Abstract
Recent studies have shown that accessory proteins that interact with the apical Na+/H+ exchanger NHE3 are a vital part of the dynamic nature of the Na+/H+ exchanger regulation. We have identified MAST205, a microtubule-associated serine/threonine kinase with a molecular mass of 205 kDa that interacts with NHE3. MAST205 contains a S/T kinase domain and a PDZ domain that mediates interaction with NHE3. Northern blot analysis showed that MAST205 is highly expressed in human and rat kidney. Expression in opossum kidney (OK) cells showed that MAST205 is predominantly expressed in the apical membrane of the cells. Immunohistochemical studies demonstrated the presence of MAST205 at the apical region of the renal proximal tubules. Heterologous expression of MAST205 in OK cells inhibited endogenous NHE3 activity, and this inhibition required the presence of the kinase domain of MAST205, since deletion of the kinase domain or a dominant-negative mutant of MAST205 did not affect the activity of NHE3. Consistent with these results, we found that MAST205 phosphorylated NHE3 under in vitro conditions. However, overexpression of MAST205 did not affect expression of NHE3 proteins, suggesting that the effect of MAST205 was not mediated by a decrease in NHE3 expression. These findings suggest that MAST205 regulates NHE3 activity and, although the precise mechanism is yet to be determined, MAST205 appears to inhibit NHE3 activity through a phosphorylation-dependent mechanism.
Keywords: sodium/hydrogen exchange, kinase
the mammalian proximal tubules reabsorb the majority of the filtered NaCl and HCO3− (19, 20). The Na+/H+ exchanger NHE3 localized in the brush-border membrane of the renal proximal tubule is the key protein that mediates transcellular reabsorption of Na+ and HCO3− in the kidney. A wide range of stimuli have been identified as regulators of NHE3, including endothelin, dopamine, and parathyroid hormone (20). In recent years, a number of studies identified accessory proteins that interact with NHE3 to either directly or indirectly regulate the activity of NHE3. These include Na+/H+ exchanger regulatory factors, NHERF1 and NHERF2, the scavenger receptor megalin, dipeptidyl peptidase IV, and calcinurin homologous protein (4, 8, 9, 24, 36). In addition, pharmacological disruption of actin cytoskeleton severely inhibits Na+/H+ exchange activity, implying dynamic interaction of NHE3 with the actin cytoskeleton (26).
There has been much interest in the regulation of transport proteins and membrane receptors by the proteins containing one or more PDZ (PSD-95/Dlg/ZO-1) domains. The PDZ domain-containing proteins play important regulatory roles, including ion transport, recycling of membrane proteins, targeting of proteins to the surface membrane, receptor-mediated signaling, and maintenance of the epithelial cell barrier (6, 10, 18, 27, 34). Previous studies have shown that NHERF1 and NHERF2 regulate NHE3 activity by PDZ-mediated interaction that assembles multiple signaling proteins (13, 34, 35). The genetic deletion of NHERF1 promotes internalization of sodium phosphate cotransporter, demonstrating a role of PDZ proteins in the apical targeting of integral proteins (25).
We have previously utilized a yeast two-hybrid system to screen a WI-38 human lung fibroblast library using the entire COOH terminus of NHE3 as bait (11, 36). One of the clones that interacted with NHE3 exhibited ~90% identity to mouse microtubule-associated serine/threonine kinase with a molecular mass of 205 kDa (MAST205; see Ref. 29). In this study, we report the initial characterization of regulation of NHE3 by MAST205. We show that overexpression of MAST205 in opossum kidney (OK) cells inhibited NHE3 activity, and this inhibition requires the presence of the kinase domain, raising the possibility of phosphorylation-dependent regulation of NHE3.
METHODS AND MATERIALS
Cell culture
OK cells were cultured in a 1:1 mixture of DMEM and Ham’s F-12 supplemented with 10% FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin at 37°C in a 95% air-5% CO2 atmosphere. PS120/NHE3V fibroblasts, PS120 fibroblasts stably expressing rabbit NHE3 with an antibody epitope derived from vesicular stomatitis virus glycoprotein (VSVG) fused at the COOH terminus, were grown in DMEM supplemented with 10% FBS, 100 μg/ml streptomycin, 100 U/ml penicillin, and 400 μg/ml G418 (36).
Yeast two-hybrid system
Yeast two-hybrid screen of a human library using cDNA encoding the entire cytoplasmic tail (CT) of NHE3 (amino acids 475–832) was previously described (36). Among 15 clones isolated from the initial screen, W78 and W98 were identical clones of ~2.4 kb in length in pJG4–5 and showed 90% identity in nucleotide sequences to mouse MAST205. W78 and W98 were rescued from the yeast colonies and were cotransformed into EGY48 with pEG202 harboring NHE3 CT (pEG:C3) or NHE1 CT (pEG:C1) to reconfirm the specificity of the interaction (11). The pEG:C1 was kindly provided by Dr. Jacques Pouyssegur at the University of Nice. Positive interaction was indicated by auxotrophic growth of yeast on a minimal plate lacking uracil, histidine, and leucine (11).
Northern blot analysis
The rat Multiple Tissue Northern blot and the human Multiple Tissue Expression (MTE) blot purchased from BD Bioscience were hybridized with the MAST205 probe using ExpressHyb solution according to the recommendation by the manufacturer. The EcoRI-XbaI fragment of mouse MAST205 was labeled with [α-32P]dATP by nick translation.
Western immunoblotting
Cells were rinsed three times with ice-cold PBS buffer and lysed by sonication in lysis buffer composed of 50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, 1% Triton X-100, and protease inhibitors (one complete Mini EDTA-free protease inhibitor cocktail tablet/10 ml; Roche Applied Science). The lysates were cleared by centrifugation at 14,000 g at 4°C for 10 min. Rat kidney membranes were prepared from Sprague-Dawley rats. This study was approved by the Institutional Animal Care and Use Committee at Emory University. Renal tissues were homogenized (1 g/10 ml) in 10 mM HEPES, pH 7.5, 250 mM sucrose, 2 mM EDTA, and protease inhibitors using a Polytron at a setting of five with three 10-s bursts (Brinkmann Instruments). Homogenate was spun at 750 g for 30 min at 4°C to separate cell debris. Supernatant was centrifuged at 100,000 g for 1 h at 4°C. The final pellet containing crude membranes was resuspended in the same buffer. Protein concentration was determined by the bicinchoninic acid assay (Sigma). The equal amount of lysate in 2 × sample buffer (125 mM Tris · HCl, pH 6.8, 2% SDS, 20% glycerol, and 0.6 M β-mercaptoethanol) was resolved by 6% SDS-PAGE, and Western immunoblotting was performed as previously described (34). Ab3927 was used at 1:3,000 dilution. The monoclonal anti-opposum NHE3 antibody, 3H3, was kindly provided by Dr. Daniel Biemesderfer at Yale University. The polyclonal antibody against NHERF1 was previously described (16).
Preparation of an antiserum against MAST205
cDNA corresponding to amino acids 721–970 of mouse MAST205 was amplified by PCR using AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA) and the paired primers [5′ -GGATCCAGAGCCTGTTGCCAAT GG-3′ and 5′ -GCTCAGAGGTTGCTGAA(A/C)AG(C/T)TC-3′ ]. The PCR product was then cloned into pET30 (Novagen, SanDiego, CA), expressed as hexahistidine (His6)-tagged fusion proteins in Escherichia coli, and affinity purified with Ni2+-nitrilotriacetic acid (Ni-NTA) resin, as suggested by the manufacturer (Qiagen). The anti-MAST antiserum against the recombinant protein was produced by immunizing New Zealand White rabbits by Covance Research Products (Denver, PA). This polyclonal antiserum is referred to as Ab3927. Ab3927 was then affinity purified by using Affi-Gel 10 (Bio-Rad), activated N-hydroxysuccinimide esters cross-linked to agarose gel bead support, as previously described (12).
