of chimeras immunized with NP-Ficoll, their phenotype was not that of germinal center B cells. First, as shown above, many of the NP-specific B cells were present in follicles that lack germinal centers, and even when they were in follicles with germinal centers, many were not in cell cycle (Fig. 1F). On the basis of immunohistology, these cells lack the germinal center B cell-associated features of Bcl6 expression and peanut agglutinin binding (data not shown). It is a theoretical possibility that hemopoietic cell chimerism was established by lymph-node lymphocyte transfer, although this hypothesis is unlikely because no host stem-cell depletion was undertaken before cell transfer. If this phenomenon had occurred, it could have led to B lymphopoiesis and the recruitment of naïve B cells into response. This possibility is addressed and excluded as an explanation for the persistence of plasmablasts in the response to NP-Ficoll in experiments described in the last section of Results.
There Are Sufficient B1b Cells in Lymph Nodes to Reconstitute the B1b Cell Compartment of Chimeras.
The experiments described above raise the suspicion that the long-term response of chimeras might be sustained by B1 cells because these, unlike B2 cells, are known to have the capacity for self-renewal in the periphery (23). A recent article has highlighted the ability of B1b cell clones to provide potent long-lasting TI-protective Ab responses against a relapsing fever bacterium, Borrelia hermsii (24). Furthermore, this study showed the expansion of antigen-specific clones of B1 cells. At first, it seemed that our choice of peripheral lymph-node cells as a source of donor cells for chimera construction did not favor this conclusion; for, as expected, the B cells in peripheral lymph-node suspensions were overwhelmingly IgDhigh IgMvariable recirculating B cells (Fig. 1B); such IgDhigh cells are known not to respond to NP-Ficoll in vivo (6, 17), although they can give rise to marginal zone cells (8, 25–27) that can respond to this antigen (6, 8, 28). Flow cytometry of the donor B220+ lymphocytes showed that <2% of B cells have an IgMhigh IgDlow phenotype that characterizes B1 cells and marginal zone B cells (Fig. 1B). Despite this result, analysis of peritoneal exudate cell (PEC) suspensions from chimeras showed that an IgM+, IgDlow/−, CD11b+, CD23low B1 population emerges over time in RAG-1−/− mice that have received peripheral lymph-node cells (see Fig. 4A, which is published as supporting information on the PNAS web site). In comparison to PEC from WT mice, a lower proportion of the IgM+ PEC in these chimeras expressed CD5 (see Fig. 4B), indicating that lymph-node cells predominantly reconstituted the B1b compartment. The small number of B1 cells transferred compared to the numbers of B1 cells recovered in the PEC of chimeras seems likely to reflect proliferation after transfer.
Small Numbers of B1 Cells Endow RAG-1−/− Mice With the Ability to Mount Long-Term Ab Responses to NP-Ficoll Without Reconstituting the Marginal Zone or Recirculating B Cell Pools.
To investigate the possible contribution of B1 cells to the persistent response to NP-Ficoll, a new series of chimeras were constructed by transferring into RAG-1−/− mice small numbers of B1 cells with minimal numbers of B2 cells (Fig. 2A). The aim was to create a B1 cell pool but not recirculating and marginal zone B2 cell pools. The chimeras constructed with PEC enriched for B1 cells and depleted of B2 cells received 105 IgM+ PEC depleted of IgD-expressing cells. A second group of chimeras received 105 IgM+ PEC depleted of CD5-expressing cells. This group was used to test whether B1b cells were able to support long-term responses to NP-Ficoll. Positive control groups were included that received either 106 total PECs (a mixture of B1 and recirculating B cells) or, as in the first experiments, 107 peripheral lymph-node cells (mainly recirculating B cells but also, as shown above, some B1 cells). A final group of chimeras received 105 IgDhigh PECs (recirculating cells depleted of B1 cells). The phenotypes of representative donor populations are shown in Fig. 2A. Chimeras were immunized i.p. with NP-Ficoll 14 and 42 days after cell transfer and were finally assessed on day 48.
Serological analysis of the response of the different chimeras to NP-Ficoll is shown in Fig. 2B. The chimeras constructed with IgD− IgM+ PEC produced IgM and IgG3 Ab after immunization with NP-Ficoll (Fig. 2B). A similar serum Ab response was detected in the other groups of chimeras, except those constructed by transfer of 105 IgD+ PEC, which failed to mount an NP-specific Ab response to NP-Ficoll. In addition to the specific Ab response to NP-Ficoll, it can be seen that 14 days after cell transfer and before primary immunization natural IgM, but not IgG3, anti-NP Ab is present in the serum of all chimeras except those constructed with IgD+ cells.
