Abstract
We identified a homozygous missense mutation in the noncatalytic subunit (RAB3GAP2) of RAB3GAP that results in abnormal splicing in a family with congenital cataracts, hypogonadism, and mild mental retardation (Martsolf syndrome). Recently, mutations in the catalytic subunit of RAB3GAP (RAB3GAP1), a key regulator of calcium-mediated hormone and neurotransmitter exocytosis, were reported in Warburg micro syndrome, a severe neurodevelopmental condition with overlapping clinical features. RAB3GAP is a heterodimeric protein that consists of a catalytic subunit and a noncatalytic subunit encoded by RAB3GAP1 and RAB3GAP2, respectively. We performed messenger RNA–expression studies of RAB3GAP1 and RAB3GAP2 orthologues in Danio rerio embryos and demonstrated that, whereas developmental expression of rab3gap1 was generalized (similar to that reported elsewhere in mice), rab3gap2 expression was restricted to the central nervous system. These findings are consistent with RAB3GAP2 having a key role in neurodevelopment and may indicate that Warburg micro and Martsolf syndromes represent a spectrum of disorders. However, we did not detect RAB3GAP2 mutations in patients with Warburg micro syndrome. These findings suggest that RAB3GAP dysregulation may result in a spectrum of phenotypes that range from Warburg micro syndrome to Martsolf syndrome.
Rab proteins (which belong to the Ras family of small G proteins) are prime regulators of vesicular membrane transport in both the exocytic and endocytic pathways. The active forms of Rab proteins have multiple functions in cargo selection and as scaffolds for the sequential assembly of effectors required for vesicle budding, cytoskeletal transport, and target membrane fusion (Takai et al. 2001; Zerial and McBride 2001). The four members of the Rab3 subfamily (Rab3A, Rab3B, Rab3C, and Rab3D) have been implicated in regulated exocytosis of neurotransmitters and hormones (Takai et al. 1996; Schluter et al. 2002; Li and Chin 2003; Sudhof 2004). The activity of Rab3 proteins is tightly regulated by RabGDI (GDP dissociation inhibitor), Rab3GEP, and RAB3GAP. The latter two determine the balance of active (GTP) to inactive (GDP) forms, and RAB3GAP specifically converts active Rab3-GTP to the inactive -GDP form (Fukui et al. 1997; Wada et al. 1997; Nagano et al. 1998). Rab3A is the most abundantly expressed Rab protein in the brain and is present in virtually all synapses. Through binding to its effector Rim, Rab3A has a critical role in the release of neurotransmitter vesicles (Li and Chin 2003; Sudhof 2004). Rab3A, Rab3B, and Rab3C are also expressed in endocrine tissues, and Rab3B is expressed at high levels in the anterior pituitary, where it has been implicated in gonadotrophin release (Tasaka et al. 1998; Schluter et al. 2002). Recently, we found germline-inactivating mutations in the catalytic subunit of Rab3GAP (RAB3GAP1 [Ensembl accession number ENSG00000115839; GenBank accession number D31886]) in 12 of 18 kindreds with Warburg micro syndrome (MIM 600118) (Aligianis et al. 2005). This severe autosomal recessive disorder is characterized by ocular defects (microphthalmos, microcornea, congenital cataracts, and optic atrophy) and neurodevelopmental ones (microcephaly, cortical gyral abnormalities such as pachygyria and polymicrogyria, hypoplasia of the corpus callosum, severe mental retardation, and spastic cerebral palsy) and hypothalamic hypogenitalism (Warburg et al. 1993; Rodriguez et al. 1999; Megarbane et al. 1999; Nassogne et al. 2000; Ainsworth et al. 2001; Derbent et al. 2004; Graham et al. 2004). Linkage to RAB3GAP1 was excluded in some families without mutations, confirming locus heterogeneity. RAB3GAP is a heterodimeric complex consisting of a 130-kDa catalytic subunit, encoded by RAB3GAP1 on chromosome 2q21.3, and a 150-kDa noncatalytic subunit (Fukui et al. 1997; Nagano et al. 1998), the gene for which—RAB3GAP2 (Ensembl accession number ENSG00000118873; GenBank accession number AF004828)—is located on chromosome 1q41. Previously, we did not detect mutations in RAB3GAP2 in six families with Warburg micro syndrome without RAB3GAP1 mutations (Aligianis et al. 2005). However, to further investigate the potential role of RAB3GAP2, we (1) analyzed neurodevelopmental expression of RAB3GAP1 and RAB3GAP2 in a model organism and (2) undertook further RAB3GAP2 mutation analysis in Warburg micro syndrome and in the related Martsolf syndrome (MIM 212720), which shares clinical features but is a milder disorder.
Expression of RAB3GAP1 and RAB3GAP2 orthologues in zebrafish.—To further investigate the potential neurodevelopmental role of RAB3GAP2, we compared the expression patterns of RAB3GAP1 and RAB3GAP2 orthologues in developing and adult zebrafish. Two ESTs showing similarities to RAB3GAP1 and RAB3GAP2 were identified through BLAST searches (zebrafish rab3gap1 [GenBank accession number AI629291] and rab3gap2 [GenBank accession number CF348222]), and riboprobes were prepared for in situ hybridization studies.
