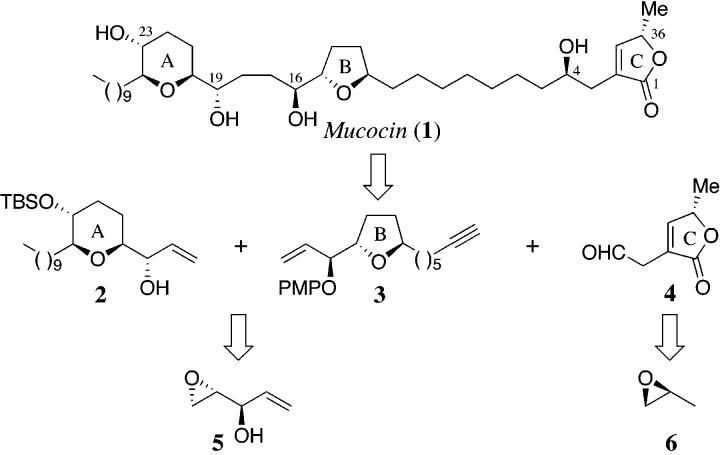
The potent antitumor agent mucocin (1) was isolated from the leaves of Rollinia mucosa (jacq.) Baill. (Annonaceae) by McLaughlin and co-workers in 1995.1-3 This agent has exquisite selectivity for the inhibition of A-549 (lung cancer) and PACA-2 (pancreatic cancer) solid tumor cell lines with potency 10,000 times that of adriamycin (doxorubicin). Annonaceous acetogenins selectively inhibit cancerous cells through the blockage of the mitochondrial complex I (NADH-ubiquinone oxidoreductase), and the inhibition of the plasma membrane NADH oxidase, which depletes ATP and induces apoptosis (programmed cell death) in malignant cells.4
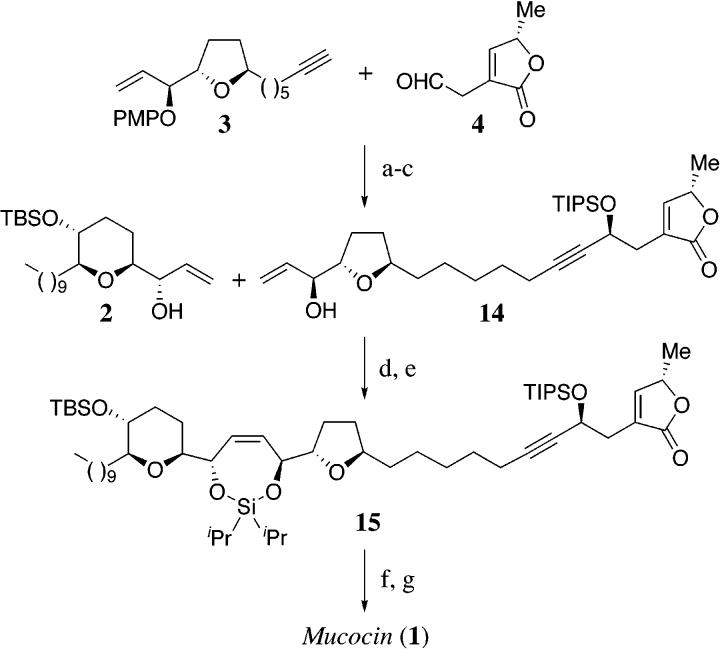
In a program directed toward the construction of nonadjacent tetrahydrofuran containing acetogenins, we have developed a new approach to the construction of C2-symmetrical 1,4-diols, using a temporary silicon-tethered (TST) ring-closing metathesis (RCM) homo-coupling reaction.5 Herein, we now describe a novel and expeditious synthesis of mucocin (1), which utilizes the TST-RCM cross-coupling reaction (Scheme 1).6,7 This approach capitalizes on the localized C2-symmetry and thereby permits the construction of 2 and 3 from a common synthetic intermediate, the known homoallylic epoxide 5.8 We further envisioned that the C4-C5 bond could be formed by enantioselective addition of the alkyne 3 to the aldehyde 4, thereby providing a new strategic disconnection for this class of biologically important molecules.9 The key feature of this approach is the utilization of a triply convergent strategy, that can be adapted to facilitate the synthesis of related annonaceous acetogenins, resulting in one of the most expeditious syntheses of a complex acetogenin developed to date.
Scheme 1.