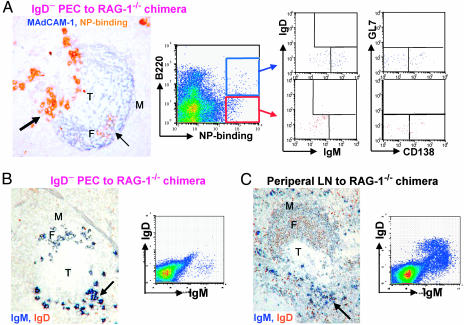
The spleens of the chimeras reconstituted with IgD− IgM+ PEC had NP-specific plasmacytoid cells in extrafollicular foci (Fig. 3A). Between 39% and 64% of these cells had switched to IgG3 (see Table 1, which is published as supporting information on the PNAS web site) and consistently ≈10% of these were Ki-67+. In addition, NP-specific B cells were found in splenic B cell follicles of these chimeras (Fig. 3A). These cells are probably correspond to B220+ NP-binding B cells identified by flow cytometric analysis of these spleens (Fig. 3A), which are a mixture of IgM+ and, presumably switched, IgM− cells. No cells expressed IgD, nor the germinal center B cell marker GL7, whereas some cells expressed intermediate levels of syndecan consistent with them being plasmablasts.
Fig. 3.
Transfer of 105 PEC depleted of IgD+ cells enables RAG-1−/− mice to sustain long-term responses to NP-Ficoll without restoring the recirculating and marginal zone pools of B2 cells. (A) Analysis of NP-specific cells in the spleen of a chimera constructed 48 days before with 105 IgD-depleted PEC. The photomicrograph shows IgM+ plasmacytoid cells (large arrow). Stromal elements in a follicle (F) and marginal sinus (M) are stained blue for mucosal addressin cell-adhesion molecule expression; There is a cluster of NP-specific B cells in the follicle (small arrow), but none of these cells are located in the marginal zone, which lies outside the marginal sinus. Flow cytometry shows analysis of IgM, IgD, GL7, and CD138 expression by NP-binding cells gated into B220high and B220low subsets. The spleen was taken 48 days after cell transfer with immunizations with NP-Ficoll on days 14 and 42. (B) IgM and IgD expression in the spleen of a chimera constructed with 105 IgD-depleted PEC. (C) IgM and IgD expression in the spleen of a chimera constructed with 107 peripheral lymph-node (LN) cells. (B and C) The chimeras were also immunized with NP-Ficoll on days 14 and 42 after cell transfer and analyzed on day 48; The arrow points to clustered IgM+ plasmacytoid cells in the red pulp. T, T zone.
Analysis of the B cell compartments in these chimeras constructed with 105 PEC depleted of IgD+ B cells shows that they have small numbers of IgD− IgM+ B cells in the follicles (Fig. 3B and see Table 1). IgD+ cells were not found in their follicles, and this accords with flow cytometry of these spleens, which revealed the presence of IgM+- but not IgD-expressing B cells (Fig. 3B and see Table 1). Despite the presence of IgD− IgM+ B cells in follicles, no B cells were found in the marginal zone. This finding contrasts markedly with chimeras constructed by transfer of 107 peripheral lymph-node cells. These chimeras have follicles with many IgD+ B cells and well developed IgDlow/−, IgM+ marginal zone B cell populations (Fig. 3C). The absence of IgD+ B cells or B cells in the marginal zone of the chimeras constructed with IgD− IgM+ PEC indicates that hemopoietic chimerism leading to primary B lymphopoiesis had not been established.
Analysis of the spleens from the other groups of chimeras shows that all of the chimeras that produced an Ab response to NP-Ficoll had NP-specific plasmacytoid cells in their spleen (Fig. 3A and see Table 1). Many of these cells had switched to produce IgG3. These chimeras also had NP-specific B cells in their splenic follicles. On the basis of flow cytometry, these cells were mainly IgM+ IgD−. Although NP-binding cells from chimeras constructed with IgD-depleted PEC or CD5-depleted PEC did not express surface IgD, a significant minority of the NP-binding B cells from chimeras constructed by transfer of 107 lymph-node cells or 106 PEC expressed IgD (Table 1).
Taken together, these transfer experiments indicate that B1b cells can give rise to persistent extrafollicular responses to NP-Ficoll. At this stage, the participation of B1a cells, i.e., those B1 cells that express CD5 are not excluded. Recirculating IgDhigh B cells seem unlikely to contribute to these long-term responses, although as mentioned above, large numbers of recirculating cells will reconstitute a marginal zone population, which gives a response to NP-Ficoll (17); however, this response is likely to be short-term.