Confocal microscopy
Transfected cells were fixed in 3% paraformaldehyde and permeabilized in 0.2% Triton X-100. Cells were blocked in 15% goat serum-PBS-1% BSA and incubated with the anti-MAST205 antibody for 1 h at room temperature (1:1,000 dilution), followed by Alexa Fluor 488-conjugated secondary antibody and Alexa Fluor 568-phalloidin (Molecular Probes). The monolayers were mounted with Prolong Antifade and examined using an LSM510 confocal microscope (Carl Zeiss Microimaging).
Immunohistochemistry
Kidneys from male Sprague-Dawley rats were perfused with 4% paraformaldehyde, dissected, and incubated in the same solution. Tissues were embedded in paraffin blocks and cut into 0.5- to 0.8-μm sections. Sections were deparaffinized with xylene two times for 8 min each and hydrated in a series of (100–0%) ethanol. Antigen unmasking was performed through microwave treatment in a citrate buffer (pH 6.0), and endogenous peroxidase was quenched with 0.3% hydrogen peroxide for 30 min. Subsequent washes were performed in PBT buffer (140 mM NaCl, 15 mM phosphate buffer, pH 7.3, and 0.1% Tween 20) three times for 5 min each. Nonspecific blocking was obtained by incubation in PBS with 10% normal goat serum for 30 min at 37°C. Adjacent sections were then incubated overnight at 4°C with the primary antibody Ab3927 (1:500 dilution) or the antibody K1A (1:500) that recognizes the electrogenic Na-HCO3 cotransporter (NBCe1; see Ref. 3). After washes with PBT buffer, tissues were treated with biotinylated anti-rabbit secondary antibody using the Vectastain Elite ABC kit (Vector Laboratories) for 1 h at room temperature. Sections were washed and incubated in a peroxidase solution using the Aminoethyl Carbazole Substrate Kit (Zymed Laboratories). The sections were stained with a hematoxylin and mounted with glycerol.
Plasmid constructs
The plasmid constructs of MAST205 in pcDNAI-neo have been described previously (29). These include pcDNAI-neo harboring the entire MAST205 (MAST), the NH2 terminus (5B: amino acids 1–411), and the PDZ and kinase domains together (BP: amino acids 412–1455; Fig. 1). MΔ5 was generated by subcloning the 4,025-bp BamHI-XbaI fragment (amino acids 412–1734) into pcDNA3.1HisB. PC was made by subcloning the 2,420-bp NheI-XbaI fragment (amino acids 947–1734) into pcDNA3.1HisB. The kinase-dead Δ5 (KMΔ5) was generated by mutation of 482Lys of MΔ5 to Met using the QuickChange site-directed mutagenesis kit according to the recommendation by the manufacturer (Stratagene). The presence of the K482M mutation was confirmed by nucleotide sequencing.
Fig. 1.
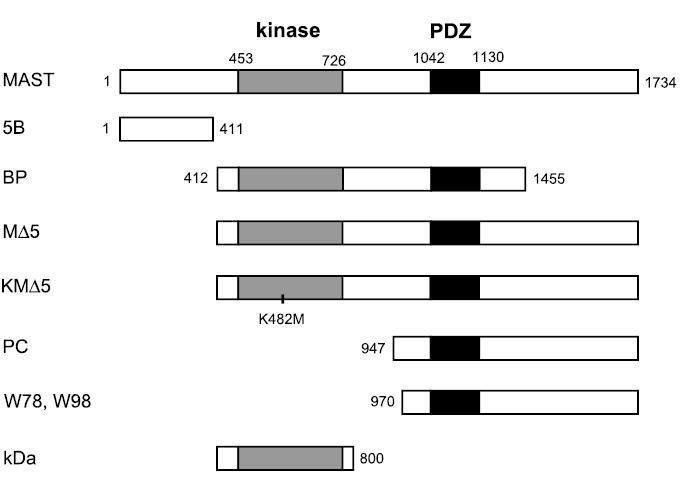
Microtubule-associated serine/threonine kinase with a molecular mass of 205 kDa (MAST205) and its derivatives used in the study. MAST, 5B, and BP are in pcDNA1-neo, whereas MΔ5, KMΔ5, and PC are cloned in pcDNA3.1-HisB. KD is cloned in pET30. The numeric values indicate the amino acid numbers. W78 and W98 are the partial human MAST205 genes identified by yeast two-hybrid screen in pJG4–5. The location of the K482M mutation in KMΔ5 is marked. See text for definitions.
In vitro interaction
The COOH-terminal domain of NHE3 (NHE3C: amino acids 475–832) cloned in pET16b (34) was expressed and radiolabeled with 20 μCi [35S]Met (Amersham) using the TNT in vitro Transcription-Translation System according to the manufacturer (Promega). The [35S]Met-labeled NHE3C protein was purified by immobilizing on Ni-NTA resins. The MAST205 constructs in pcDNAI-neo were similarly labeled with [35S]Met. The labeled MAST constructs (20 μl) were incubated with the immobilized NHE3C for 2 h at 4°C in 10 mM Tris, pH 7.2, 150 mM NaCl, and 0.2% Tween 20. At the end of incubation period, the Ni beads were washed three times with the same buffer, bound proteins were separated by 6% SDS-PAGE, and the gel was dried. Autoradiography was carried out using the Typhoon phosphoimager (Amersham).
In vivo interaction
OK cells grown to 80% confluence in 10-cm culture dishes were transfected with MΔ5 or PC cloned in pcDNA3.1-HisB using Lipofectamine2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s recommendation. As a control, cells were transfected with the vector alone. Posttransfection (48 h), cell lysate was prepared in lysis buffer as described above. An equal amount (500 μg) of lysate was incubated with Ni-NTA beads for 2 h at 4°C with constant rotation. At the end of incubation period, the Ni-NTA beads were washed three times with lysis buffer, and bound proteins were separated by SDS-PAGE. The presence of copurified NHE3 was detected by Western blot using the 3H3 antibody.
Na+-dependent intracellular pH recovery
The Na+-dependent changes in intracellular pH (pHi) by NHE3 were determined using the ratiofluorometric, pH-sensitive dye 2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein AM (BCECF-AM; Molecular Probe), as previously described (16). Briefly, 80% confluent cells on cover slips were transfected with different constructs of MAST205 or vector as a control. Forty-eight hours later, cells were loaded with 6.5 μM BCECF-AM, and the cover slips were mounted on a perfusion chamber mounted on an inverted microscope and superfused with NH4 buffer (40 mM NH4Cl, 90 mM NaCl, 20 mM HEPES, 5 mM KCl, 1 mM TMA-PO4, 2 mM CaCl2, 1 mM MgSO4, and 18 mM glucose) and subsequently with TMA buffer (130 mM TMA-Cl, 20 mM HEPES, 5 mM KCl, 1 mM TMA-PO4, 2 mM CaCl2, 1 mM MgSO4, and 18 mM glucose). Na buffer was then reintroduced to drive Na-dependent pH recovery. Calibration of the fluorescence signal was performed using the K+/H+ ionophore nigericin as described previously (16). The microfluorometry was performed on a Nikon TE200 inverted microscope with a Nikon CFI Super Fluor ×40 objective, coupled to a Lambda 10-2 filter wheel controller equipped with a multiwavelength filter set designed for BCECF. Photometric data were acquired using the Metafluor software (Universal Imaging). Na+/H+ exchange rate was described by the rate of pHi recovery, which was calculated by determining slopes along the pHi recovery by linear least-square analysis over a minimum of 9 s.
