Abstract
In contrast to commonly used serotype 5 based adenovirus (Ad) vectors, Ads containing fibers derived from B-group serotype 35 (Ad5/35) efficiently transduce human DCs ex vivo and appear to target antigen-presenting cells after intravenous injection into baboons. Based on this, Ad5/35 vectors could be valuable tools for immunotherapy and vaccination. On the other hand, a number of studies indicate that signaling through the B-group Ad receptor, CD46, can cause tolerance or immuno-suppression. Since mice do not express CD46 in a human-like pattern, we studied the in vivo properties of Ad5/35 in transgenic mice that express CD46 in a pattern and at a level similar to humans. Hypersensitivity assays and analyses of frequencies of regulatory T-cells and T-cell responses did not indicate that Ad5/35 injection exerts detrimental effects on the host's immune system. An Ad5/35 vector expressing a model antigen was able to trigger a strong T-cell response against the test antigen after intramuscular injection. Overall, compared to Ad5 vectors, Ad5/35 vectors had a better safety profile, reflected by lower serum levels of pro-inflammatory cytokines.
Introduction
Adenovirus (Ad) vectors have been used for vaccination in pre-clinical models (for a review see: [1]) and, recently also in humans [2]. Ad based vaccines have a number of advantages over naked DNA vaccines or vaccines based on other viruses such as poxvirus or alphavirus. These advantages include the ability to easily prepare high-titer stocks of purified virus and the remarkable efficiency of each step in the Ad cell/nucleus entry process leading to high-level transgene expression. Furthermore, it is thought that Ads can provide an adjuvant effect in stimulation of antigen-specific immune responses. Interaction of Ad capsids with cellular receptors induces expression of pro-inflammatory cytokines/chemokines, such as TNFα, IFNγ, IL-1, IL-6, IL-12, MCP-1 and 2, which results in recruitment of effector cells of the innate and adaptive immune system to the site of infection [3]. These cytokines also activate functions of antigen-presenting cells (APCs). Furthermore, presentation of Ad proteins from the incoming Ad particle and/or de novo expression of Ad proteins in APC could help in activation of T-cells specific to the vaccination antigen.
A critical factor in each vaccination strategy is the efficient uptake of vaccination vectors and antigen expression in professional APCs, such as dendritic cells (DC). DC are located strategically at the interface of potential pathogen entry sites. They capture antigens and migrate into secondary lymphoid tissues, where they activate both helper T-cells and cytotoxic T-lymphocytes. They also interact with B-cells and probably NK cells. Current vectors for gene transfer into human DCs, including the commonly used serotype 5 Ad vectors, are inefficient or cytotoxic. The poor transduction of human DCs with Ad5-based vectors is due to low-level expression of CAR [4]. In contrast, earlier studies have shown that Ad vectors containing B-group Ad serotype 35 fibers (Ad5/35) efficiently transduce human DCs ex vivo [4] and appear to target APC after intravenous injection into CD46-transgenic mice and baboons [5]. Based on this, Ad5/35 vectors could be valuable tools for immunotherapy and vaccination.
Vectors containing B-group Ad fibers, including Ad5/35 vectors, use CD46 for initial cellular attachment [6]. CD46 is a membrane glycoprotein that protects cells from complement damage. There are four major isoforms of CD46 (BC1, BC2, C1 and C2), depending on the alternative splicing of a region encoding an extracellular domain and the choice between one or two cytoplasmic tails, Cyt-1 and Cyt2. CD46 is also a receptor for measles virus laboratory strains, for human herpes virus 6, and for certain pathogenic bacteria [7]. In humans, CD46 is expressed on all nucleated cells at a low level. Although Ad5/35 vectors efficiently transduce DC in vitro and potentially in vivo, a number of findings argue against the utility of these vectors for in vivo vaccination. i) CD46 signaling (upon binding of CD46-monoclonal antibodies, recombinant complement factor C3b, or measles virus hemagglutinin) can induce immunosuppression depending on the nature of its cytoplasmic tail, with Cyt-1 (C1 isoform) engagement suppressing inflammation, and Cyt-2 (C2 isoform) increasing it [8]. ii) Studies of an Ad35 outbreak leading to pneumonia and sepsis revealed a transient neutropenia, which could have been directly caused by Ad35 infection [9]. iii) Measles virus and HHV6, which also use CD46 as a receptor, can cause transient immunosupression at the level of dendritic cell precursors, bone marrow stromal cells or CD34+ myeloid progenitors [10].
The majority of published in vivo studies with B-group fiber containing Ad vectors have been done in mice [11-16]. Since mice do not express CD46 in a human-like pattern [17], these studies might not be predictive for Ad5/35 behavior in humans. We therefore studied the in vivo properties of Ad5/35 in transgenic mice that express CD46 in a pattern and at a level similar to humans. This strain of CD46 transgenic mice has been extensively used in studies with measles virus (which also uses CD46 as receptor) and in studies on the role of CD46 activation on immune responses [18, 19]. Specifically, we evaluated the effect of Ad5/35 on the host's immune system and the ability of an Ad5/35 vector to induce immune responses against a model antigen.