Discussion
Ab responses to NP-Ficoll are associated with plasmablasts that persist for months in extrafollicular foci (20). This finding differs strikingly from conventional TD extrafollicular Ab responses in which plasmablasts are only present for a few days (19). The chimeras constructed with small numbers of IgD− PEC lacked B2 cells, indicating that primary B lymphopoietic capacity had not been established. Consequently, the persistence of NP-specific plasmablasts after immunization could not have been attributable to continued recruitment of naïve B cells. At this stage, it is not possible to exclude whether plasmablast persistence is attributable to long-term self-renewal. An alternative possibility is that the NP-specific B cells that accumulate in follicles in these responses are involved in maintaining the pool of plasmablasts. These two possibilities are not mutually exclusive. The location of these NP-specific B cells distinguishes them from conventional memory B cells generated in response to NP-protein conjugates that typically localize to the marginal zone (29) and to the blood and bone marrow (30). Follicular localization opens the possibility that these TI-2 antigen-induced B cells might be recruited back into the response by NP-Ficoll held as immune complex on follicular dendritic cells (20).
Recently, Haas et al. (31) have proposed distinct roles for B1a and B1b cells on the basis of different selection requirements for these two populations during ontogeny. They found that mice overexpressing CD19 produced B1a cells with few B1b cells and noted that these mice had high levels of natural Ab. By contrast, CD19-deficient mice were found to lack B1a cells and natural Ab but had B1b cells and mounted Ab responses against the TI-2 antigen type-3 pneumococcal capsular polysaccharide. In our studies, the excellent response to NP-Ficoll by chimeras reconstituted with 105 CD5− peritoneal exudate B cells is consistent with this conclusion. Nevertheless, these chimeras did produce as much background (natural) anti-NP IgM as chimeras reconstituted with whole-PEC preparations. It may be that very small numbers of contaminating CD5+ B cells were able to expand to produce normal levels of Ab in these chimeras. An alternative possible explanation is that WT B1b cells did produce natural Ab but that this function was impaired if the B1b cells lacked CD19.
The B1 cell clones involved in long-term Ab responses might give rise to pathology. For example, the protracted antigen-driven TI Ab responses may provide the cellular basis for auto-Ab production in diseases such as Guillain–Barré syndrome. Nonmutated IgM and IgG Abs (IgM and IgG3 in mice) against a glycolipid motif found on Campylobacter jejuni, which crossreacts with neural gangliosides, mediate this relatively common self-limiting paralytic disease (32). These Abs persist for longer than would be expected from a short-lived TI-extrafollicular Ab response. Another situation in which these responses by B1b cells might give rise to disease lies in the continued production of plasmablasts by clones responding NP-Ficoll or similar persistent antigens. This sort of response presents a potential danger for the development of B cell neoplasms, which are associated with serial acquisition of genetic changes. This theory is well exemplified in the plasmacytomas of BALB/c mice, which only develop after protracted exposure to mineral oil given into the peritoneal cavity (33). The role of antigen in the development of these plasmacytomas can be inferred from the relative protection of mice rendered pathogen-free from plasmcytoma development (34). These neoplasms often show signs of receptor editing but only approximately half show Ig V-region gene mutations (35). At least those neoplasms without these mutations might have been generated during Ab responses not involving a germinal center reaction. The peritoneal location of these plasmacytomas has led to the suggestion that these neoplasms may arise from B1 cells. The continued production of plasmablasts by B1b cell clones described in the present article appears to provide a situation in which plasmablasts could acquire the sequential genetic changes associated with plasmacytoma development.
Materials and Methods
Animals and Immunizations.
RAG1−/− mice 6 to 8 weeks of age on a C57BL/6 background were obtained from the Biomedical Services Unit (University of Birmingham) and were kept under specific pathogen-free conditions at all times. Rag1−/− mice were reconstituted, as indicated in the text, with congenic WT cells from C57BL/6 mice (Harlan Olac, Bicester, U.K.) by i.v. injection; 2 weeks later the chimeras received their first dose of NP-Ficoll (Biosearch Technologies, Novato, CA; 30 μg in sterile PBS) i.p. They were challenged again with NP-Ficoll at times specified in the text. All experiments involving animals were carried out under license from the British Home Office, which was granted after local animal ethics committee approval.
Cell Purification and Adoptive Transfer.