Surface biotinylation
Surface biotinylation of NHE3 was performed as previously described (2, 13). Briefly, cells grown in 10-cm petri dishes were rinsed three times in PBS followed by borate buffer (154 mM NaCl, 7.2 mM KCl, 1.8 mM CaCl2, and 10 mM H3BO3, pH 9.0). Hereafter, all procedures were performed at 4°C. Cells were then incubated for 40 min with 1.5 mg NHS-SS-biotin (Pierce) in borate buffer. Unbound NHS-SS-biotin was quenched with Tris buffer (20 mM Tris, pH 7.4, and 120 mM NaCl). Cells were then rinsed with PBS, scraped, solubilized in 1 ml of the lysis buffer described above, and sonicated for 20 s. The lysate was agitated for 30 min and spun to remove insoluble cell debris. An aliquot was retained as the total fraction representing the total cellular NHE3 protein. The equal amounts of supernatant (500 μg) were incubated with streptavidin-agarose beads (Pierce) for 1 h. The beads were washed at least three times with lysis buffer and one time with Tris buffer. Immobilized biotinylated proteins, representing the surface fraction, were separated by boiling in 5 × sample buffer. The total and surface fractions were resolved by SDS-PAGE, and Western blotting was performed using the 3H3 antibody. NHE3 protein abundance was quantified by densitometry.
In vitro phosphorylation assay
The kinase domain (KD) of MAST205 was subcloned in pET30a. KD was expressed as His-tagged protein and purified from E. coli using Ni2+ -agarose resins. PS120/NHE3V fibroblasts grown in 10-cm petri dishes were serum deprived overnight and lysed in lysis buffer supplemented with phosphatase inhibitors (in mM: 1 EGTA, 1 EDTA, 1 sodium orthovanadate, 10 sodium fluoride, 10 sodium pyrophosphate, and 25 β-glycerophosphate), as described above. NHE3V was immunoprecipitated using the monoclonal antibody P5D4 against the VSVG tag (14). The immobilized NHE3 was incubated with 0.2 μg of the purified KD for 15 min at 30°C in 20 mM HEPES, pH 7.5, 10 mM MgCl2, 100 μM ATP, 1 μM dithiothreitol, and 10 μCi [γ-32P]ATP. The samples were then resolved by SDS-PAGE, and phosphorylation of NHE3 was analyzed by autoradiography.
Statistics
Densitometric analyses were performed on the Typhoon phosphoimager using the Image Quant program. Statistical significance was assessed by ANOVA. Results were considered statistically significant at P < 0.05.
RESULTS
Interaction of MAST205 with NHE3
A yeast two-hybrid screen using the COOH-terminus of NHE3 was previously described (36). Among the genes isolated from this screen were two identical clones, W78 and W98, that were ~2,400 bp in length. These clones showed ~90% identity in nucleotide sequence to the mouse MAST205 gene, which was previously cloned as a protein interacting with microtubules (29). Mouse MAST205 is 1,734 amino acid residues long and consists of a Ser/Thr protein kinase domain and a PDZ domain. W78 and W98 contain the PDZ domain and extend to the COOH terminus of MAST205 (amino acids 970–1734), but lack the kinase domain (Fig. 1). Figure 2A shows the interaction between NHE3 and W78. Coexpression of pEG:C3, the DNA-binding fusion plasmid pEG202 harboring CT of NHE3, and the clone W78 resulted in auxotropic growth of yeast on a minimal plate lacking uracil, histidine, and leucine. In comparison, CT of human NHE1, pEG:C1, did not rescue the yeast, indicating that the interaction of W78 with Na+/H+ exchangers is isoform specific. As controls, pEG:C3 plus pJG4-5 or W78 plus pEG202 did not exhibit autoactivation, as evident by the lack of auxotrophic growth.
Fig. 2.
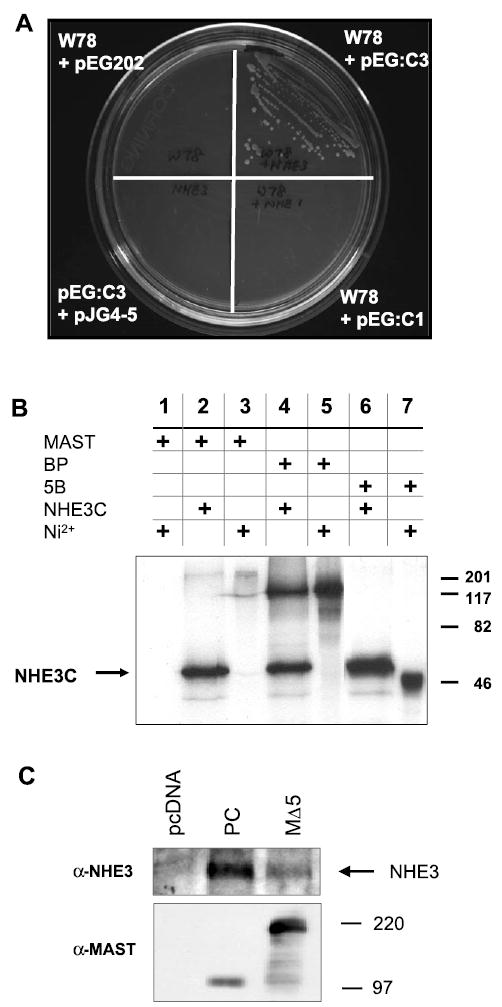
MAST205 interacts with Na+/H+ exchanger (NHE) 3. A: interaction of W78 with NHE3C in yeast. Yeast strain EGY48 was transformed with W78 with or without pEG:C3 or pEG;C1. EGY48 grew on a minimal plate lacking uracil, histidine, and leucine only in the presence of both W78 and pEG:C3 but not in the presence of pEG:C1 and W78. W78 + pEG202 or pEG:C3 + pJG4–5 did not revive the yeast. B: in vitro interaction between MAST205 and NHE3. The NHE3 COOH-terminal domain (NHE3C) was synthesized as 6xHis proteins and labeled with [35S]Met. As described in methods and materials, NHE3C immobilized on Ni2+-nitrilotriacetic acid (Ni-NTA) beads was incubated for 2 h with 20 μl [35S]Met-labeled MAST205, BP, and 5B. The protein complexes were eluded by boiling in 2 × sample buffer, resolved by SDS-PAGE, and visualized by autoradiography. The eluded protein samples were loaded in lanes 2, 4, and 6. In lanes 3, 5, and 7, 2 μl of the in vitro products were loaded to show the sizes and the relative labeling of each construct. Lane 1 shows a negative control where [35S]Met-labeled MAST205 was incubated with Ni-NTA beads without NHE3C. Similar results were obtained in three experiments. C: opossum kidney (OK) cells were transfected with pcDNA3.1-HisB, PC, or MΔ5. Transfected cells were lysed, and the expressed MΔ5 and PC proteins were affinity purified with Ni-NTA beads. Top: the presence of copurified NHE3 was detected by Western immunoblotting using a monoclonal anti-NHE3 antibody. Bottom: Western immunoblot using anti-MAST205 antiserum on lysates from transfected cells. Similar results were obtained in three experiments.