Results and Discussion
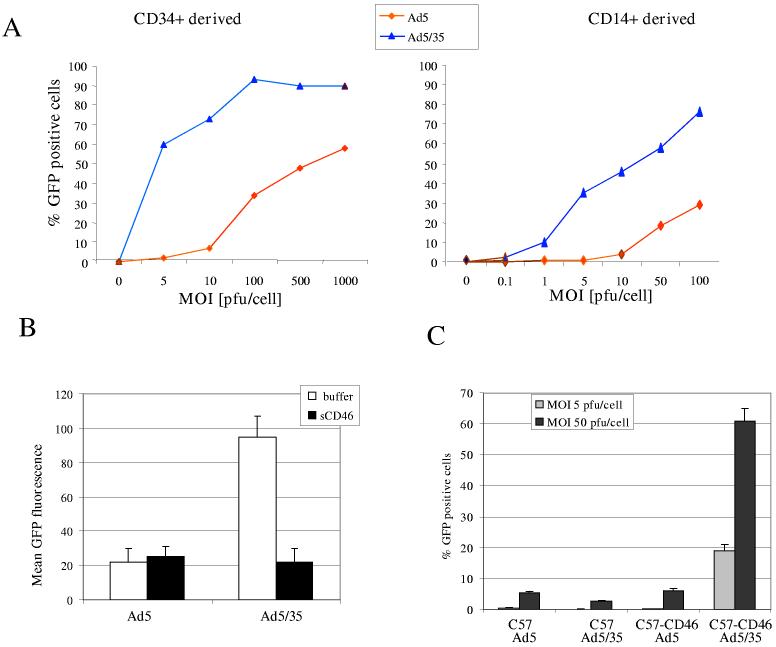
Ad5/35 transduction of dendritic cells in vitro. To corroborate the use of Ad5/35 for immunotherapy, we performed transduction studies with human DC that were generated by in vitro differentiation of CD34+ cells or CD14+ monocytes. We showed that immature human DC can be efficiently transduced at low MOIs with Ad5/35 vectors (Fig.1A). Ad5/35 transduction did not affect the expression of maturation markers (data not shown). Ad5/35 infection of CD34+ derived DCs could be efficiently blocked by soluble CD46, indicating that CD46 can mediate DC transduction (Fig.1B). We also performed transduction studies with bone marrow derived DC from C57 and C57-CD46 mice (Fig.1C). While transduction of DC with the Ad5 vector was inefficient in both wild-type and transgenic strains, Ad5/35 efficiently transduced DC from C57-CD46 but not C57 mice.
Figure 1.
Assessment of Ad5/35 vectors for transduction of dendritic cells. A) Transduction of human dendritic cells (DC) with first-generation Ad5 and Ad5/35 vectors containing a CMV-GFP expression cassette. Immature human DCs (>95% positive for CD11c) derived either from CD34+ cells (left panel) or peripheral CD14+ monocytes (right panel) were infected at different MOIs for 3 hours. GFP expression was analyzed by flow cytometry 24 hours post-infection. B) Transduction of DCs in the presence of soluble CD46. CD34+ derived DCs were transduced with Ad5-GFP or Ad5/35-GFP at an MOI of 10pfu/cell in the presence of an excess of soluble CD46 (as a competitor) and GFP expression was analyzed 24 hours later by flow cytometry. C) Transduction of DC from CD46 transgenic mice with Ad vectors. Myeloid DC from C57 and C57-CD46 mice were infected with first-generation Ad5 and Ad5/35 vectors at an MOI of 5 and 50 pfu/cell. The percentage of GFP expressing cells was analyzed 24 hours after infection by flow cytometry.
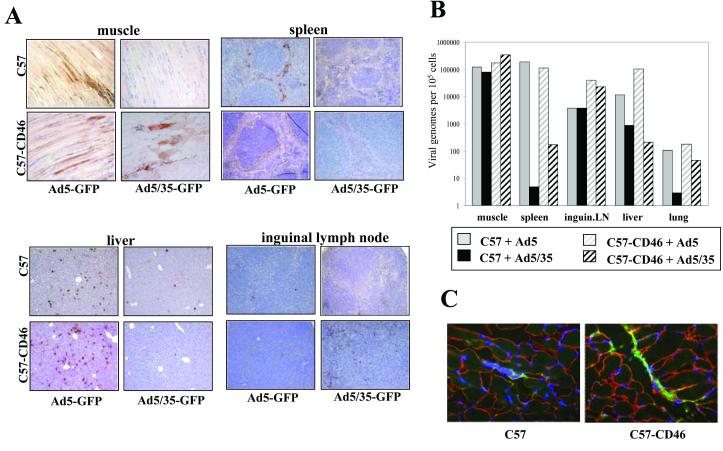
Ad5/35 vector biodistribution after intramuscular (IM) injection. To study the impact of CD46 signaling on the host's immune response to Ad5/35 or to an antigen expressed from Ad5/35 vectors, we performed the following studies in both C57Bl/6 and C57Bl/6-CD46 mice and also included Ad5 vectors (that do not interact with CD46) as controls. As a standard route for vaccination, we initially applied Ad5/35 vectors intramuscularly (IM). Biodistribution of transgene expression was assessed on organ sections 3 days after IM vector injection (Fig.2A). While Ad5.GFP efficiently transduced myocytes around the injection site in C57 and C57-CD46 mice, Ad5/35 transduced muscle cells in C57-CD46 mice but not in C57 mice. As expected Ad5 transduction was found in livers and spleens of both C57 and C57-CD46 mice at higher levels than Ad5/35 transduction. Interestingly, transduction of splenic and hepatic cells of C57-CD46 mice with Ad5/35 was slightly less efficient than in C57 mice. Furthermore, Ad5/35 appeared to transduce cells in the inguineal lymph nodes (adjacent to the injection site) in C57-CD46 mice. Ad5/35 transduction of mesenteric and lumbar lymph node cells was not detectable (data not shown). Overall, the amounts of viral genomes present in analyzed organs correlated with the observed transduction efficiency based on β-galactosidase expression (Fig.2B). Although no significant Ad5/35-mediated bGal expression was seen in muscles of C57 mice, Ad5/35 genomes were detected. The observation that Ad5/35 transduction of liver, lung and spleen was less efficient in C57-CD46 mice than C57 mice was corroborated by the genome distribution data. We speculate that a large portion of IM injected Ad5/35 particles is taken up or sequestered by CD46 transgenic muscle cells, while in C57 mice (without apparent Ad5/35 muscle transduction), more Ad5/35 particles leak into the circulation. This speculation is supported by the finding that more Ad5/35 viral particles can be found in CD46 transgenic muscles around the injection site than in muscles from non-transgenic mice (Fig.2C). Clearly, questions such as whether the interaction between Ad5/35 and CD46 on tissues changes the kinetics of viral clearance from the blood circulation or influences viral degradation have to be further studied. Importantly, upon IM injection into C57-CD46 mice, Ad5/35 transduced myocytes and lymph nodes, which potentially allows for efficient antigen presentation.
Figure 2.