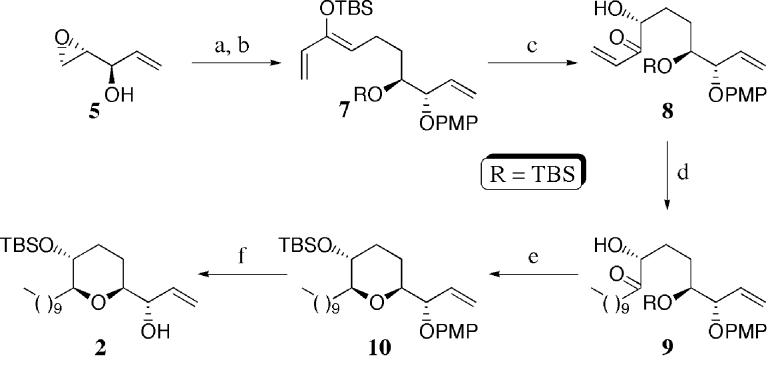
The synthesis of the 3-hydroxy-2,6-disubstituted tetrahydropyran 2 was accomplished using the novel six-step strategy outlined in Scheme 2. Mitsunobu inversion of the allylic alcohol 5 using p-methoxyphenol afforded the requisite aryl ether.10 Regiospecific ring opening of the epoxide with the homoenolate equivalent11 derived from tert-butyldimethylsilyl protected divinyl carbinol, followed by an in situ protection of the resultant secondary alcohol, afforded the differentially protected triene 7 in 96% overall yield. Chemoselective Sharpless asymmetric dihydroxylation of the triene 7 using AD-mix-β furnished the hydroxy ketone 8 in 70% yield (ds ≥99:1 by HPLC), after recycling the recovered triene 7 (2×).12 The alkyl side chain was then introduced via the conjugate addition of the cuprate derived from octylmagnesium bromide and copper cyanide to furnish the ketone 9 and thereby set the stage for the reductive etherification. Treatment of 9 with bismuth tribromide and tert-butyldimethylsilane in acetonitrile, followed by in situ protection of the secondary alcohol, furnished the tert-butyldimethylsilyl ether 10 in 93% yield (ds ≥19:1 by NMR).13 Finally, the p-methoxyphenyl ether was oxidatively cleaved with ceric ammonium nitrate (CAN) to complete the construction of 2.10
Scheme 2a.
(a)p-MeOC6H4OH,DIAD,PPh3,THF,0°C,80%;(b)(CH2=CH)2CHOTBS, sBuLi, THF,-78 °C, then TBSOTf, 2,6-lutidine, -78 to 0 °C, 96%; (c) AD-mix-β, tBuOH/H2O, MeSO2NH2, 0 °C (3×), 70%; (d) noctylMgBr, CuCN, THF, -78 °C, 65%; (e)BiBr3, tBuMe2SiH, MeCN, 0°C, then 2,6-lutidine, TBSOTf, 0°C, 93%; (f) (NH4)2Ce(NO3)6, MeCN/H2O, -5°C, 91%.
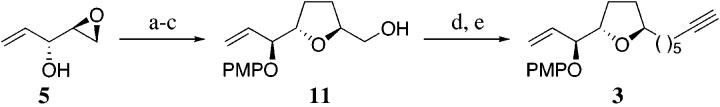
The construction of the tetrahydrofuran 3 was also initiated from the homoallylic epoxide 5, as outlined in Scheme 3. Mitsunobu inversion of 5 followed by regiospecific ring opening of the epoxide (cf. Scheme 2) with the cuprate derived from allylmagnesium bromide and catalytic copper cyanide afforded the secondary alcohol, which was subjected to a cobalt(II) catalyzed oxidative cyclization to afford the trans-2,5-tetrahydrofuran 11 in 75% overall yield (ds ≥19:1).2d,14 Conversion of the primary alcohol 11 to triflate, followed by cuprate displacement and in situ deprotection of trimethylsilyl group, furnished the B-ring fragment 3.
Scheme 3a.
(a)p-MeOC6H4OH,DIAD,PPh3,THF,0°C,80%;(b)CH2=CHCH2MgBr, CuCN, Et2O, -78°C, 90%; (c) Co(modp)2, O2, tBuOOH, iPrOH, 60°C, 83%; (d) Tf2O, Et3N, CH2Cl2, -78°C, 86%; (e) TMSC≡C(CH2)4MgBr, CuI, THF, -20 to -10°C; then MeOH, TBAF, -20°C to room temperature, 73%.
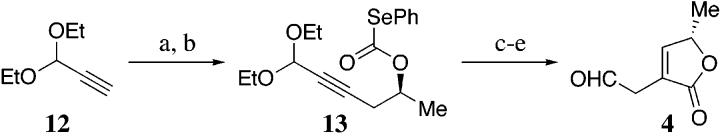
The synthesis of butenolide fragment 4 commenced with the regioselective ring opening of commercially available (S)-propylene oxide 6 (Scheme 4). Treatment of 6 with the carbanion derived from the alkyne 12 afforded the secondary alcohol, which was converted to the selenocarbonate 13 using phosgene and phenylselenol.15 The selenocarbonate 13 was subjected to standard free radical conditions, to afford the γ-butyrolactone in 80% yield. Metal-catalyzed isomerization of the exo-cyclic olefin and subsequent hydrolysis of the diethyl acetal furnished the requisite aldehyde 4 in good overall yield.