To corroborate the above results outside the context of yeast, we initially sought to express the W78 clone and mouse MAST205 as recombinant proteins in bacteria, but the yield was too low for any reliable testing. As an alternative, we utilized in vitro transcription-translation. The full-length mouse MAST205 (MAST), the kinase and PDZ domain together (BP), and the NH2-terminal domain (5B) of MAST205 (Fig. 1) were synthesized and labeled with [35S]Met by in vitro transcription-translation. Concomitantly, the entire CT of NHE3 (NHE3C) was also synthesized in vitro and purified by its affinity to Ni-NTA beads. The immobilized [35S]NHE3C was then incubated with the MAST205 constructs. Figure 2B shows that NHE3C interacted with MAST (lane 2) and BP (lane 4), both of which contain the PDZ domain. The interaction of NHE3C with MAST205 appears weak compared with that with BP, but this is reflective of the inefficient in vitro synthesis of MAST205 (lane 3), yielding a smaller amount of MAST205. NHE3C did not interact with the NH2-terminus of MAST205, 5B (lane 6), and no interaction of MAST205 to Ni-NTA was observed (lane 1).
To further demonstrate the interaction between MAST205 and NHE3, we performed in vivo interaction assay using lysates prepared from transfected OK cells. For this analysis, MΔ5 and PC (Fig. 1) cloned in pcDNA3.1-HisB, permitting their expression as 6xHis-tagged proteins, were used to transfect OK cells. As a control, OK cells were transfected with pcDNA3.1HisB that produces a small protein of 52 amino acids with the His-tag. MΔ5 and PC were then affinity purified from the transfected cells using Ni-NTA beads. The presence of copurified NHE3 was accessed by Western immunoblotting using the monoclonal antibody 3H3 specific for OK NHE3. Figure 2C, top, shows that NHE3 copurified with MΔ5 and PC, but not in the sham-transfected cells, further demonstrating the interaction between MAST205 and NHE3. Figure 2C, bottom, is a representative Western blot using the polyclonal anti-MAST205, Ab3927, showing expression of PC and MΔ5 in transfected OK cells (the characterization of the anti-MAST205 is described later). The expression level of MΔ5 appears much higher than that of PC, and the amount of NHE3V pulled down does not readily correlate with the expression levels of MΔ5 and PC, raising a potential concern on quantification of the pulldown assay. The antigen used to generate Ab3827 spans the region between amino acids 721 and 970, which marginally overlaps with PC (amino acids 947–1734); hence, we attribute this difference to the partial recognition of the PC protein by the antibody, and the expression of PC is significantly underrepresented in the Western blot.
Expression of MAST205 mRNA in human and rat
One criterion for being an NHE3-interacting protein is its expression in the kidney and intestine where NHE3 is predominantly expressed. As previously shown, MAST205 is highly expressed in the rat testis, but its expression is not limited to the testis (29). Instead, all rat tissues, including kidney, express varying amounts of MAST205 mRNA (Fig. 3A). This broad expression of MAST205 transcripts was confirmed by using the human MTE array blot, which contains normalized quantities of tissue-specific mRNAs (Fig. 3B). In particular, MAST205 is most abundant in the kidney (7A and 11C), testis (8F), adrenal gland (9C), and the general regions of the hindbrain (1G, 1H, 2 E–H). In addition, MAST205 is expressed in the small intestine and colon at lower levels.
Fig. 3.
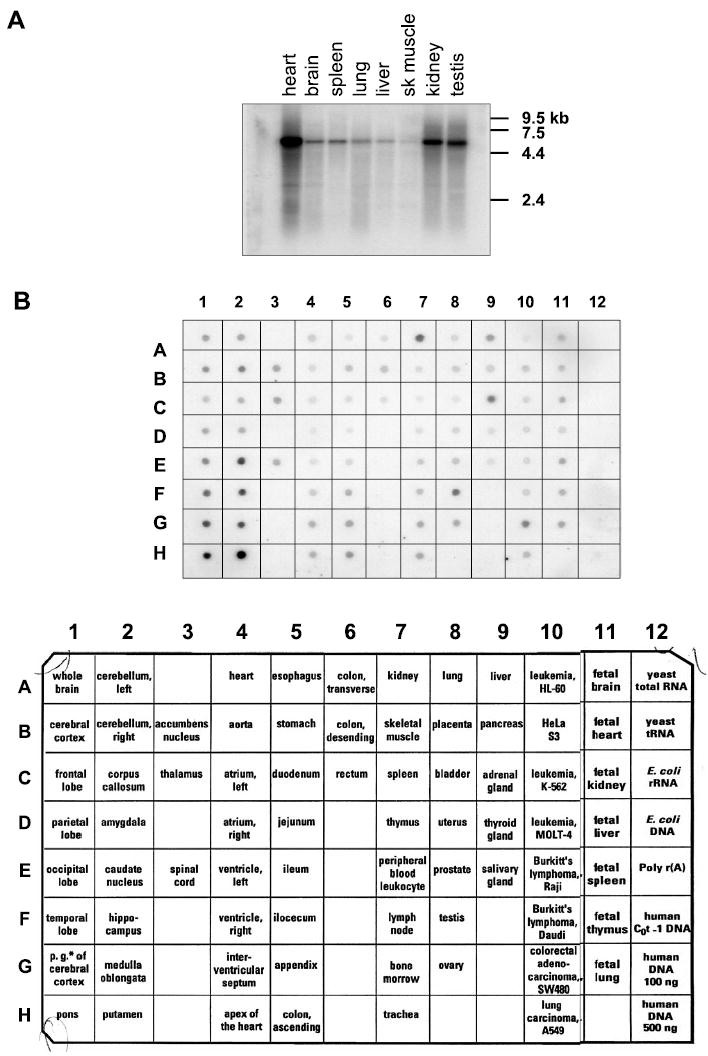
Expression of MAST205 transcript. A: Northern analysis of MAST205 mRNA on a blot containing mRNA from 8 rat tissues showing the presence of ~5.5 kb transcript in all tissues. B: multiple tissue expression (MTE) array of MAST205. Strongest signals were obtained in kidney (7A), testis (8F), adrenal gland (9C), and brain (1F–H, and 2E–H). Description of the RNA spotted on the MTE array is shown on bottom. Columns 1–11: poly(A)+RNA samples from human tissues; column 12: controls, poly(A)+RNA and DNA.
Expression of MAST205 protein
As described above, the anti-MAST205 antiserum was generated against a recombinant protein corresponding to a region flanking the kinase and PDZ domains (amino acids 721–970) of MAST205. Figure 4 shows a Western immunoblot against lysate from OK cells transfected with MAST205 or pcDNA. This antiserum strongly reacted with a protein of 220 kDa in MAST205 transfected OK cells but not in the sham-transfected cells. In addition to MAST205, a faint band of ~70 kDa is observed. The identity of this protein is not known, but this band was present in the sham-transfected control. Figure 4 also indicates that MAST205 is absent in OK cells. The lack of MAST205 protein is consistent with the inability to detect MAST205 transcript by Northern blot (data not shown).
Fig. 4.