Biodistribution of Ad mediated transgene expression and vector genomes in C57 and C57-CD46 mice after intramuscular injection of Ad5.GFP and Ad5/35.GFP. A) GFP expression in organ sections 72 hours after intramuscular injection of 5×109 pfu of Ad5.GFP and Ad5/35.GFP. Tissue sections were analyzed for GFP expression by immunohistochemistry with anti-GFP antibodies. B) Quantitative comparison of viral genomes present in major organs at 72 hours after intramuscular Ad injection. The genome concentration was expressed as the number of viral genomes per 107 cells (assuming that the mass of a diploid human genome is 6pg). qPCR results for vector genomes were equalized based on qPCR data for an endogenous (two copies/genome) mouse GAPDH gene. The average of 3 independent tissue samples is shown. Standard deviation was less than 10%C) Detection of Ad5/35 particles around the injection site. Mice were injected IM with 5×109pfu Ad5/35.GFP in 40μl of PBS. One hour after injection, mice were sacrificed. Sections (8μm) of muscle tissue around the injection site were analyzed by immunofluorescence. Viral particles were detected using anti-Ad5 hexon-FITC antibodies (green). Muscle cells were stained with anti-laminin antibodies (red). Cell nuclei are blue.
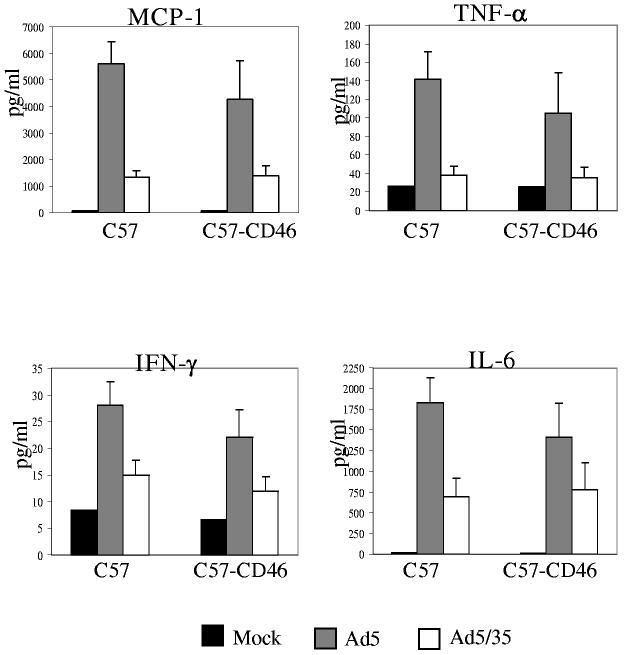
Innate immune responses after IM Ad injection. In vivo application of Ad5 vectors results in the release and expression of pro-inflammatory cytokines, which in turn can cause hepatotoxicity, increased vascular permeability, leukocyte infiltration into sites of infection, and other symptoms of acute inflammation. On the other hand, increased levels of pro-inflammatory cytokines and chemokines are thought to contribute to the so-called “adjuvant” effect of Ad vectors in vaccination studies [20]. To assess the properties of Ad5/35 as a vaccination vector, we measured serum pro-inflammatory cytokine and chemokine levels upon IM injection of Ad vectors (Fig.3). In preliminary studies, we found that peak levels occurred at 6 hours post infusion (data not shown), in accordance with levels seen after IV injection into mice or baboons [5, 21]. Compared to animals injected with an Ad5 vector, IM injection of Ad5/35 vectors into C57 and C57-CD46 mice caused less elevation of pro-inflammatory cytokines (IL-6, TNFα, IFN-γ) and chemokines (MCP-1). For Ad5/35 injected animals, there was no significant difference between the levels in C57 and C57-CD46 mice, indicating that CD46 is not involved in Ad uptake into cytokine producing cells. This is in agreement with earlier studies where we found that Ad uptake into Kupffer cells is mediated by blood factors and that the latter process is inefficient for Ad vectors containing short-fiber shafts, such as Ad5/35 [21, 22].
Figure 3.
Innate immune responses upon IM injection of Ad5/35 into mice. Plasma levels of MCP-1, TNF-α, IFN-γ, and IL-6 at 6 hours after IM injection of Ad vectors. Mice were injected with PBS (Mock) or with 5×109 pfu of Ad5.GFP or Ad5/35.GFP. Plasma samples from three individual mice per virus were collected and analyzed in duplicate by cytometric bead array for cytokine and chemokine levels.
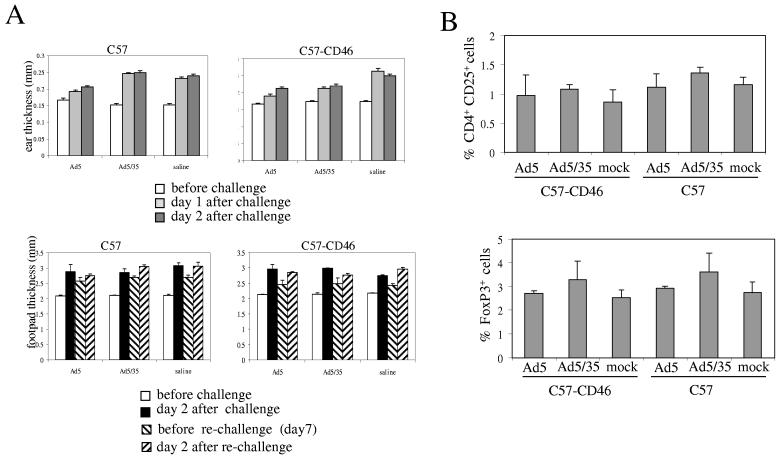
Assessment of the immune status after Ad5/35 injection. In an attempt to assess the host's immune response to Ad5/35 vectors, we studied two types of T cell–dependent inflammatory reaction: delayed type hypersensitivity (DTH) to keyhole limpet hemocyanin (KLH) mediated by CD4+ T cells, and contact hypersensitivity (CHS) induced by epicutaneous exposure to the hapten dinitrofluorobenzene (DNFB) and mediated by CD8+ T cells. Six hours after Ad5 and Ad5/35 injection into C57 and C57-CD46 mice, animals were injected with DNFB or KLH. Ear and foot pad thickness was measured upon re-challenge with the hapten (Fig.4A). After re-challenge, no Ad5/35 mediated inhibition of CHS and DTH responses were seen.
Figure 4.