Scheme 4a.
(a)S-Propylene oxide 6, nBuLi, HMPA, THF, -30 °C; (b) COCl2, Et3N, C6H6, 0 °C to room temperature, then PhSeH, pyridine, THF/C6H6, 0 °C to room temperature, 60% overall yield from 12; (c) nBu3SnH, AIBN, C6H6, Δ, 8O%; (d) RhH(CO)(PPh3)3, C6H6, 85 °C, 84%; (e) HCOOH, pentane, 0 °C, 9O%.
Scheme 5 outlines the manner in which the three fragments were assembled to complete the synthesis of mucocin (1). The enantioselective addition of the alkynyl zinc reagent derived from 3 to the aldehyde 4 furnished the propargylic alcohol in 81% yield with excellent selectivity (ds = 20:1 by HPLC).9,16 Protection of the alcohol as the triisopropylsilyl ether followed by deprotection of the p-methoxyphenyl ether afforded the allylic alcohol 1410 and thereby set the stage for the TST-RCM cross-coupling reaction. The construction of the mixed bis-alkoxy silane was achieved from the allylic alcohol 2 through the treatment with excess diisopropyldichlorosilane to afford the mono-alkoxychlorosilane, followed by the removal of the excess silylating agent and addition of the second allylic alcohol 14. Ring-closing metathesis of the silicon-tethered diene using stoichiometric Grubbs' catalyst furnished 15 in 83% yield and completed the construction of the carbon skeleton of mucocin (1) (Scheme 5).17 The synthesis was concluded with the fluoride-mediated deprotection of 15, followed by chemoselective reduction with diimide.18 The spectroscopic data and optical rotation of synthetic mucocin (1) were identical in all respects to the values reported for the natural substance [1H/13C NMR, IR, [α]26D -16.O (c = 0.25, CH2Cl2)].
Scheme 5a.
(a)3, Et2Zn, PhMe, Δ, then (R)-BINOL, Ti(OiPr)4, THF, 4,0 °C, 81%; (b) TIPSOTf, pyridine, DMAP, THF, 0 °C, 96%; (c) (NH4)2Ce(NO3)6, MeCN/H2O, -10 °C, 91%; (d) 2, iPr2SiCl2 (xs), CH2Cl2, imidazole, 0 °C to room temperature, then 14, imidazole, 0 °C to room temperature, 74%; (e) Grubbs' catalyst (1.8 equiv), 1,2-DCE, Δ, 83%; (f) HF/MeCN, CH2Cl2, room temperature, 91%; (g) TsNHNH2, NaOAc, 1,2-DME/H2O, Δ, 95%.
In conclusion, we have accomplished an enantioselective total synthesis of the annonaceous acetogenin (—)-mucocin (1) using a triply convergent 12-step sequence (longest linear sequence) in 13.6% overall yield. This approach represents the first application of the temporary silicon-tethered (TST) ring-closing metathesis (RCM) cross-coupling reaction and the enantioselective alkyne/aldehyde addition in the synthesis of a complex annonaceous acetogenin. Finally, the synthesis highlights the utility of the bismuth tribromide-mediated reductive etherification for the construction of 3-hydroxy-2,6-disubstituted tetrahydropyrans.
Supplementary Material
Acknowledgment.
This work is dedicated to Professor Philip D. Magnus on the occasion of his 6Oth birthday. We sincerely thank the National Institutes of Health (GM58877) for generous financial support. We also thank Johnson and Johnson for a Focused Giving Award and Pfizer Pharmaceuticals for the Creativity in Organic Chemistry Award. The Camille and Henry Dreyfus Foundation is thanked for a Camille Dreyfus Teacher-Scholar Award (P.A.E.).
Footnotes
Note Added after ASAP. In the version posted 11/5/O3, in Scheme 2 the absolute configuration for the secondary tert-butyldimethylsilyl ether in 7, 8, and 9 was incorrect. The version posted 11/11/O3 and the print version are correct.