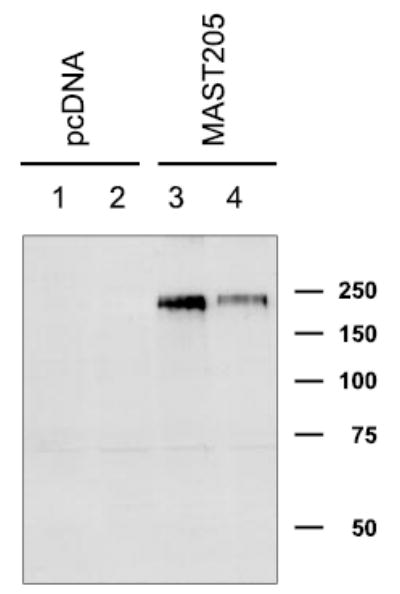
Characterization of anti-MAST205 antiserum. OK cells were transiently transfected with pcDNA3.1HisB or MAST205. A representative Western immunoblot using the anti-MAST205 antiserum (Ab3927) shows the presence of a 220-kDa protein in MAST205 (lanes 3 and 4) but not in pcDNA-transfected cells (lanes 1 and 2).
We next determined the subcellular localization of MAST205 by indirect immunofluoresence microscopy on OK cells transiently transfected with MAST205, MΔ5, or pcDNA3.1HisB. Staining of OK cells with the anti-MAST antiserum (green in Fig. 5A) revealed that MAST205 and MΔ5 were present throughout the cytosol and also at the plasma membrane. There was no appreciable signal in the sham-transfected OK cells. The vertical x–z sectional scan of the transfected cells (Fig. 5B) showed that MAST205 and MΔ5 are more abundantly expressed on the apical side of the cells than the basolateral surface.
Fig. 5.
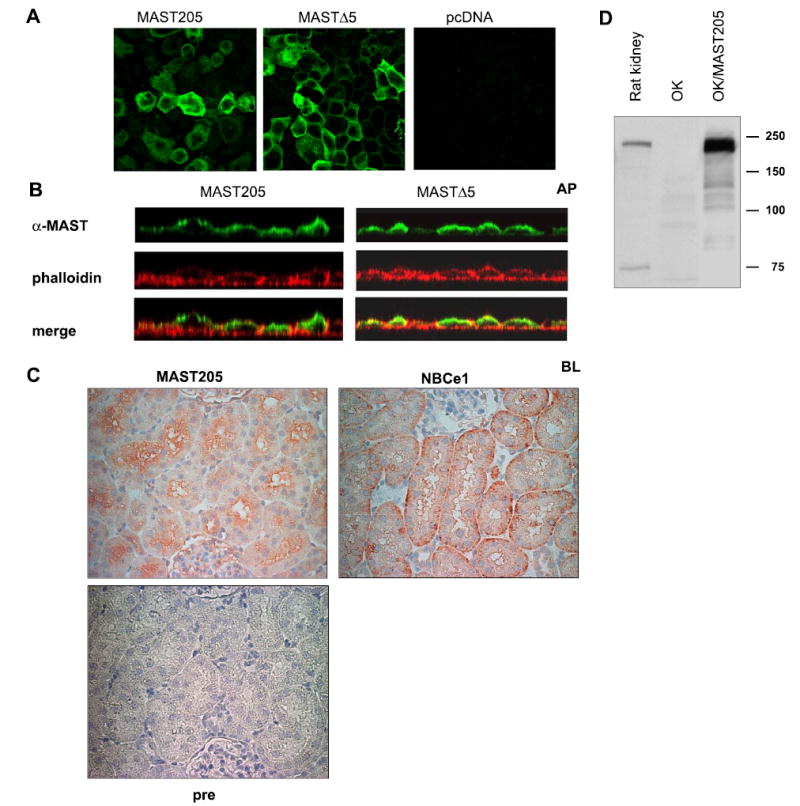
Subcellular localization of MAST205. A: monolayers of transfected OK cells were fixed and stained with anti-MAST205 antiserum followed by Alexa Fluor 488-conjugated secondary antibody (green). Focal plane views of MAST205-, MΔ5-, or pcDNA3.1HisB-transfected cells are shown. B: vertical sectional views of MAST205- or MΔ5-transfected cells are shown. Actin was labeled with phalloidin (red) to show the location of the apical (AP) and basolateral (BL) membrane. The presence of MAST205 and MΔ5 at the apical membrane is evident as yellow fluorescent signals, indicating the colocalization of MAST205 and actin. C: immunohistochemical staining of rat renal proximal tubules with the anti-MAST205, anti-NBCe1, or preimmune (pre) serum. MAST205 is expressed at the brush-border membrane. As a control, the basolateral staining of NBCe1 is shown. No staining with the preimmune serum was observed. D: Western immunoblot using the anti-MAST205 antibody on lysates prepared from rat kidney, OK, and OK cells transfected with mouse MAST205.
To ascertain whether MAST205 is expressed in the apical membrane of the nephron segment where NHE3 is known to exist, immunohistochemical staining of rat kidney was performed. In the proximal tubules, the MAST205 antibody predominantly stained the brush-border membranes (Fig. 5C) where NHE3 is located (5). As a control, the antibody against Na+/HCO3− cotransporter NBCe1 labeled the basolateral membrane of the proximal tubules. Preimmune serum did not stain the tubules. Consistent with the immunohitochemical detection of MAST205 in rat kidney, the presence of MAST205 in rat kidney was confirmed by Western immunoblotting using the anti-MAST205 antiserum (Fig. 5D).
MAST205 inhibits NHE3 activity
To determine a functional role of MAST205 in regulation of NHE3 activity, we expressed MAST205 in OK cells. Although our initial strategy was to obtain a stably transfected cell line, the expression of MAST205 was lost rapidly within two to three passages for undefined reasons. To circumvent this problem, we transiently expressed MAST205 in OK cells seeded on cover slips and determined NHE3 activity at 48 h posttransfection. As a control, cells were transfected with the vector alone. We estimated the transfection efficiency to be ~60–70% over several attempts, as determined by immunofluorescence staining (data not shown). Because we could not identify transfected cells, we reasoned that we could determine the effect of MAST205 by sampling a large number of cells. Specifically, we determined the rate of sodium-dependent pHi recovery on several pools of cells, each pool containing a minimum of seven cells. For each cover slip, at least 10 traces were collected, and a minimum of three cover slips was studied for each set of the experiment. Alternative to transient transfection, cells transfected with MAST205 were initially selected by G418, and surviving cells were reseeded on cover slips to be used in the subsequent transport assay. Both approaches gave similar results, and the expression of MAST205 in transfected cells was concomitantly tested by Western immunoblot. Figure 6A shows that expression of MAST205 resulted in a significant decrease in NHE3 activity compared with the sham-transfected cells. Similarly, expression of MΔ5 that lacked the NH2-terminal region of MAST205 inhibited the basal carrier activity of NHE3 (Fig. 6B).
Fig. 6.
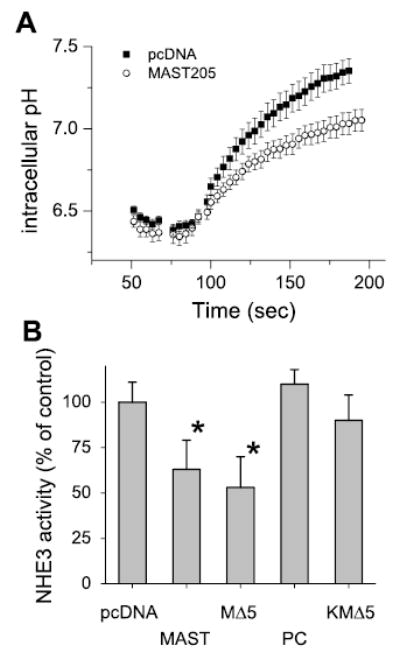
Expression of MAST205 decreased NHE3 activity in OK cells. A: OK cells were transfected with MAST205 or pcDNA3.1HisB as a control. Representative traces of Na-dependent pHi recovery are shown. Similar results obtained from 8 sets of experiments; each set consists of at least 3 cover slips studied. B: OK cells were transfected with pcDNA, MAST205, MΔ5, PC, or KMΔ5; n = 5. *P < 0.01 compared with pcDNA-transfected control. Results are presented as means (bars) and SD (error bars).