Absence of immunosuppression upon Ad5/35 infectionA) CD4- and CD8 mediated hypersensitivity reactions. C57 or CD57-CD46 mice were injected intramuscularly with saline, 5×109pfu of Ad5.GFP or Ad5/35.GFP. Upper panel: Contact hypersensitivity: Six hours after virus injection mice were sensitized with dinitrofluorobenzene. Six day later, mice were challenged and the ear thickness was measured before challenge and 24 and 48 hours after challenge. Lower Panel: Delayed type hypersensitivity: Six hours after virus injection, mice were sensitized with KLH. Six day later, mice were challenged and the food pad thickness was measured before challenge and 48 hours after challenge. To assess potential long-term effects of Ad injection, mice were re-challenged with KLH and foot pad thickness was measured before and after re-challenging. (N=3)B) Analysis of regulatory T-cells. C57 or CD57-CD46 mice were injected with 5×109pfu of Ad5.GFP or Ad5/35.GFP. Seven days later splenocytes and lymph nodes were harvested and analyzed by flow cytometry for the percentage of CD4+CD25+ and FoxP3+ cells (N=3).
Kemper et al reported that co-engagement of CD3 and CD46 in the presence of IL-2 can induce regulatory T-cells (Tregs), which potentially can suppress adaptive immune responses or induce tolerance [23]. Human and murine Tregs are CD4+CD25+ and express a number of other markers including Forkhead P3 (FoxP3). We therefore analyzed whether Ad5/35 interaction with CD46 can induce Tregs in mice. C57 and C57-CD46 mice were injected with 5×109 pfu of Ad5.GFP or Ad5/35.GFP and seven days later cell suspensions from spleen and draining lymph nodes were analyzed by flow cytometry for the presence of CD4+CD25+ and FoxP3+ cells (Fig. 4B). There were no significant differences between the experimental groups, indicating that Ad5/35 does not increase the frequency of Tregs in CD46 transgenic mice.
T-cell response against a model antigen and Ad5/35 vector particles. To study the ability of Ad5/35 vectors to induce an immune response against a test antigen, we constructed Ad5 and Ad5/35 vectors that expressed HBeAg under the control of the CMV promoter (which is known to be active in DCs). HBeAg is the secreted form of the Hepatitis B core antigen and is considered to be a target for immunotherapy of HBV associated hepatocarcinoma [24].
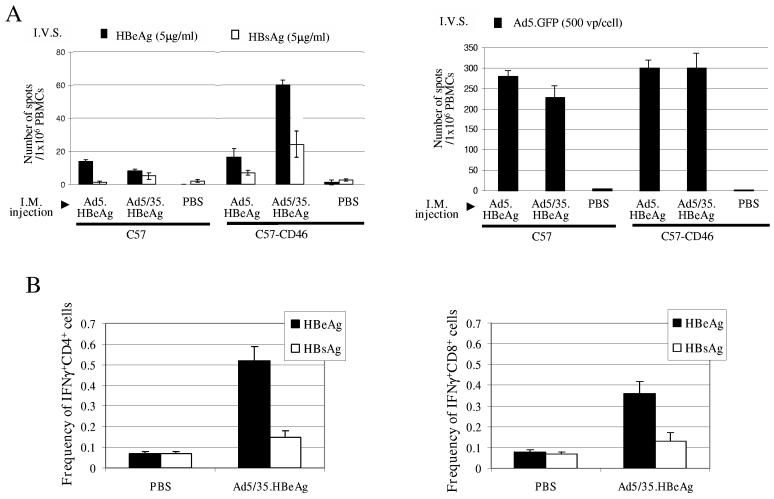
HBeAg expression upon in vitro infection of DCs from C57-CD46 mice was confirmed by Immunoprecipitation/Western blot analysis (data not shown). C57 and C57-CD46 mice were mock-injected or IM injected with 5×109 pfu of Ad5.HBeAg or Ad5/35.HBeAg. Three weeks later, splenocytes were isolated and the frequency of IFNγ producing T-cells was analyzed by ELISpot assay upon in vitro sensitization with HBeAg and, as a negative control, HBsAg (Fig.5A). Injection of Ad5/35.HBeAg into C57-CD46 mice resulted in a significantly higher frequency of HBeAg specific T-cells, compared to all other groups (60 vs <15spots per 106 PBMC, p<0.05). We also determined the frequency of anti-Ad5 specific T-cells in splenocytes of test mice (Fig.5A, right panel). For in vitro sensitization we infected splenocytes with Ad5.GFP at an MOI of 500vp/cell. All Ad injected animals, regardless of the Ad vector or genetic background, demonstrated a high frequency of anti-Ad T-cells, which was 5 to 10 fold higher than the frequency of HBeAg specific T-cells. Furthermore, we used intracellular cytokine (IFNγ) staining to assess whether anti-HBeAg T-cell responses observed in ELIspot assays were CD4+ or CD8+ mediated. Figure 5B shows that Ad5/35.HBeAg injection into C57-CD46 mice induces both antigen specific CD4 and CD8 responses.
Figure 5.