The zebrafish plasmids were linearized with SalI and were transcribed with SP6-RNA polymerase, and riboprobes were purified using quick spin columns (Roche) and were stored in 50% formamide at −70°C. In situ hybridization studies on cryostat sections were performed as described elsewhere (Costagli et al. 2002). Sections were then mounted in glycerol. Images were captured with a Polaroid digital camera connected to a Nikon Optiphot-2 microscope, by use of ×4, ×10, and ×20 Plan-apo lenses. Digital images were stored as 1,600 ×1,200 pixels at a resolution of 300 dpi and were manually arranged, to form composite pictures, with Adobe Photoshop 5.5. Danio rerio aged 2 and 4 wk from the University College London fish facility were used in all experiments. Fish were raised at 28°C with a cycle of 14 h light and 10 h darkness. Animals were handled in accordance with U.K. and European Union regulations for laboratory animals. Animals were terminally anesthetized with 0.3% tricaine methane sulphonate (MS222 [Sigma]) and were fixed overnight in 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. They were then rinsed in PB and were equilibrated in 10% and 20% sucrose in PB at 4°C for 48 h. Tissue was embedded in OCT compound (Agar), was frozen on dry ice, and was cut serially at 20 μm in coronal and sagittal planes. Sections were collected on Superfrost slides (BDH) and were stored at −70°C in sealed boxes until ready to use.
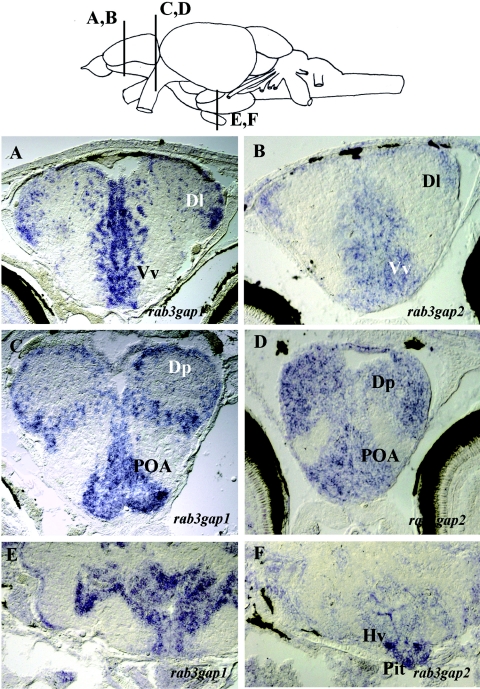
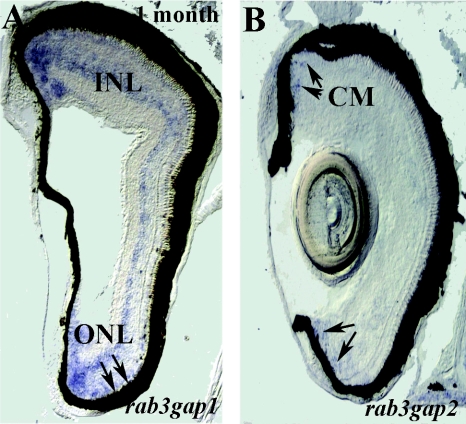
We found that, whereas rab3gap1 was ubiquitously expressed during development, expression of rab3gap2 started during larval stages (3–4 d postfertilization), and transcripts could be clearly detected only in the CNS (data not shown). The analysis of its expression pattern and the comparison with rab3gap1 expression was conducted in 3–4-wk-old zebrafish (figs. 1 and 2). Both subunits were expressed in the CNS in areas with high neuronal density, and were excluded from the regions enriched with fiber tracts and neuropil. In the forebrain of 3–4-wk-old zebrafish, the transcripts of both rab3gap subunits were largely coexpressed in the dorsal (predominantly in the region marked as “Dl” in fig. 1A and 1B) and ventral (“Vv” in fig. 1A and 1B) telencephalon, in the posterior region of the dorsal telencephalon (“Dp” in fig. 1C and 1D), and in the preoptic area (POA) (fig. 1C and 1D). In addition, rab3gap1 was strongly expressed throughout the hypothalamic region (fig. 1E), whereas rab3gap2 expression in the hypothalamus was restricted to the ventral zone (“Hv” in fig. 1F) and the pituitary (“Pit” in fig. 1F). In the eye, rab3gap1 (fig. 2A) was strongly expressed throughout the internal nuclear layer (INL) and was weakly expressed in the photoreceptors, whereas the expression of rab3gap2 (fig. 2B) was confined to the remnants of the ciliary margin (CM), the proliferative zone of the retina.
Figure 1.
Brain expression patterns of rab3gap1 and rab3gap2 in zebrafish. In the forebrain of 3–4-wk-old zebrafish, the transcripts of both rab3gap subunits are largely coexpressed in the dorsal (predominantly in Dl [A and B]) and ventral (Vv [A and B]) telencephalon, in the posterior region of the dorsal telencephalon (Dp [C and D]), and in the POA (C and D). In addition, rab3gap1 is strongly expressed throughout the hypothalamic region (E), whereas rab3gap2 expression in the hypothalamus is restricted to the ventral zone (Hv [F]) and the pituitary (Pit [F]).