Supporting Information Available: Spectral data and detailed experimental procedures for all of the synthetic intermediates (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Shi G, Alfonso D, Fatope MO, Zeng L, Gu Z-M, Zhao G.-x., He K, MacDougal JM, McLaughlin JL. J. Am. Chem. Soc. 1995;117:10409. [Google Scholar]
- (2). For total syntheses of mucocin (1), see:; (a) Neogi P, Doundoulakis T, Yazbak A, Sinha SC, Sinha SC, Keinan E. J. Am. Chem. Soc. 1998;120:11279. [Google Scholar]; (b) Baäurle S, Hoppen S, Koert U. Angew. Chem., Int. Ed. 1999;38:1263. doi: 10.1002/(SICI)1521-3773(19990503)38:9<1263::AID-ANIE1263>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]; (c) Takahashi S, Nakata T. J. Org. Chem. 2002;67:5739. doi: 10.1021/jo020211h. [DOI] [PubMed] [Google Scholar]; (d) Takahashi S, Kubota A, Nakata T. Angew. Chem., Int. Ed. 2002;41:4751. doi: 10.1002/anie.200290038. [DOI] [PubMed] [Google Scholar]
- (3). For alternative synthetic approaches to the 3-hydroxy 2,6-disubstituted tetrahydropyran ring of mucocin (1), see:; (a) Evans PA, Roseman JD. Tetrahedron Lett. 1997;38:5249. [Google Scholar]; (b) Evans PA, Murthy VS. Tetrahedron Lett. 1999;40:1253. [Google Scholar]; (c) Takahashi S, Fujisawa K, Sakairi N, Nakata T. Heterocycles. 2000;53:1361. [Google Scholar]
- (4).(a) Ahammadsahib KI, Hollingworth RM, McGovren JP, Hui YH, McLaughlin JL. Life Sci. 1993;53:1113. doi: 10.1016/0024-3205(93)90547-g. [DOI] [PubMed] [Google Scholar]; (b) Morre DJ, de Cabo R, Farley C, Oberlies NH, McLaughlin JL. Life Sci. 1995;56:343. doi: 10.1016/0024-3205(94)00957-0. [DOI] [PubMed] [Google Scholar]
- (5). For examples of silicon-tethered ring-closing metathesis homo-coupling reactions, see:; (a) Fu GC, Grubbs RH. J. Am. Chem. Soc. 1992;114:7324. [Google Scholar]; (b) Evans PA, Murthy VS. J. Org. Chem. 1998;63:6768. doi: 10.1021/jo9811524. [DOI] [PubMed] [Google Scholar]
- (6). For examples of silicon-tethered ring-closing metathesis cross-coupling reactions, see:; (a) Hoye TR, Promo MA. Tetrahedron Lett. 1999;40:1429. [Google Scholar]; (b) Van de Weghe P, Aoun D, Boiteau J-G, Eustache J. Org. Lett. 2002;4:4105. doi: 10.1021/ol0268438. [DOI] [PubMed] [Google Scholar]
- (7). For recent reviews on temporary silicon-tethered strategies, see:; (a) White JD, Carter RG. In: Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. Fleming I, editor. Georg Thieme Verlag; New York: 2001. pp. 371–412. [Google Scholar]; (b) Skrydstrup M. In: Science of Synthesis: Houben-Weyl Methods of Molecular Transformations. Fleming I, editor. Georg Thieme Verlag; New York: 2001. pp. 439–530. [Google Scholar]
- (8).Schreiber SL, Schreiber TS, Smith DB. J. Am. Chem. Soc. 1987;109:1525. [Google Scholar]
- (9).(a) Lu G, Li X, Chan WL, Chan ASC. Chem. Commun. 2002. p. 172. [DOI] [PubMed] [Google Scholar]; (b) Gao G, Moore D, Xie R-G, Pu L. Org. Lett. 2002;4:4143. doi: 10.1021/ol026921r. [DOI] [PubMed] [Google Scholar]
- (10).Fukuyama T, Laird AA, Hotchkiss LM. Tetrahedron Lett. 1985;26:6291. [Google Scholar]
- (11). For the ring opening of dioxirane, which leads to a regioisomeric mixture, see:; Oppolzer W, Snowden RL. Tetrahedron Lett. 1976;17:4187. [Google Scholar]
- (12).Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem. ReV. 1994;94:2483. [Google Scholar]
- (13).(a) Evans PA, Cui J, Gharpure SJ, Hinkle RJ. J. Am. Chem. Soc. 2003;125:11456. doi: 10.1021/ja036439j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Evans PA, Cui J, Gharpure SJ. Org. Lett. 2003;5:3883. doi: 10.1021/ol035438t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Inoki S, Mukaiyama T. Chem. Lett. 1990. p. 67. [Google Scholar]
- (15).Evans PA, Murthy VS. Tetrahedron Lett. 1998;39:9627. [Google Scholar]
- (16).Extensive modification of the reported procedure was necessary to achieve optimum selectivity
- (17). The construction of trans-1,4-silaketals using ring-closing metathesis is known to be challenging, see:; Evans PA, Cui J, Buffone GP. Angew. Chem., Int. Ed. 2003;42:1734. doi: 10.1002/anie.200250486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Marshall JA, Chen M. J. Org. Chem. 1997;62:5996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.