Analysis of frequencies of IFNγ-producing T-cells specific to a model antigen or adenovirus vector.A) ELIsport analyses of IFNγ-producing T-cells frequencies. C57 or C57-CD46 mice were injected intramuscularly with 5×109 pfu of Ad5.HBeAg, Ad5/35.HBeAg, or PBS. Twelve days later, spleen cells of naïve syngeneic animals were obtained and pulsed with 5μg/ml of recombinant HBeAg and HBsAg (as a negative control) (left panel) or transduced with 25pfu (=500 viral particles) of Ad.5GFP (right panel). On day 14, vaccinated animals were sacrificed, splenocytes were collected and 1×106 cells were mixed with 1×106 ex vivo pulsed splenocytes for in vitro sensitization. After 24 hours of incubation in 96 well plates, cells were plated in anti-IFNγ-coated wells of an ELIspot plate. Twenty-four hours later, plates were washed and the spots of IFNγ producing T-cells were counted. The number of spots was expressed as the average +/− the standard deviation. N=3 animals per group.B) Frequency of IFNγ+ CD4+ cells (left panel) and IFNγ+ CD8+ cells (right panel) analyzed by intracellular cytokine staining. C57-CD46 mice were injected intramuscularly with 5×109 pfu of Ad5/35.HBeAg, or PBS. Twelve days after immunization, splenocytes were harvested and cultured with recombinant HBeAg and (control) HBsAg for 5 hours in the presence of Brefeldin. Cells were then stained for CD4 and CD8 and intracellular IFNγ. Shown is the percentage of IFNγ+CD4+ in all CD4+ cells and IFNγ+CD8+ in all CD8+ cells. (N=3)
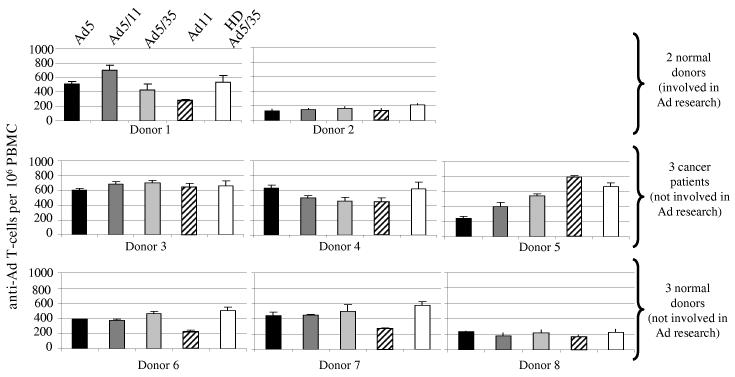
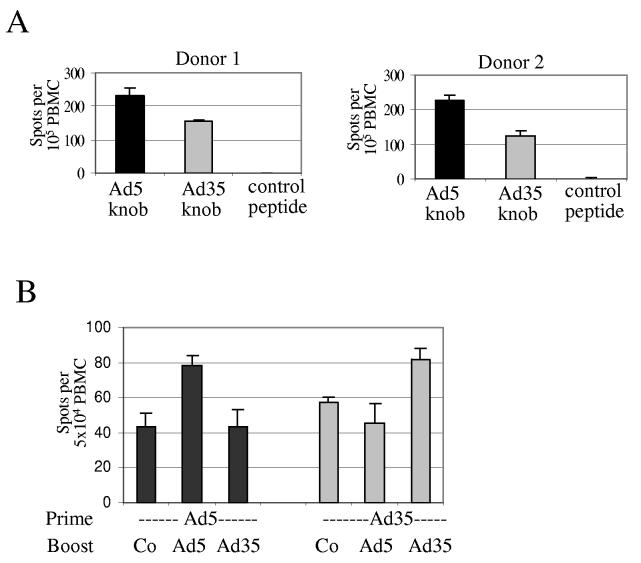
Memory anti-Ad T-cell responses in humans. A potential problem for the application of Ad vectors (including Ad5/35) for induction of T-cell responses, particularly against relatively weak antigens such as tumor-associated antigens, is the high immunogenicty of Ad vectors resulting in induction of anti-Ad T-cells at a frequency that potentially might affect the T-cell response to the vaccination antigen. The latter may be even more problematic in humans as a result of pre-existing anti-Ad T memory responses [25]. To illustrate this, we analyzed the frequencies of IFNγ producing Ad-specific T-cells in human PBMC from 8 donors (Fig.6). In this study, we compared E1/E3-deleted Ad5/35 vectors (which are known to express viral proteins in transduced cells) with helper-dependent Ad5/35 vectors (which are devoid of all viral genes). Since most T-cells are thought to be directed against immunodominant Ad5 hexon epitopes [25], Ad5/35 vectors (containing Ad5 hexons) will probably induce the same T-cell responses. In our study, we found strong T-cell responses in all samples upon infection of human PBMC with Ad5 and Ad5/35 vectors. The responses to the first-generation Ad5/35 vectors were comparable to those induced by the HD-Ad5/35 vector. The latter indicates a response to Ad capsid epitopes from the incoming particles. Frequencies of anti-Ad11 IFNγ producing T-cells were comparable to those seen for the other vectors. Previous studies reported memory T-cell responses against Ad hexon protein [25-28]. Using a similar experimental approach as described for Fig. 6 and recombinant Ad5 and Ad35 fiber knob domains for pulsing of PBMC, we found memory T-cell also against the fiber knob domains, whereby the frequency of anti-Ad35 knob T-cell was significantly less than that of Ad5 knob specific T-cells (Fig7A). Another experiment with recombinant fiber knobs suggested that Ad5 and Ad35 knob specific T-cells did not cross-react (Fig.7B). This was not surprising considering that the homology between Ad5 and Ad 35 fiber knobs is less than 30% (on the amino acid level).
Figure 6.
Analysis of memory T-cells against Ad vectors in human PBMCs by ELIspot for IFNγ expressing T-cells.PBMCs were obtained from normal donors that have never done research with Ad vectors, from normal donors that worked with Ad vectors, and from Senegalese, stage III breast cancer patients (before chemotherapy). Cells were transduced with the indicated Ad vectors at an MOI of 200 viral particles/cell and the number of IFNγ expressing T-cells was analyzed by ELIspot 24 hours later. N=3
Figure 7.
Cross-reactivity of anti-fiber knob T-cells.A) Memory T-cells against fiber knob domains. PBMC from two normal donors that are involved in adenovirus research were incubated with 0.2μg/ml of recombinant Ad5 or Ad35 fiber knob domains (produced in E.coli) or control peptide (HBeAg) and the frequency of IFNγ expressing T-cells was analyzed by ELIspot 24 hours later. (N=3) B) Crossreactivity of T-cells against fiber knobs. T-cells were primed by incubating PBMC from a healthy donor with recombinant Ad5 or Ad35 fiber knob. Subsequently T-cells were incubated for 7 days in the presence of IL-2 and then boosted with dendritic cells pulsed with control peptide (Co), Ad5, or Ad35 fiber knob. The frequency of IFNγ expressing T-cells was analyzed by ELIspot 24 hours later. (N=3)
In summary, our data indicate that, compared to Ad5 vectors, Ad5/35 have a better safety profile (less induction of pro-inflammatory cytokines) and induce stronger CD4 and CD8 T-cells to a model antigen (which is most likely due to a more efficient transduction of APC). Hyposensitivity assays as well as analysis of Treg and T-cell responses did not indicate that Ad5/35 injection induces immunosuppression in CD46 transgenic mice. Several explanations might account for the discrepancies between the reports listed above and our data. i) The binding domains of CD46 ligands within CD46 used in the study by Marie et al. are different (C3b, anti-CD46 antibodies) or only partially overlap (measles H) with the Ad35 binding domain [29], which might have different downstream effects in terms of signaling. ii) Ad5/35 uses CD46 for internalization, while the other ligands do not, which again might result in different intracellular signaling. In agreement with our results in C57-CD46 mice, a recent study in baboons demonstrated no suppression of host immune responses upon Ad5/35 vector injection [12, 16]. iii) Immunosuppression by measles virus and wt Ad35 might be the result of viral replication in and cytolysis of infected cells, which does not occur with the first generation vectors used in our model.