Figure 2.
Eye expression patterns of rab3gap1 and rab3gap2 in zebrafish. In the eye, rab3gap1 (A) is strongly expressed throughout the INL and in the CM and is only weakly expressed in the photoreceptors (arrows; outer nuclear layer [ONL]), whereas the expression of rab3gap2 (B) is confined to the remnants of the CM, the proliferative zone of the retina.
Identification of a RAB3GAP2neurodevelopmental phenotype.—To investigate the role of RAB3GAP2 in human neurodevelopmental disease, we investigated families with clinical features overlapping those seen in Warburg micro syndrome, and we identified a homozygous germline RAB3GAP2 mutation in all three children of a family with a Martsolf syndrome–like phenotype.
Clinical report.—The proband (subject IV-1; see fig. 3) was the first child of consanguineous Pakistani parents. He was born at 38 wk by cesarean section, with a birth weight of 1.87 kg, and was noted to have congenital cataracts, microphthalmia, micropenis, and cryptorchidism at birth. Bilateral cataract extraction was performed at age 6 wk. At age 5 mo, he was hypotonic, and his motor development was delayed. He gained head control at age ∼1 year and sat at age 2 years, at which time a brain CT scan was reported as normal. He toe walked from age ∼3.5 years, when spastic diplegia was noted. His first words were at age ∼3 years. At age 11 years, he had mild learning difficulties, was microcephalic (occipitofrontal head circumference [OFC] 49 cm at age 8 years; <3rd percentile) and walked with a walker. He was bilingual and attended special school. Eye examination showed small pupils, aphakia, hypermetropia, and controlled secondary glaucoma. Visual acuity was poor (2/650 right eye [RVA] and 3/60 left eye [LVA]). There were no distinctive facial dysmorphisms.
Figure 3.
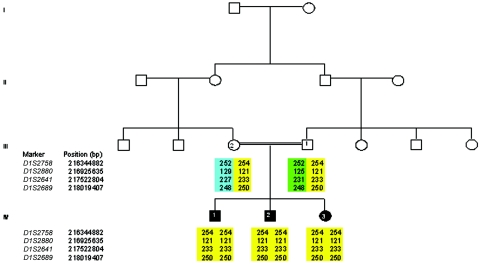
Pedigree of family and fine mapping for markers flanking the RAB3GAP2 gene (which is located between 216712122 and 216834147 bp) on chromosome 1. The parents are in generation III, and the affected siblings are in generation IV: IV-1, IV-2, and IV-3. The disease haplotype is shown in yellow.
Subject IV-3, the proband’s sister, was born by normal delivery at 39 wk after an uncomplicated pregnancy. Her birth weight was 3.060 kg, and her OFC was 34 cm (<10th percentile); she had dense bilateral cataracts and microphthalmia. After cataract surgery at age 4 mo, she developed secondary glaucoma, which required a vitrectomy and peripheral iridectomy. Prior to the glaucoma, her fundal examination was normal. Postsurgery visual acuity was reduced (RVA 6/38; LVA 6/120). Hypotonia was noted in infancy, and she later developed spastic diplegia. She had global developmental delay and sat at age 14 mo, stood with support at age 17 mo, walked with support at age 3 years, and developed speech at age 2 years. At age 5 years, she had moderate learning difficulties and required special schooling, but she was bilingual in English and Punjabi. Growth was normal (height and weight at the 50th percentile), but she had borderline microcephaly, with an OFC just >3rd percentile. She had a low anterior hairline and was hirsute, but she did not have any distinctive facial dysmorphisms.
Subject IV-2 was born, at term, with congenitally corrected transposition of the great vessels. His birth weight was 2.4 kg (50th percentile), and OFC was 33 cm (<10th percentile). He had micropenis and bilateral cryptorchidism, congenital cataracts, and microphthalmia. Left cataract removal was performed at age 2 mo. Immediately postoperatively, he suffered a cardiac arrest of unknown etiology, and, following resuscitation and artificial ventilation, he was found to have severe hypoxic ischemic encephalopathy with convulsions. His ischemic encephalopathy has been associated with profound global developmental delay and spastic quadriplegia. CT and magnetic resonance imaging (MRI) scans showed cerebral atrophy in a pattern consistent with hypoxic damage, and electroencephalogram showed poor background activity, with minimal variability and very little central activity. He was facially hirsute but did not have any dysmorphic facial features.