To determine whether the decrease in Na−/H−exchange activity is caused by the interaction with the PDZ domain of MAST205, we determined NHE3 activity in the cells transfected with PC that contained the PDZ and the carboxyl domains but lacked the kinase domain. In contrast to MAST205 and MΔ5, the expression of PC did not attenuate NHE3 activity despite its ability to bind to NHE3 via the PDZ domain. These results suggest that the PDZ interaction alone is not sufficient for the decreased activity of NHE3. To test whether the kinase domain of MAST205 was necessary for the observed effect, we generated a kinase-dead MΔ5 (KMΔ5) by mutating Lys at 482 to Met (K482M) (Fig. 1). K482 is the invariant lysine within the ATP-binding motif of S/T protein kinases. This mutation has previously been shown to function as a dominant negative kinase (38). Unlike MAST205 or MΔ5, the expression of KMΔ5 failed to inhibit NHE3 activity (Fig. 6B). The lack of effect by KMΔ5 on NHE3 activity was not because of the loss of interaction with NHE3, since the binding to NHE3 was not affected by the K482M mutation in KMΔ5 (data not shown). These results suggest that the inhibition of NHE3 activity by MAST205 requires the functional kinase activity of MAST205.
MAST205 phosphorylates NHE3 in vitro
Because the above data suggest the necessity of the kinase motif in the inhibition of NHE3 activity, we tested whether NHE3 was a substrate for phosphorylation by MAST205 under in vitro conditions. Our attempts to affinity purify MAST205 protein from MAST205-transfected OK lysates was unsuccessful because of a significant level of nonspecific kinase activity associated with the purification. Therefore, we engineered MAST205 in bacteria by expressing the KD as a recombinant protein. We used PS120/NHE3V fibroblasts as the source of NHE3 protein since we were less constrained by the amount of the monoclonal P5D4 available to us (36). Figure 7 shows that 87-kDa NHE3V affinity purified from PS120/NHE3V fibroblasts (lane 1) was phosphorylated by KD. The phosphorylation of NHE3V was mediated by KD since the immunoprecipitated NHE3V did not autophosphorylate in the absence of KD (lane 2). The lack of labeling was not related to an insufficient amount of immunoprecipitated NHE3V in this sample, since the Western immunoblot using anti-VSVG antibody (Fig. 7, bottom) showed the presence of comparable amounts of NHE3V in both lanes 1 and 2. The 87-kDa protein being NHE3V was further supported by the absence of the labeled band in the control immunoprecipitation from PS120 lysate (lane 3). Interestingly, MAST205 did not phosphorylate myelin basic protein (lane 4), which was incidentally used as a positive control.
Fig. 7.
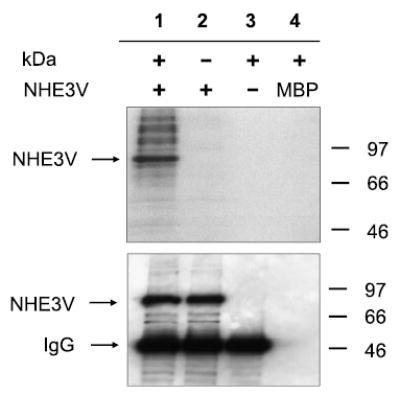
MAST205 phosphorylated NHE3 in vitro. The kinase domain (KD) of MAST205 was bacterially engineered and incubated with NHE3V immunoprecipitated from PS120/NHE3V cells. Lane 1, NHE3 + KD; lane 2, NHE3 alone; lane 3, KD + immunoprecipitants from PS120; lane 4, KD + myelin basic protein (MBP). Top: autoradiograph. Bottom: Western immunoblot using the monoclonal P5D4 against the vesicular stomatitis virus glycoprotein tag showing the presence of NHE3V in lanes 1 and 2. Similar results were obtained from three separate experiments.
MAST205 does not affect NHE3 expression
Our results thus far demonstrate that expression of MAST205 in OK cells reduces NHE3 activity. However, the mechanism underlying this inhibition remains unclear despite the phosphorylation of NHE3 by MAST205. We contemplated whether the expression of MAST205 affected NHE3 protein abundance in OK cells. To test this possibility, OK cells transfected with MΔ5, KMΔ5, PC, or the vector alone were examined for the total cellular level of NHE3. Figure 8A shows that the expression of the MAST construct did not affect the NHE3 protein abundance in transfected cells.
Fig. 8.
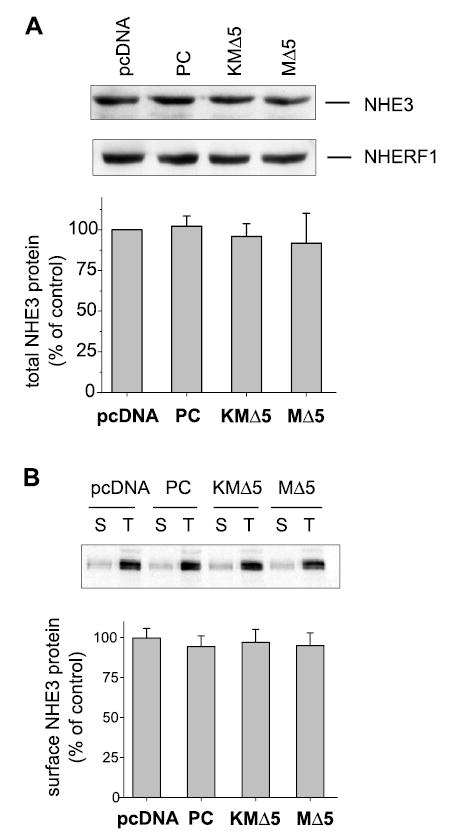
Effect of MAST205 on NHE3 protein abundance in OK cells. A: OK cells were transfected with pcDNA, PC, MΔ5, or KMΔ5. Total cellular NHE3 protein abundance (40 μg) was determined on lysates by Western immunoblot using the OK NHE3 antibody 3H3. A representative immunoblot is shown. Equal loading of the samples was tested by determining the amount of NHERF1 by Western blot. Bottom: quantification of NHE3 expression normalized to NHERF1 expression level from 6 different experiments. Results are presented as means ± SD. B: cell surface NHE3 was determined by biotin-labeled and streptavidin-precipitated NHE3 from lysates prepared from transfected OK cells. Total protein (60 μg) and surface-biotinylated proteins (all streptavidin precipitated) were loaded, and the amounts of NHE3 were determined by immunoblot. A representative immunoblot and the quantification from three sets of experiments are shown. Results are presented as means (bars) and SD (error bars); n = 3.
To determine whether MAST205 expression affects the surface targeting of NHE3, the surface membrane expression of NHE3 was determined by surface biotinylation to isolate NHE3 on the plasma membrane. Surface proteins were biotinylated and precipitated with strepavidin-agarose beads. NHE3 on the surface was then probed by Western immunoblot. Quantification of NHE3 surface expression by biotinylation has successfully been adapted to OK cells (7, 8). Comparison of pcDNA-, MΔ5-, KMΔ5-, and PC-transfected cells shows that the relative surface membrane expression of NHE3 was not altered significantly by expression of MAST205 or its derivatives (Fig. 8B).