In this study we also show that Ad5, as well as Ad5/35, vectors induce a strong anti-Ad T-cell response that is at least an order of magnitude higher than the anti-HBeAg response. Considering the concept of competition between T-cell clones and space in the thymus [30], this might be problematic if T-cell responses against weak antigens such as tumor-associated antigens have to be induced. In an attempt to address this problem we tested a vector derived from another Ad serotype (Ad11) with less seroprevalence of neutralizing antibodies in the human population, however, we found the same T-cell responses in humans. This is probably due to cross-reactivity of T-cells with Ad serotypes, specifically to immunodominant hexon epitopes which are conserved among Ad serotypes [31]. To address this problem, we are currently attempting to modify immunodominant epitopes within the Ad capsid. Notably, the immunodominance of Ad antigens might not be as problematic if T-cell responses against strong antigens such as HIV antigens to be raised.
Materials and Methods
Cells. Peripheral blood mononuclear cells (PBMC) from normal donors were obtained after leukapheresis and provided by Dr. Nora Disis (Tumor Vaccine Group, Department of Oncology, University of Washington, Seattle, WA). PBMC from Senegalese cancer patients were purified from heparinized blood by density gradient centrifugation using Ficoll-Hypaque gradients. Monocyte derived dendritic cells (DC): PBMC were re-suspended in Iscove modified Dulbecco medium (IMDM) medium (Invitrogen, Carlsbad, CA) and plated in 6-well plates at 1 × 107 cells/well for 1 hour. After 1 hour, non-adherent cells were washed off and AIMV media containing 2mM Lglutamine, 100U/ml penicillin, 100μg/ml streptomycin, 800U/ml GM-CSF (Avigen, Seattle, WA) and 500 IU/ml interleukin-4 (R&D systems, Minneapolis, MN) was added. Cells were left for 5-7 days and media was replaced on day 3. CD34+ derived DC: Human CD34+ peripheral blood derived cells were provided by Dr. Shelly Heimfeld (Fred Hutchinson Cancer Research Center, Seattle, WA). Standard protocols for ex vivo differentiation of CD34+ progenitors into DCs with GM-CSF, IL-4, flt3-ligand, and TNFα were used. Mouse DCs from bone marrow: Mouse bone marrow cells were cultured in RPMI 1640, 50μM β-mercaptoethanol, 10% inactivated FCS, and 200U/ml rmGM-CSF for 8 days. On the day of the experiment, 1 × 105 DCs were plated in 24 well plates and incubated with the indicated viruses overnight. At 24 hours post infection, GFP expression was assessed by flow cytometry.
Viruses. Ad5.GFP and Ad5/35.GFP are first-generation E1/E3 deleted adenovirus vectors expressing GFP [32]. Ad5.HBeAg and Ad5/35.HBeAg express the HBV core antigen under the control of the CMV promoter [33]. Ad11.GFP is an E1-deleted Ad serotype 11 vector, expressing GFP under the control of the CMV promoter [34]. HD-Ad5/35.GFP is a helper-dependent (HD) Ad5/35 vector expressing GFP under the control of the CMV promoter [35]. The contamination with helper virus genomes in the HD-Ad5/35.GFP preparation used in this study was less than 0.1%. Viral vectors were produced and propagated following standard procedures [36]. Viruses were banded in CsCl gradients, dialyzed, and stored in aliquots as described previously. Titers were determined by plaque titration in 293 cells as described [36] and the contamination level of wild type (wt) virus was examined by real-time PCR as described [32]. For all first-generation Ad5 and Ad5/35 vectors used in this study, the ratio of viral particles to pfu was 20:1. Only vector preparations that contained less than one wt viral genome in 106 genomes were used in these studies. All vectors were free of endotoxin.
Recombinant fiber knob domains. Ad5 and Ad35 knobs were produced in E.coli and purified as described elsewhere [37]. The knob domains were dialyzed against 5mM KCl, 10% glycerol, 10mM MgCl2 and stored at −20°C at a stock concentration of 5mg/ml.
sCD46 Competition Assays. 10 μg sCD46 expression plasmid (courtesy JP Atkinson) was transfected onto 293-T cells. The supernatant was collected 72 h post transfection. This supernatant was diluted with fresh growth medium 1:1 and incubated with virus in a volume of 200 μl at room temperature for 1 h before adding to cells for 30 min at 4 °C. Cells were washed once, plated, and examined 24 h later for GFP expression by flow cytometry.
Mice. All experiments involving animals were conducted in accordance with the institutional guidelines set forth by the University of Washington. All mice were housed in specific-pathogen-free facilities. C57Bl/6 mice (“C57”) were obtained from Charles River (Wilmington, MA). CD46 transgenic C57Bl/6 mice line MCP8B (C57-CD46) were generously provided by Dr. Branka Horvat (INSERM, Paris, France) [18]. (This line has been crossed into the C57Bl/6 background for 9 generations.) These mice express CD46 at levels similar to human cells. The transgene is a CD46 C1 isoform under the control of the ubiquitously active hydroxymethyl-glutaryl coenzyme A reductase (HMGR) promoter. CD46 DNA positive mice were identified by PCR on tail DNA using the following primers: F-CD46: 5′-GGTCAAATGTCGATTTCCAGT-3′, R-CD46: 5′-AATCACAGCAATGACCCAAA-3′. For intramuscular injection, Ad vectors in 50μl of PBS were injected into the hamstring muscle.
Quantitative PCR (qPCR) for vector genomes. Three days after virus injection, mice were anaesthetized, the heart was canulated and mice were exsanguinated and flushed with 20ml PBS. Tissue samples were harvested under conditions that minimize cross-contamination. Organ samples were processed for histological analyses and snap frozen in liquid nitrogen. DNA was subsequently extracted from snap frozen tissues and analyzed for viral DNA using primers specific for viral DNA. Samples were analyzed using a Lightcycler and the SYBR Green kit, and the following primers were used for viral DNA: F-L4: 5′-TGCAAGATACCCCTATCCTG-3′, R-L4: 5′CCTGTTGCAGAGCGTTTGC-3′. Purified Ad DNA was used as external standards. Samples were equalized for DNA input using control primers against mouse GAPDH: F-mGAPDH: 5′-ATCACTGCCACCCAGAAGAC-3′, R-mGAPDH: 5′-CACATTGGGGTAGGAACAC-3′.