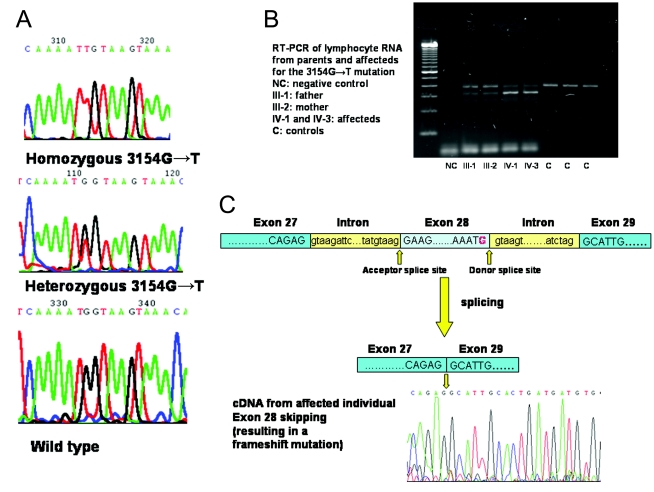
Molecular genetic analysis.—A 10-cM genomewide linkage scan was performed on DNA from all three affected individuals in this family, by use of the Research Genetics version 10 mapping panel, as described elsewhere (Aligianis et al. 2002, 2005). All exons and intron-exon boundaries were amplified by PCR and then were sequenced, by direct sequencing, using the ABI3730 capillary sequencer. The sequencing primers for RAB3GAP1 and PCR conditions are included in table 1. Linkage to RAB3GAP1 was excluded, but all three affected individuals were homozygous for a 16-cM region between markers GATA124F08 and D1S549 that contained the RAB3GAP2 locus (fig. 3). Additional microsatellite markers flanking the noncatalytic subunit of RAB3GAP2 (D1S2880, D1S2641, and D1S2689) were typed and confirmed homozygous-by-descent. We then proceeded to sequence the 36 exons and intron-exon boundaries of RAB3GAP2 and detected a homozygous 3154G→T (Gly1051Cys) missense substitution in all three affected individuals (fig. 4A). Both parents were heterozygous for this substitution, and it was not found in the 270 ethnically matched control chromosomes. Since the substitution is adjacent to the exon 28 splice-donor site, RNA was obtained from lymphocytes from two of the affected children and their parents. Lymphocyte RNA revealed that this mutation resulted in two transcripts (fig. 4B). Sequencing of the cDNA synthesized from these transcripts showed that the splice-site mutation resulted in exon 28 skipping and a frameshift (fig. 4C). Sequencing analysis of DNA from another family with Martsolf syndrome, reported elsewhere (Hennekam et al. 1988), did not detect a mutation in RAB3GAP2 or RAB3GAP1, and linkage analysis excluded linkage to both genes. Also, we did not detect a RAB3GAP2 mutation in an additional two families with Warburg micro syndrome without RAB3GAP1 mutations.
Table 1.
PCR Primers and Conditions Used for RAB3GAP2 Sequencing[Note]
| Primer(5′→3′) | ||||
| Exon(s) | Forward | Reverse | Product Size | MgCl2(mmol/liter) |
| 1 | GCGAGTAGGAGACTCGAACC | GGCTAGAGCTATTGGGGCTC | 290 | 1 |
| 2 | AAAATGTCAGCTTGACAGGG | TCAGCATTATACAGGGGTTGG | 260 | 1 |
| 3 | CACCTAATCCATTTTCTCTTCTATCC | CTCTCACCCCTTGAGACAGG | 275 | 1.5 |
| 4 | TCACCTACAAGTCAAATAATTTCATAC | AATCATATTCTAAGGGGAAAGAGC | 246 | 1 |
| 5 | CACAATGCAGGTCCAGTCAG | GCATCAAATAAAACTGGTTCACAG | 288 | 1 |
| 6–7 | GCTAACTAACTGTGCCACAAGAC | TCCCACTAGCAAGCCAAAAG | 706 | 1 |
| 8 | GACATACTTAATTCCATAAGCAGGTG | GGGTGTTGGGTGGTTATGTTAC | 286 | 1 |
| 9 | CTTGATCCTGGAAGGTGGAG | ACGTGCATTGTCTTGGTGTG | 464 | 1.5 |
| 10 | AAGGCTAAGCAAGTTACCATATCC | TTTAGCATCTTAGAATGGAACTGAAG | 321 | 1 |
| 11–12 | TCAGCAAATCAACCAAGCAC | CTTTTCAAAGCATTTTAAAGTAAGC | 484 | 1 |
| 13 | AACATTGCTCAACTATTGGGG | GCCTATTTCATTTATGCATTTCG | 293 | 1 |
| 14 | TACATGCCAGGCACTCACAG | TAGCTTTTCTTACCCCACCC | 482 | 1 |
| 15 | CAAACCTAGGAACTAGGAAAAGTTAGC | TGATAATAATGTTGGGTTTCCTAGC | 305 | 1 |
| 16–17 | TTCAGCCTCTCATGTTTGGG | TGTTGTTTCCTTTGTCTTTAGACTG | 684 | 1 |
| 18 | CACATTAAAGTAACATGATGGTATCAC | TGGGGTAGTGAAAGTTTGGG | 289 | 1 |
| 19 | AGCACAGAGAGCTAGCAGGG | TGAAAGCAAAGCCTGTTTATG | 350 | 1 |
| 20 | TGCTATATGTTCCTACCATGTTTTC | TTTCAGTTCAGATAATGTGGGTG | 350 | 1 |
| 21 | CTGACACTTCTGGGTGACATTC | TTGTGATTCAATAAGATTGTTAGTCC | 233 | 1 |
| 22 | TTTTGTGACATTTCAAACACG | GCTGTCTAGGAAAGTGTAAGATTTTG | 238 | 1 |
| 23 | AAGGAAAGCATCCCCTTAGC | TTGATTCAGAACAAAGATGACTAAAAG | 434 | 1 |
| 24 | CTGAGCGTGGAACTGACC | ACCCTCATGTTTCATCCAGC | 578 | 1 |