DISCUSSION
In this work, we identified MAST205 as a protein that regulates the transport activity of NHE3. MAST205 appears to be an ideal regulatory protein with structural elements to modulate NHE3 activity. It contains a PDZ domain to interact with NHE3 and a kinase motif to phosphorylate NHE3 or other accessory proteins. We showed that MAST205 specifically interacts with NHE3 through its PDZ domain. Our studies also demonstrated that coexpression of MAST205 resulted in an inhibition of NHE3 activity, and this regulation was dependent on the presence of a functional kinase motif in MAST205.
Despite the cloning of MAST205 more than a decade ago, the physiological role of MAST205 is not clear. MAST205 has initially been identified as a protein that interacts with microtubules and potentially regulates the function of microtubule in mouse testis (29). However, any role of MAST205 on the structural integrity of microtubules or regulation of MAST205 by microtubules is not known. In fact, MAST205 does not directly bind tubulin but rather interacts indirectly via other microtubule-associated proteins, further suggesting that MAST205 may not be regulated by the microtubule network (29). However, a broader role of MAST205 can be inferred from the recent identification of proteins interacting with MAST205 (1, 10, 17, 38). These include β2-syntrophin at the neuromuscular junction that may link MAST205 to the cortical actin cytoskeleton (17). The type IIa Na-dependent phosphate transporter in the renal proximal tubule is shown to interact with MAST205 (10). Recent studies suggested a role of MAST205 in interleukin-12 synthesis and NF-κB activation via interaction with TRAF6 (32, 38). MAST205 also interacts with protocadherin LKC, which induces contact inhibition of cell proliferation (23).
NHE3 is predominantly expressed in the proximal tubule apical membrane (5). Consistent with its interaction with NHE3, MAST205 is highly expressed in the kidney, as demonstrated by Northern blots. The immunohistochemical studies showed MAST205 is located at the apical region of the renal tubules. Overexpression in OK cells located MAST205 throughout the cytoplasm, but its apical localization was also demonstrated. A recent study using Madin-Darby canine kidney cells (MDCK) showed that MAST205 can be recruited to the plasma membrane by coexpression of a member of the cadherin superfamily, protocadherin LKC (23). Whether the apical localization of MAST205 is driven by its interaction with NHE3 remains to be determined. Despite the presence of MAST205 in the kidney, the presence of MAST205 in OK cells could neither be demonstrated by Northern nor Western blot. In contrast, MAST205 mRNA was detected in MDCK cells and the NHE3-expressing human colonic Caco-2 cells (data not shown). The lack of MAST205 expression in OK is perplexing. However, there is precedent that the species difference in nucleotide or protein sequences could explain the absence of a detectable signal in OK cells. For example, NHE3 mRNA from rat, rabbit, and human do not cross-reacts with OK NHE3 mRNA (21).
NHE3 is regulated by a number of hormones that are coupled to protein kinases (20). There are putative phosphorylation sites in the cytoplasmic domain of NHE3, and deletion of the cytoplasmic domain abolished the regulatory features of NHE3 despite the retention of transport activity. These findings suggest that phosphorylation of NHE3 may play a role in regulation of NHE3 activity. Although regulation of transport activity does not always correlate with changes in NHE3 phosphorylation, there are convincing data to suggest that phosphorylation of NHE3 is essential for protein kinase A-dependent regulation (15, 31, 33, 37). Our findings that the kinase domain of MAST205 is required for the inhibition of NHE3 activity and phosphorylation of NHE3 in vitro suggest that the regulation of NHE3 by MAST205 is probably phosphorylation dependent. The catalytic domain of MAST205 shows limited structural resemblance to the ACG group of protein kinases and the substrate specificity of MAST205 is not known. Our preliminary attempts to define the phosphorylation sites in NHE3 showed that MAST205 phosphorylates NHE3 at multiple locations (data not shown). It is noteworthy that Walden and Cowan (29) reported the presence of a major phosphorylated protein with a molecular mass of 75 kDa within the MAST205-containing immune complexes (29). Given the resolution limits of SDS-PAGE, it is tempting to consider that the size of ~75 kDa is within the molecular mass range of NHE3, which is highly expressed in testis (28). However, a kinase could phosphorylate NHE3 at different sites with varying efficiency under in vitro and in vivo conditions, as in the case of phosphorylation of NHE3 by protein kinase C (31). We cannot preclude the possibility that MAST205 may not phosphorylate NHE3 in vivo and may indirectly regulate NHE3 activity via phosphorylation of another protein.
During the course of these studies, we attempted to assay the kinase activity of MΔ5, after treatment with various agonists, including phorbol ester, cAMP, insulin, and epidermal growth factor. However, we were not able to observe a consistent pattern in the kinase activity. Although this problem is likely related to the limited amounts of the affinity-purified kinase resulting in a relatively low signal-to-noise ratio, there remains a possibility that the enzymatic activity of MAST205 may not acutely respond to the mitogenic stimuli. Instead, MAST205 might be subjected to transcriptional regulation. For example, it has been shown that MAST205 is highly expressed in developing spermatids, and its expression is also controlled by the class II myosin heavy chain transactivator in B cell (22, 30).
In summary, we have identified MAST205 as a protein that interacts with NHE3. Given that MAST205 contains a PDZ domain to interact with NHE3 and a kinase motif to phosphorylate NHE3, it raises the possibility that MAST205 may phosphorylate NHE3. Expression of MAST205 in OK cells resulted in a significant inhibition of NHE3 activity. The absence of inhibition by the kinase-dead KMΔ5 suggests that MAST205 may inhibit NHE3 activity by a phosphorylation-dependent mechanism.
Acknowledgments
We are extremely grateful to Dr. Daniel Biemesderfer for the monoclonal anti-NHE3 antibody and helpful discussion. We thank the Emory Epithelial Pathobiology Research Development Center [supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant DK-064399] for assistant with confocal microscopy.
Footnotes
GRANTS
This work was supported by grants to C. C. Yun (NIDDK Grant DK-061418 and the American Heart Association Southeast Affiliate Grant-in-aid) and I. Choi (American Heart Association Beginning Grant-In-Aid).