Histological analyses. GFP expression was visualized on paraffin sections by immunohistochemistry using a mouse anti-GFP antibody (1:200; Clontech, BD Biosciences, San Diego, CA, USA). Binding was visualized using the ABC method (Vector Laboratories, Burlingame, CA, USA). Ad particles were detected on muscle sections using goat anti-Ad5 hexon-FITC antibodies (Chemicon, Temecula, CA). Muscle cells were stained with rabbit –anti-laminin (DAKO, Denmark) and goat-anti-rabbit-AlexaFlour568 (Molecular Probes, Eugene, OR). Cell nuclei were stained with DAPI (Sigma, St. Louis, MO).
Cytokines. Serum samples were analyzed by Cytometric Bead Array, using a “Mouse Inflammatory Cytometric Bead Array” (BD Biosciences, Palo Alto, CA) as described elsewhere [21].
Hypersensitivity assays. CHS and DTH were analyzed six hours after virus injections. For CHS, hapten dinitrofluorobenzene (DNFB) was diluted in acetone: olive oil (4:1) before use, and 25 μl of 0.5% DNFB solution was applied to a shaved ventral skin area (sensitization phase). After 6 days, mice received 10 μl of a nonirritant concentration of DNFB applied on both sides of the left ear and the solvent alone on the right ear (effector phase). Ear thickness was monitored before challenge and 24 and 48 hours after challenge, in a blinded study. DTH response to KLH was measured by a conventional footpad swelling assay. Mice were sensitized by subcutaneous injection with 200μl of 300μg limpet hemocyanin (KLH) emulsified with complete Freund's adjuvant (CFA) (1:1, v/v). After 6 days, mice were challenged by subcutaneous injection in the left hind footpad with 150μg of KLH diluted in PBS. The right footpad was injected with PBS alone. Footpad thickness was measured before challenge and after challenge.
ELIspot assay. C57 or C57-CD46 mice were intramuscularly injected with 5×109 pfu of Ad5.HBeAg, Ad5/35.HBeAg, or saline. Twelve days later, spleen cells of naïve syngeneic animals were obtained and pulsed with 5μg/ml of recombinant HBeAg or HBsAg, or transduced with 500 viral particles/cell (25pfu/cell) of Ad5.GFP. On day 14, vaccinated animals were sacrificed, their spleen cells were collected and 1×106 cells were mixed with 1×106 of ex vivo pulsed splenocytes for in vitro sensitization (I.V.S.). After 24 hours of incubation in 96 well plates, cells were plated at a concentration of 105 cells/100 μl into wells of 96-well hydrophobic polyvinylidene difluoride-backed plates (Millipore, Bedford, Mass.), previously coated with 50μl of 10μg/ml anti-IFNγ monoclonal antibody (1-D1K, mouse immunoglobulin G1; Mabtech, Nacka, Sweden) overnight at 4°C. As a positive control, phytohemagglutinin (Sigma, St. Louis, MO) was added to two wells at a final concentration of 1 μg/ml. All responses were tested in triplicate. Plates were incubated overnight, washed with phosphate-buffered saline containing 0.05% Tween 20, and incubated at room temperature for 2 hours with biotinylated anti-IFNγ monoclonal antibody at 1μg/ml (7-B6-1, mouse immunoglobulin G1; Mabtech). Binding was developed using the Vectastain ABC Elite kit (PK-6100; Vector Laboratories, Burlingame, CA).
T-cell cross-reactivity to fiber knobs. Human PBMC were incubated with Ad5 or Ad35 fiber knob (1μg/ml) or PBS. Upon pulsing T-cells were separated and expanded for 7 days in the presence of 5ng/ml IL-2. Simultaneously, DC were derived from peripheral monocytes as described elsewhere [34]. DC were incubated with 0.2μg/ml of Ad5 or Ad35 fiber knobs or control peptide. A total of 5×103 of DCs were mixed with 5×104 T-cells and incubated for 24 hours for IFNγ ELIspot assays.
Intracellular cytokine staining. Splenocytes from immunized animals were incubated with 5 μg/ml of recombinant HBeAg or HBsAg protein for 5 hours in 96-well U-bottom plates with 106 cells/well in the presence of 50 U/ml of human recombinant IL-2 and 1 μl/ml of Brefeldin A (Golgistop, Pharmingen, La Jolla, CA) (to block the intracellular transport processes of cytokine proteins in the endoplasmic reticulum or Golgi complex). After incubation, the cells were washed and surface-stained with an FITC-conjugated rat anti-mouse CD8 or CD4 antibody (Pharmingen, La Jolla, CA) followed by fixation/permeabilization (Cytofix/Cytoperm, Pharmingen) and intracellular staining for IFN-γ (PE-conjugated rat anti-mouse IFN-γ, Pharmingen) according to the manufacturer's instructions. After washing, cells were examined by two-color flow cytometry and CellQuestPro software. Percentage of responding cells is calculated counting a minimum of 50,000 events (CD8+ or CD4+ T cells).
Analysis of Tregs. Treg analysis was performed as described elsewhere [38]. Briefly, Splenocytes were analyzed by flow cytometry using the following antibodies: rat Mab anti-FoxP3-PE (clone FHK16s, eBioscience), rat Mab anti-CD4-PE (Pharmingen), and rat Mab anti-CD25-FITC (all from eBioscience). All samples were treated with Fc-block (CD16/CD32). Corresponding isotope controls yielded no significant staining.
Acknowledgments
We thank Steve Roffler for critical discussions. We are grateful to Si-Yi Chen, Branka Horvat, Nora Disis, and Shelly Heimfeld for providing valuable material. This study was supported by NIH grants CA080192, HLA078836, HL-00-008 and grants from the Doris Duke and Avon Charitable Foundations.