| 25–26 | GCTATTTAATTAGACGGTCCCC | AAACACTGCCCTGTGTGTTG | 522 | 1 |
| 27 | TCTTTAAATTCACATCCTGTTAAATC | TCTCCATTGATAATGGTAAAACC | 219 | 1 |
| 28 | TGAGGATTTAGAGTCCTTCTTTATG | TTCATGCTTAGTGGCAATTTAGAC | 493 | 1.5 |
| 29 | AATGATAAAAGGCAAAGTTCACC | ACTTATAGCGCCATTTACGG | 247 | 1 |
| 30 | TGCAATGGTTTGCAGAATATG | TTTGGCTGACAGTAACTATAGCAG | 206 | 1 |
| 31 | AAATATGAAAATCCATGTGTCTGC | ATGAATTTGCACTTTGCTGC | 387 | 1 |
| 32 | TGTCCTTGCGATAGAAAGTAGTC | GGGGATCACTGGGTTTCATC | 391 | 1 |
| 33 | GCAAAAGGCTTCATTGGAAAC | TGACAGAAAAGCTAACAATACTTGG | 402 | 1 |
| 34 | CCAGGCACTCAAGAAAACATC | CTTCACTGGCAACCCCTTTC | 299 | 1.5 |
| 35_1 | CAGCTGTGAGGTAGGTTTGC | GCTGCCACCTGGGAGTTC | 470 | 1 |
| 35_2 | TGAACCCATTCTTCCTCCTC | GACCTGGAATTTTATGGGAAAAG | 513 | 1 |
Note.— Annealing temperature was 60°C for all sequencing.
Figure 4.
The 3154G→T mutation in RAB3GAP2. A, Mutations in genomic DNA in RAB3GAP2, shown by sequence traces (nucleotide exchange) from affected individuals (top), carrier parents (middle), and healthy control individuals (bottom). B, An agarose gel showing the RT-PCR products, with the two affected individuals having a smaller transcript than the controls. The parents of subject IV-1 have a normal size and shortened product, in keeping with their carrier status. C, Location of the donor splice-site mutation and sequence chromatogram from the smaller transcript in subject IV-1, showing that exon 28 is skipped. The mutation is shown in red.
Germline mutations in RAB3GAP2 have not been reported elsewhere. Although it has been shown that the noncatalytic subunit does not influence RAB3GAP catalytic activity, our findings suggest a critical role for Rab3GAP2 in human development. The expression of rab3gap2 during zebrafish embryogenesis is consistent with the neurodevelopmental phenotype of RAB3GAP2 mutation in humans. Elsewhere, we found that, in mouse embryos, Rab3gap1 showed a low level of general expression throughout the embryo from E10 to E12. In addition to this continued generalized expression, there was also prominent expression in a number of organ systems, including the CNS and peripheral nervous system, at E14.5 (Aligianis et al. 2005). Consistent with this, we found that rab3gap1 is ubiquitously expressed during zebrafish development. However, an unexpected and intriguing finding was the observed differences between rab3gap1 and rab3gap2 expression patterns in developing zebrafish. Thus, rab3gap2 expression was restricted to the CNS, which suggests that this dictates the localization of Rab3gap activity during embryogenesis and hence explains the developmental phenotype of Warburg micro syndrome despite ubiquitous rab3gap1 expression. We note that hypothalamic hypogonadism is a feature of Warburg micro syndrome and that, in zebrafish, rab3gap1 is strongly expressed throughout the hypothalamic region. In contrast, rab3gap2 expression in the hypothalamus is restricted to the ventral zone and the pituitary. In the eye, rab3gap1 was strongly expressed throughout the INL and in the photoreceptors, whereas rab3gap2 expression was confined to the remnants of the CM. We note that, in fish, the retina continues to grow with the eye throughout the life of the animal, and new retinal cells are added at the CM from neural glial stem cells. Further comparative studies of RAB3GAP1 and RAB3GAP2 expression in mice and humans may provide further insights into the relationship between these expression patterns and the Warburg micro syndrome and Martsolf syndrome phenotypes.