Total PECs were obtained from washes of the peritoneal cavity with cold RPMI medium 1640. Cells were stained with IgD and IgM fluorochrome-conjugated Ab and positively selected for IgDhigh IgMvariable and IgDlow/− IgMhigh with a MoFlow flow cytometric sorter (DAKO). CD5− cells were negatively selected from total PEC suspension by magnetic bead separation (Miltenyi Biotec, Bisley, U.K.).
Flow Cytometry Reagents.
After isolation, single-cell suspensions from donor mice or chimeras were stained with the following Ab conjugates: B220-biotin, CD11b-FITC, CD138-phycoerythrin, CD5-biotin, IgD-phycoerythrin, IgM-FITC (all substances were obtained from BD Biosciences, San Diego, CA). The monoclonal Ab GL-7, which binds to a molecule expressed by germinal center B cells (36), was a kind gift of G. Kelsoe (Duke University, Durham, NC) and was used as a biotin conjugate. Biotin was detected by using streptavidin-CyChrome (BD Biosciences). NP-binding cells were identified by using a locally prepared NP-phycoerythrin conjugate. The cells were analyzed by using a FACScalibur flow cytometer (BD Biosciences).
ELISA.
For testing NP-specific Abs, NP15-BSA-coated 96-well plates were used. The serum Abs from chimeras were detected by alkaline phosphatase-conjugated goat anti-mouse IgM or IgG3 (Southern Biotech, Birmingham, AL), and the enzyme bound to plates was developed with p-nitrophenylphosphate substrate (Sigma–Aldrich). For standardization, a positive control was run along with test samples in all plates. The titers for serum samples were calculated as the log serum concentration required to achieve 30% maximum OD.
Immunohistochemistry Staining.
This process was carried out as described in refs. 20 and 37. Briefly, 5-μm, acetone-fixed frozen sections were stained for IgD, mucosal addressin cell-adhesion molecule (38), NP-binding, and expression of the proliferation-associated nuclear antigen Ki-67 (39). NP-binding cells were detected by using NP-conjugated sheep IgG and biotinylated donkey anti-sheep secondary Ab. The detection of NP-specific cells was revealed in a streptavidin-alkaline phosphatase system. Mucosal addressin cell-adhesion molecule was also stained by using streptavidin-alkaline phosphatase. IgD and Ki-67 were detected by using immunoperoxidase. The stained sections were viewed under ×20 objective, and the number of positively stained cells per mm2 of section was determined by using a 10-mm graticule.
Supplementary Material
Acknowledgments
This work was funded by grants from the British Medical Research Council.
Abbreviations
- TI
thymus-independent
- TD
thymus-dependent
- NP
(4-hydroxy-3-nitrophenyl)-acetyl
- RAG-1
recombinase-activating gene 1
- PEC
peritoneal exudate cell.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. J. Exp. Med. 1972;136:931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mond J. J., Scher I., Mosier D. E., Baese M., Paul W. E. Eur. J. Immunol. 1978;8:459–463. doi: 10.1002/eji.1830080703. [DOI] [PubMed] [Google Scholar]
- 3.Hazlewood M., Nusrat R., Kumararatne D. S., Goodall M., Raykundalia C., Wang D. G., Joyce H. J., Milford-Ward A., Forte M., Pahor A. Clin. Exp. Immunol. 1993;93:157–164. doi: 10.1111/j.1365-2249.1993.tb07959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler J. C., Breiman R. F., Lipman H. B., Hofmann J., Facklam R. R. J. Infect. Dis. 1995;171:885–889. doi: 10.1093/infdis/171.4.885. [DOI] [PubMed] [Google Scholar]
- 5.Kaczmarski E. B. Commun. Dis. Rep. CDR Rev. 1997;7:R55–R59. [PubMed] [Google Scholar]
- 6.Lane P. J., Gray D., Oldfield S., MacLennan I. C. Eur. J. Immunol. 1986;16:1569–1575. doi: 10.1002/eji.1830161216. [DOI] [PubMed] [Google Scholar]
- 7.Martin F., Oliver A. M., Kearney J. F. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 8.Vinuesa C. G., Sunners Y., Pongracz J., Ball J., Toellner K.-M., Taylor D., MacLennan I. C., Cook M. C. Eur. J. Immunol. 2001;31:1340–1350. doi: 10.1002/1521-4141(200105)31:5<1340::AID-IMMU1340>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 9.Coico R. F., Bhogal B. S., Thorbecke G. J. J. Immunol. 1983;131:2254–2257. [PubMed] [Google Scholar]
- 10.Shih T. A., Meffre E., Roederer M., Nussenzweig M. C. Nat. Immunol. 2002;3:570–575. doi: 10.1038/ni803. [DOI] [PubMed] [Google Scholar]