References
- 1.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J. AIDS research. Merck reemerges with a bold AIDS vaccine effort. Science. 2001;292:24–25. doi: 10.1126/science.292.5514.24. [DOI] [PubMed] [Google Scholar]
- 3.Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 4.Rea D, et al. Highly efficient transduction of human monocyte-derived dendritic cells with subgroup B fiber-modified adenovirus vectors enhances transgene-encoded antigen presentation to cytotoxic T cells. J Immunol. 2001;166:5236–5244. doi: 10.4049/jimmunol.166.8.5236. [DOI] [PubMed] [Google Scholar]
- 5.Ni S, et al. Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum Gene Ther. 2005;16:664–677. doi: 10.1089/hum.2005.16.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 7.Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens' magnet. J Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider-Schaulies S, ter Meulen V. Modulation of immune functions by measles virus. Springer Semin Immunopathol. 2002;24:127–148. doi: 10.1007/s00281-002-0101-3. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez MP, et al. Outbreak of adenovirus 35 pneumonia among adult residents and staff of a chronic care psychiatric facility. J Infect Dis. 1997;176:760–763. doi: 10.1086/517295. [DOI] [PubMed] [Google Scholar]
- 10.Manchester M, et al. Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J Virol. 2002;76:6636–6642. doi: 10.1128/JVI.76.13.6636-6642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barouch DH, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 12.Ophorst OJ, et al. An adenoviral type 5 vector carrying a type 35 fiber as a vaccine vehicle: DC targeting, cross neutralization, and immunogenicity. Vaccine. 2004;22:3035–3044. doi: 10.1016/j.vaccine.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Sakurai F, Mizuguchi H, Yamaguchi T, Hayakawa T. Characterization of in vitro and in vivo gene transfer properties of adenovirus serotype 35 vector. Mol Ther. 2003;8:813–821. doi: 10.1016/s1525-0016(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 14.Seshidhar Reddy P, et al. Development of adenovirus serotype 35 as a gene transfer vector. Virology. 2003;311:384–393. doi: 10.1016/s0042-6822(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 15.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 2002;62:1063–1068. [PubMed] [Google Scholar]
- 16.Xin KQ, et al. Prime-boost vaccination with plasmid DNA and a chimeric adenovirus type 5 vector with type 35 fiber induces protective immunity against HIV. Gene Ther. 2005 doi: 10.1038/sj.gt.3302590. [DOI] [PubMed] [Google Scholar]
- 17.Kemper C, et al. Membrane cofactor protein (MCP; CD46) expression in transgenic mice. Clin Exp Immunol. 2001;124:180–189. doi: 10.1046/j.1365-2249.2001.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marie JC, et al. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- 19.Marie JC, et al. Mechanism of measles virus-induced suppression of inflammatory immune responses. Immunity. 2001;14:69–79. doi: 10.1016/s1074-7613(01)00090-5. [DOI] [PubMed] [Google Scholar]
- 20.Bronte V. Genetic vaccination for the active immunotherapy of cancer. Curr Gene Ther. 2001;1:53–100. doi: 10.2174/1566523013348931. [DOI] [PubMed] [Google Scholar]
- 21.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol. 2004;78:5368–5381. doi: 10.1128/JVI.78.10.5368-5381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shayakhmetov DM, et al. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemper C, et al. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 24.Milich DR, et al. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol. 1988;141:3617–3624. [PubMed] [Google Scholar]
- 25.Olive M, et al. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- 26.Bakay M, et al. Cross-reactivity between human adenoviruses in delayed-type hypersensitivity. Apmis. 2005;113:197–202. doi: 10.1111/j.1600-0463.2005.apm1130307.x. [DOI] [PubMed] [Google Scholar]
- 27.Leen AM, et al. Conserved CTL epitopes on the adenovirus hexon protein expand subgroup cross-reactive and subgroup-specific CD8+ T cells. Blood. 2004;104:2432–2440. doi: 10.1182/blood-2004-02-0646. [DOI] [PubMed] [Google Scholar]
- 28.Leen AM, et al. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood. 2004;103:1011–1019. doi: 10.1182/blood-2003-07-2449. [DOI] [PubMed] [Google Scholar]
- 29.Gaggar A, et al. Localization of regions in CD46 that interact with adenovirus. J Virol. 2005;79:7503–7513. doi: 10.1128/JVI.79.12.7503-7513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockinger B, Barthlott T, Kassiotis G. The concept of space and competition in immune regulation. Immunology. 2004;111:241–247. doi: 10.1111/j.1365-2567.2004.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heemskerk B, et al. Extensive cross-reactivity of CD4+ adenovirus-specific T cells: implications for immunotherapy and gene therapy. J Virol. 2003;77:6562–6566. doi: 10.1128/JVI.77.11.6562-6566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernt K, et al. A new type of adenovirus vector that utilizes homologous recombination to achieve tumor-specific replication. J Virol. 2002;76:10994–11002. doi: 10.1128/JVI.76.21.10994-11002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You Z, et al. Induction of vigorous helper and cytotoxic T cell as well as B cell responses by dendritic cells expressing a modified antigen targeting receptor-mediated internalization pathway. J Immunol. 2000;165:4581–4591. doi: 10.4049/jimmunol.165.8.4581. [DOI] [PubMed] [Google Scholar]
- 34.Stone D, et al. Development and assessment of human adenovirus type 11 as a gene transfer vector. J Virol. 2005;79:5090–5104. doi: 10.1128/JVI.79.8.5090-5104.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shayakhmetov DM, et al. Genome size and structure determine efficiency of postinternalization steps and gene transfer of capsid-modified adenovirus vectors in a cell-type-specific manner. J Virol. 2004;78:10009–10022. doi: 10.1128/JVI.78.18.10009-10022.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlson CA, Shayakhmetov DM, Lieber A. An Adenoviral Expression System for AAV Rep78 Using Homologous Recombination. Mol Ther. 2002;6:91–98. doi: 10.1006/mthe.2002.0634. [DOI] [PubMed] [Google Scholar]
- 37.Shayakhmetov DM, et al. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J Virol. 2003;77:3712–3723. doi: 10.1128/JVI.77.6.3712-3723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Paolo N, et al. Effect of adenovirus mediated heat shock protein expression and oncolysis in combination with low-dose cyclophosphamide treatment on anti-tumor immune responses Cancer Research 2005. under revision [DOI] [PMC free article] [PubMed] [Google Scholar]