The precise mechanisms whereby RAB3GAP1 and RAB3GAP2 mutations cause human disease is unclear. In Warburg micro syndrome, RAB3GAP1 mutations are associated with microgenitalia that may result from hypothalamic hypogonadotropinism and disordered neurotransmitter vesicle release. Ocular and neurodevelopmental defects and functional deficits might result either from abnormal neurotransmitter vesicular transport and exocytosis and/or from abnormal neurotrophic vesicle release during human development. In total, we analyzed 26 families with Warburg micro syndrome and two families with Martsolf syndrome for mutations in RAB3GAP1 and RAB3GAP2. In 18 of 26 families with Warburg micro syndrome, we identified RAB3GAP1 mutations but no RAB3GAP2 mutations, which confirms that Warburg micro syndrome is a heterogeneous condition. However, a homozygous RAB3GAP2 missense mutation that resulted in aberrant splicing was identified in one family with Martsolf syndrome (three siblings), but no RAB3GAP1 or RAB3GAP2 mutations were found in our other Martsolf kindred (Aligianis et al. 2005). The RAB3GAP2-mutation phenotype was milder than that seen with RAB3GAP1 mutations in Warburg micro syndrome. Martsolf syndrome was reported in 1978 (Martsolf et al. 1978) in two brothers of Polish-Jewish origin with severe mental retardation, cataracts, short stature, primary hypogonadism, and minor digital and cephalic abnormalities. Since then, there have been several case reports of children with congenital cataracts, mental retardation, and hypogonadism to which the eponym Martsolf syndrome has been attached. These have confirmed autosomal recessive inheritance and further delineated the condition’s clinical features (table 2) (Sanchez et al. 1985; Hennekam et al. 1988; Strisciuglio et al. 1988; Harbord et al. 1989). The minor features that have been described as being part of the condition can include brachycephaly, lax finger joints, talipes valgus, a pouting mouth, maxillary retrusion, and slight hirsutism. Although facial dysmorphisms have been described, these are subtle, and Harbord et al. (1989) described a family with Martsolf syndrome that did not have any distinctive dysmorphic features (members were microcephalic, with small jaws and slight hirsutism). Martsolf syndrome has many features in common with Warburg micro syndrome, but the ocular and neurodevelopmental defects are less severe in Martsolf syndrome. At present, it is unclear whether the milder phenotype observed in our family with an RAB3GAP2 mutation (compared with that observed for RAB3GAP1 mutations causing Warburg micro syndrome) is because either (1) the p130 subunit is more critical than the p150 subunit for Rab3GAP function or (2) the “leaky” nature of the splicing defect caused by the RAB3GAP2 mutation allowed some normal protein to be produced and so ameliorated the clinical phenotype.
Table 2.
A Comparison of the Clinical Features of Micro Syndrome, the Reported Martsolf Cases, and the Family Described in the Present Report[Note]
| Characteristic | Micro Syndrome | Hennekam | Strisciuglio | Sanchez | Harbord | Martsolf | Current Familya |
| Ethnicity | Non-Jewish | Sephardic Jews | Pakistani | Polish Jews | Pakistani | ||
| Consanguinity | No | Status unknown | Yes | Yes | Yes | ||
| No. of affected individuals | 2 | 2 | 2 | ||||
| Sex and last-reported age | M, 12 years; F, 17 years | M, 13 mo | M, 9 years; M, 15 d | F, 9 mo; M, 9 mo | M, 28 years; M, 25 years | M, 11 years; F, 6 years | |
| Intrauterine growth retardation | No | Yes | No | No | No | No | No |
| Postnatal growth retardation | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| OFC at birth or percentile | ⩽50th | 25th | 35 cm; 33 cm | 34 cm | 34 cm | ||
| Postnatal microcephaly | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Seizures | Variable | No | No | No | Yes, 1 of 2b | No | |
| Developmental delay | Very severe | Moderate | Severe | Severe | Yes | Severe | |
| Truncal hypotonia | Yes | Yes | Yes | ||||
| Limb spasticity and spastic cerebral palsy | Yes | Yes | Yes | Increased reflexes and extensor plantar responses | Yes | ||
| Speech | None | Yes | Some | 4 years and 7 years | 3 years | ||
| Walking | Very rare | Yes | 4 years | 3 years | |||
| Eye signs | Microcornea, microphthalmia, congenital cataracts, optic atrophy, atonic pinpoint pupils | Bilateral posterior cortical cataracts at ages 9 and 10 years; normal fundi | Congenital cataracts | Congenital cataracts, punctiform pupils due to iridocrystalline adherences | Fundi normal; bilateral cataracts at ages 6 and 8 mo | Bilateral lacy lenticular cataracts at ages 14 and 12 years, fundi normal | Congenital cataracts? Microphthalmia |
| Electrophysiology | Normal electroretinogram, absent visual evoked potential | Normal electroretinogram; failed visual evoked potential | Abnormal electroretinogram | ||||