- 11.Vinuesa C. G., Cook M. C., Ball J., Drew M., Sunners Y., Cascalho M., Wabl M., Klaus G. G., MacLennan I. C. J. Exp. Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amlot P. L., Grennan D., Humphrey J. H. Eur. J. Immunol. 1985;15:508–512. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura H., Minato N., Nakano T., Honjo T. Int. Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi K., Melchers F., Shimizu T. Eur. J. Immunol. 2005;35:957–963. doi: 10.1002/eji.200425853. [DOI] [PubMed] [Google Scholar]
- 15.Sato S., Steeber D. A., Tedder T. F. Proc. Natl. Acad. Sci. USA. 1995;92:11558–11562. doi: 10.1073/pnas.92.25.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsitsikov E. N., Gutierrez-Ramos J. C., Geha R. S. Proc. Natl. Acad. Sci. USA. 1997;94:10844–10849. doi: 10.1073/pnas.94.20.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinuesa C. G., Sze D. M.-Y., Cook M. C., Toellner K.-M., Klaus G. G. B., Ball J., MacLennan I. C. M. Eur. J. Immunol. 2003;32:297–305. doi: 10.1002/immu.200310003. [DOI] [PubMed] [Google Scholar]
- 18.Guinamard R., Okigaki M., Schlessinger J., Ravetch J. V. Nat. Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 19.Sze D. M., Toellner K.-M., Garcia de Vinuesa C., Taylor D. R., MacLennan I. C. J. Exp. Med. 2000;192:813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinuesa C. G., O’Leary P., Sze D. M., Toellner K.-M., MacLennan I. C. M. Eur. J. Immunol. 1999;29:1314–1323. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Humphrey J. H. Immunol. Lett. 1985;11:149–152. doi: 10.1016/0165-2478(85)90161-0. [DOI] [PubMed] [Google Scholar]
- 22.Taccioli G. E., Rathbun G., Oltz E., Stamato T., Jeggo P. A., Alt F. W. Science. 1993;260:207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- 23.Herzenberg L. A. Immunol. Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- 24.Alugupalli K. R., Leong J. M., Woodland R. T., Muramatsu M., Honjo T., Gerstein R. M. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Dammers P. M., de Boer N. K., Deenen G. J., Nieuwenhuis P., Kroese F. G. Eur. J. Immunol. 1999;29:1522–1531. doi: 10.1002/(SICI)1521-4141(199905)29:05<1522::AID-IMMU1522>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Kumararatne D. S., MacLennan I. C. Eur. J. Immunol. 1981;11:865–869. doi: 10.1002/eji.1830111104. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava B., Quinn W. J., III, Hazard K., Erikson J., Allman D. J. Exp. Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balazs M., Martin F., Zhou T., Kearney J. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y. J., Zhang J., Lane P. J., Chan E. Y., MacLennan I. C. Eur. J. Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 30.McHeyzer-Williams L. J., McHeyzer-Williams M. G. Annu. Rev. Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 31.Haas K. M., Poe J. C., Steeber D. A., Tedder T. F. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Boffey J., Nicholl D., Wagner E. R., Townson K., Goodyear C., Furukawa K., Conner J., Willison H. J. J. Neuroimmunol. 2004;152:98–111. doi: 10.1016/j.jneuroim.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Potter M., Wiener F. Carcinogenesis. 1992;13:1681–1697. doi: 10.1093/carcin/13.10.1681. [DOI] [PubMed] [Google Scholar]
- 34.Byrd L. G., McDonald A. H., Gold L. G., Potter M. J. Immunol. 1991;147:3632–3637. [PubMed] [Google Scholar]
- 35.Diaw L., Siwarski D., Coleman A., Kim J., Jones G. M., Dighiero G., Huppi K. J. Exp. Med. 1999;190:1405–1416. doi: 10.1084/jem.190.10.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi Y., Dutta P. R., Cerasoli D. M., Kelsoe G. J. Exp. Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunningham A. F., Serre K., Toellner K.-M., Khan M., Alexander J., Brombacher F., MacLennan I. C. Eur. J. Immunol. 2004;34:686–694. doi: 10.1002/eji.200324510. [DOI] [PubMed] [Google Scholar]
- 38.Briskin M. J., McEvoy L. M., Butcher E. C. Nature. 1993;363:461–464. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- 39.Gerdes J., Dallenbach F., Lennert K., Lemke H., Stein H. Hematol. Oncol. 1984;2:365–371. doi: 10.1002/hon.2900020406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.