| Vision | Light perception only | Decreased | Visual attention after cataract surgery | ||||
| Ptosis | Yes | ||||||
| Deep-set eyes | Yes | ||||||
| Brachycephaly | Yes; some also have plagiocephaly | Yes | Yes | Brachiplagiocephaly | Yes | Yes | |
| Face | Beaked nose/prominent root of nose | Mild hypoplastic maxilla | Low nasal bridge, mild micrognathia, low posterior hairline | High forehead, flat superciliary ridges, micrognathia; when older, flat maxilla and prognathism | No distinct dysmorphic features, no maxillary retrusion or pouting lips | Maxilla hypoplastic, philtrum short, low posterior hairline | No distinct dysmorphic features, no maxillary retrusion or pouting lips |
| Mouth | Malaligned teeth | High, arched palate | High, arched palate | Malaligned teeth, pouting lower lip | |||
| Hirsutism | Some | Mild | Yes | No | Yes | ||
| Low frontal hairline | Yes | Yes | |||||
| Genital abnormalities | When examined, features have included labial and clitoral hypoplasia (female); micropenis and some cryptorchidism, hypospadius, and bifid scrotum (male) | Male bilateral cryptorchidism, micropenis; female, none noted | Male bilateral cryptorchidism but normal-length penis | Male cryptorchidism, micropenis | Female normal; male undescended testes | Male small testes | Male micropenis, cryptorchidism; female, none noted |
| Ears | Large anteverted ears | Posteriorly rotated | Posteriorly rotated, low set with prominent antihelices | Large | Large | ||
| Lax finger joints | Yes | No | Yes | ||||
| Brain imaging | Normal in some cases, variable hypoplasia, agenesis of the corpus callosum, mild generalized cerebral atrophy, cortical gyral abnormalities, pachygyria, polymicrogyria | Normal MRI in female | Microencephaly with cortical and central atrophy and slightly dilated lateral ventricles; brain biopsy showed normal structure | Generalized cerebral and brainstem atrophy and delayed myelination | Moderate cerebral atrophy | Normal CT scan | |
| Nerve-conduction studies | Can have sensorimotor neuropathy | Failed | |||||
| Muscle biopsy | Normal | ||||||
| Cardiac | Normal | Normal | Cardiomyopathy, cardiac failure | ||||
| Other abnormalities | Short palms, club foot 1 of 2b | Pectus excavatum | Pectus excavatum, thoracic scoliosis, sparse hair | Short palms, talipes valgus, short ulnae |
Note.— Only subjects IV-1 and IV-3 are included, since their brother’s (IV-2) learning difficulties are attributable to his cardiac arrest and subsequent severe hypoxic ischemic encephalopathy.
The family described in the present study.
One of the two children included in the study.
Our findings have (1) demonstrated ocular and CNS expression specificity for RABGAP2, (2) demonstrated genetic heterogeneity in Martsolf syndrome phenotypes, (3) linked Warburg micro syndrome and Martsolf syndrome phenotypes such that novel genes for one disorder may be considered as candidate genes for the other, and (4) expanded the phenotypic spectrum of Rab3GAP dysfunction. Further mutation analysis of RAB3GAP1 and RAB3GAP2 in neurodevelopmental disorders associated with Warburg micro/Martsolf syndrome–like ocular and gonadal defects will provide insights into the phenotypic consequences of Rab3GAP dysfunction in human disease.
Acknowledgments
We thank the U.K. Birth Defects Foundation, the Medical Research Council, and the Wellcome Trust for funding. We are most grateful to the families who helped with this research. All participants gave informed consent, and the research was approved by the South Birmingham Research Ethics Committee.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- Ensembl, http://www.ensembl.org/ (for human RAB3GAP1 [accession number ENSG00000115839]) and RAB3GAP2 [accession number ENSG00000118873)
- Genbank, http://www.ncbi.nlm.nih.gov/Genbank/ (for human RAB3GAP1 [accession number D31886], human RAB3GAP2 noncatalytic subunit [accession number AF004828], rab3gap1 [accession number AI629291], and rab3gap2 [accession number CF348222])
- Online Mendelian Inheritance in Man (OMIM), http://ncbi.nlm.nih.gov/Omim/ (for Warburg micro syndrome and Martsolf syndrome)
References
- Ainsworth JR, Morton JE, Good P, Woods CG, George ND, Shield JP, Bradbury J, Henderson MJ, Chhina J (2001) Micro syndrome in Muslim Pakistan children. Ophthalmology 108:491–497 10.1016/S0161-6420(00)00540-6 [DOI] [PubMed] [Google Scholar]
- Aligianis IA, Forshew T, Johnson S, Michaelides M, Johnson CA, Trembath RC, Hunt DM, Moore AT, Maher ER (2002) Mapping of a novel locus for achromatopsia (ACHM4) to 1p and identification of a germline mutation in the α subunit of cone transducin (GNAT2). J Med Genet 39:656–660 10.1136/jmg.39.9.656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aligianis IA, Johnson CA, Gissen P, Chen D, Hampshire D, Hoffmann K, Maina EN, et al (2005) Mutations of the catalytic subunit of RAB3GAP cause Warburg Micro syndrome. Nat Genet 37:221–223 10.1038/ng1517 [DOI] [PubMed] [Google Scholar]
- Costagli A, Kapsimali M, Wilson SW, Mione M (2002) Conserved and divergent patterns of Reelin expression in the zebrafish central nervous system. J Comp Neurol 450:73–93 10.1002/cne.10292 [DOI] [PubMed] [Google Scholar]
- Derbent M, Agras PI, Gedik S, Oto S, Alehan F, Saatci U (2004) Congenital cataract, microphthalmia, hypoplasia of corpus callosum and hypogenitalism: report and review of Micro syndrome. Am J Med Genet A 128:232–234 10.1002/ajmg.a.30109 [DOI] [PubMed] [Google Scholar]
- Fukui K, Sasaki T, Imazumi K, Matsuura Y, Nakanishi H, Takai Y (1997) Isolation and characterization of a GTPase activating protein specific for the Rab3 subfamily of small G proteins. J Biol Chem 272:4655–4658 10.1074/jbc.272.8.4655 [DOI] [PubMed] [Google Scholar]
- Graham JM Jr, Hennekam R, Dobyns WB, Roeder E, Busch D (2004) MICRO syndrome: an entity distinct from COFS syndrome. Am J Med Genet A 128:235–245 10.1002/ajmg.a.30060 [DOI] [PubMed] [Google Scholar]
- Harbord MG, Baraitser M, Wilson J (1989) Microcephaly, mental retardation, cataracts, and hypogonadism in sibs: Martsolf’s syndrome. J Med Genet 26:397–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekam RC, van de Meeberg AG, van Doorne JM, Dijkstra PF, Bijlsma JB (1988) Martsolf syndrome in a brother and sister: clinical features and pattern of inheritance. Eur J Pediatr 147:539–543 10.1007/BF00441986 [DOI] [PubMed] [Google Scholar]
- Li L, Chin LS (2003) The molecular machinery of synaptic vesicle exocytosis. Cell Mol Life Sci 60:942–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martsolf JT, Hunter AG, Haworth JC (1978) Severe mental retardation, cataracts, short stature, and primary hypogonadism in two brothers. Am J Med Genet 1:291–299 10.1002/ajmg.1320010305 [DOI] [PubMed] [Google Scholar]
- Megarbane A, Choueiri R, Bleik J, Mezzina M, Caillaud C (1999) Microcephaly, microphthalmia, congenital cataract, optic atrophy, short stature, hypotonia, severe psychomotor retardation, and cerebral malformations: a second family with micro syndrome or a new syndrome? J Med Genet 36:637–640 [PMC free article] [PubMed] [Google Scholar]
- Nagano F, Sasaki T, Fukui K, Asakura T, Imazumi K, Takai Y (1998) Molecular cloning and characterization of the noncatalytic subunit of the Rab3 subfamily-specific GTPase-activating protein. J Biol Chem 273:24781–24785 10.1074/jbc.273.38.24781 [DOI] [PubMed] [Google Scholar]
- Nassogne MC, Henrot B, Saint-Martin C, Kadhim H, Dobyns WB, Sebire G (2000) Polymicrogyria and motor neuropathy in micro syndrome. Neuropediatrics 31:218–221 10.1055/s-2000-7463 [DOI] [PubMed] [Google Scholar]
- Rodriguez Criado G, Rufo M, Gomez de Terreros I (1999) A second family with micro syndrome. Clin Dysmorphol 8:241–245 [PubMed] [Google Scholar]
- Sanchez JM, Barreiro C, Freilij H (1985) Two brothers with Martsolf’s syndrome. J Med Genet 22:308–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter OM, Khvotchev M, Jahn R, Sudhof TC (2002) Localization versus function of Rab3 proteins: evidence for a common regulatory role in controlling fusion J Biol Chem 277:40919–40929 [DOI] [PubMed] [Google Scholar]
- Strisciuglio P, Costabile M, Esposito M, Di Maio S (1988) Martsolf’s syndrome in a non-Jewish boy. J Med Genet 25:267–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC (2004) The synaptic vesicle cycle. Annu Rev Neurosci 27:509–547 10.1146/annurev.neuro.26.041002.131412 [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Matozaki T (2001) Small GTP-binding proteins. Physiol Rev 81:153–208 [DOI] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Shirataki H, Nakanishi H (1996) Rab3A small GTP-binding protein in Ca(2+)-dependent exocytosis. Genes Cells 1:615–632 10.1046/j.1365-2443.1996.00257.x [DOI] [PubMed] [Google Scholar]
- Tasaka K, Masumoto N, Mizuki J, Ikebuchi Y, Ohmichi M, Kurachi H, Miyake A, Murata Y (1998) Rab3B is essential for GnRH-induced gonadotrophin release from anterior pituitary cells. J Endocrinol 157:267–274 10.1677/joe.0.1570267 [DOI] [PubMed] [Google Scholar]
- Wada M, Nakanishi H, Satoh A, Hirano H, Obaishi H, Matsuura Y, Takai Y (1997) Isolation and characterization of a GDP/GTP exchange protein specific for the Rab3 subfamily small G proteins. J Biol Chem 272:3875–3878 10.1074/jbc.272.7.3875 [DOI] [PubMed] [Google Scholar]
- Warburg M, Sjo O, Fledelius HC, Pedersen SA (1993) Autosomal recessive microcephaly, microcornea, congenital cataract, mental retardation, optic atrophy, and hypogenitalism: micro syndrome. Am J Dis Child 147:1309–1312 [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2:107–117 10.1038/35052055 [DOI] [PubMed] [Google Scholar]