References
- 1.Adey NB, Huang L, Ormonde PA, Baumgard ML, Pero R, Byreddy DV, Tavtigian SV, Bartel PL. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res. 2000;60:35–37. [PubMed] [Google Scholar]
- 2.Akhter S, Cavet ME, Tse CM, Donowitz M. C-terminal domains of Na+/H+ exchanger isoform 3 are involved in the basal and serum-stimulated membrane trafficking of the exchanger. Biochemistry. 2000;39:1990–2000. doi: 10.1021/bi991739s. [DOI] [PubMed] [Google Scholar]
- 3.Bevensee MO, Schmitt BM, Choi I, Romero MF, Boron WF. An electrogenic Na+-HCO3− cotransporter (NBC) with a novel COOH-terminus, cloned from rat brain. Am J Physiol Cell Physiol. 2000;278:C1200–C1211. doi: 10.1152/ajpcell.2000.278.6.C1200. [DOI] [PubMed] [Google Scholar]
- 4.Biemesderfer D, Nagy T, DeGray B, Aronson PS. Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. J Biol Chem. 1999;274:17518–17524. doi: 10.1074/jbc.274.25.17518. [DOI] [PubMed] [Google Scholar]
- 5.Biemesderfer D, Rutherford PA, Nagy T, Pizzonia JH, Abu-Alfa AK, Aronson PS. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am J Physiol Renal Physiol. 1997;273:F289–F299. doi: 10.1152/ajprenal.1997.273.2.F289. [DOI] [PubMed] [Google Scholar]
- 6.Cheng J, Moyer BD, Milewski M, Loffing J, Ikeda M, Mickle JE, Cutting GR, Li M, Stanton BA, Guggino WB. A Golgi-associated PDZ domain protein modulates cystic fibrosis transmembrane regulator plasma membrane expression. J Biol Chem. 2002;277:3520–3529. doi: 10.1074/jbc.M110177200. [DOI] [PubMed] [Google Scholar]
- 7.Collazo R, Fan L, Hu MC, Zhao H, Wiederkehr MR, Moe OW. Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. J Biol Chem. 2000;275:31601–31608. doi: 10.1074/jbc.M000600200. [DOI] [PubMed] [Google Scholar]
- 8.Di Sole F, Cerull R, Babich V, Quinones H, Gisler SM, Biber J, Murer H, Burckhardt G, Helmle-Kolb C, Moe OW. Acute regulation of Na/H exchanger NHE3 by adenosine A(1) receptors is mediated by calcineurin homologous protein. J Biol Chem. 2004;279:2962–2974. doi: 10.1074/jbc.M306838200. [DOI] [PubMed] [Google Scholar]
- 9.Girardi AC, Knauf F, Demuth HU, Aronson PS. Role of dipeptidyl peptidase IV in regulating activity of Na+/H+ exchanger isoform NHE3 in proximal tubule cells. Am J Physiol Cell Physiol. 2004;287:C1238–C1245. doi: 10.1152/ajpcell.00186.2004. [DOI] [PubMed] [Google Scholar]
- 10.Gisler SM, Stagljar I, Traebert M, Bacic D, Biber J, Murer H. Interaction of the type IIa Na/Pi cotransporter with PDZ proteins. J Biol Chem. 2001;276:9206–9213. doi: 10.1074/jbc.M008745200. [DOI] [PubMed] [Google Scholar]
- 11.Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phophatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 12.Harlow E and Lane D.Antibodies: A Laboratory Manual Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 1988.
- 13.Kim JH, Lee-Kwon W, Park JB, Ryu SH, Yun CH, Donowitz M. CaP2+P-dependent inhibition of Na+/H+ exchanger 3 requires a NHE3/ E3KARP/alpha -actinin-4 complex for oligomerization and endocytosis. J Biol Chem. 2002;277:23714–23724. doi: 10.1074/jbc.M200835200. [DOI] [PubMed] [Google Scholar]
- 14.Kreis TE. Microinjected antibodies against the cytoplasmic domain of vesicular stomatis virus glycoprotein block its tranport to the cell surface. EMBO J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase. J Biol Chem. 1997;272:28672–28679. doi: 10.1074/jbc.272.45.28672. [DOI] [PubMed] [Google Scholar]
- 16.Lamprecht G, Weinman EJ, Yun CC. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem. 1998;273:29972–29978. doi: 10.1074/jbc.273.45.29972. [DOI] [PubMed] [Google Scholar]
- 17.Lumeng C, Phelps S, Crawford GE, Walden PD, Barald K, Chamberlain JS. Interactions between beta 2-syntrophin and a family of microtubule-associated serine/threonine kinases. Nat Neurosci. 1999;2:611–617. doi: 10.1038/10165. [DOI] [PubMed] [Google Scholar]
- 18.Mahon MJ, Donowitz M, Yun CC, Segre GV. Na+/H+ exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature. 2002;417:858–861. doi: 10.1038/nature00816. [DOI] [PubMed] [Google Scholar]
- 19.McDonough AA, Biemesderfer D. Does membrane trafficking play a role in regulating the sodium/hydrogen exchanger isoform 3 in the proximal tubule? Curr Opin Nephrol Hypertens. 2003;12:533–541. doi: 10.1097/00041552-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Moe OW. Acute regulation of proximal tubule apical membrane Na/H exchanger NHE-3: role of phosphorylation, protein trafficking, and regulatory factors. J Am Soc Nephrol. 1999;10:2412–2425. doi: 10.1681/ASN.V10112412. [DOI] [PubMed] [Google Scholar]
- 21.Moe OW, Miller RT, Horie S, Cano A, Preisig P, Alpern RJ. Differential regulation of Na/H antiporter by acid in renal epithelial cells and fibroblasts. J Clin Invest. 1991;88:1703–1708. doi: 10.1172/JCI115487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagarajan UM, Bushey A, Boss JM. Modulation of gene expression by the MHC class II transactivator. J Immunol. 2002;169:5078–5088. doi: 10.4049/jimmunol.169.9.5078. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki N, Takahashi N, Kojima Si Masuho Y, Koga H. Protocadherin LKC, a new candidate for a tumor suppressor of colon and liver cancers, its association with contact inhibition of cell proliferation. Carcinogenesis. 2002;23:1139–1148. doi: 10.1093/carcin/23.7.1139. [DOI] [PubMed] [Google Scholar]
- 24.Pang T, Su X, Wakabayashi S, Shigekawa M. Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J Biol Chem. 2001;276:17367–17372. doi: 10.1074/jbc.M100296200. [DOI] [PubMed] [Google Scholar]
- 25.Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting (Abstract) Proc Natl Acad Sci USA. 2002;8:8. doi: 10.1073/pnas.162232699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szaszi K, Kurashima K, Kaibuchi K, Grinstein S, Orlowski J. Role of the cytoskeleton in mediating cAMP-dependent protein kinase inhibition of the epithelial Na+/H+ exchanger NHE3. J Biol Chem. 2001;276:40761–40768. doi: 10.1074/jbc.M106724200. [DOI] [PubMed] [Google Scholar]
- 27.Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108:289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tse CM, Brant SR, Walker SM, Pouyssegur J, Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na/H exchanger isoform NHE-3. J Biol Chem. 1992;267:9340–9346. [PubMed] [Google Scholar]
- 29.Walden PD, Cowan NJ. A novel 205-kilodalton testis-specific serine/threonine protein kinase associated with microtubules of the spermatid manchette. Mol Cell Biol. 1993;13:7625–7635. doi: 10.1128/mcb.13.12.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walden PD, Millette CF. Increased activity associated with the MAST205 protein kinase complex during mammalian spermiogenesis. Biol Reprod. 1996;55:1039–1044. doi: 10.1095/biolreprod55.5.1039. [DOI] [PubMed] [Google Scholar]
- 31.Wiederkehr MR, Zhao H, Moe OW. Acute regulation of Na/H exchanger NHE3 activity by protein kinase C: role of NHE3 phosphorylation. Am J Physiol Cell Physiol. 1999;276:C1205–C1217. doi: 10.1152/ajpcell.1999.276.5.C1205. [DOI] [PubMed] [Google Scholar]
- 32.Xiong H, Li H, Chen Y, Zhao J, Unkeless JC. Interaction of TRAF6 with MAST205 regulates NF-kappaB activation and MAST205 stability. J Biol Chem. 2004;279:43675–43683. doi: 10.1074/jbc.M404328200. [DOI] [PubMed] [Google Scholar]
- 33.Yip JW, Ko WH, Viberti G, Huganir RL, Donowitz M, Tse CM. Regulation of the epithelial brush border Na+/H+ exchanger isoform 3 stably expressed in fibroblasts by fibroblast growth factor and phorbol esters is not through changes in phosphorylation of the exchanger. J Biol Chem. 1997;272:18473–18480. doi: 10.1074/jbc.272.29.18473. [DOI] [PubMed] [Google Scholar]
- 34.Yun CC, Chen Y, Lang F. Glucocorticoid activation of Na+/H+ exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem. 2002;277:7676–7683. doi: 10.1074/jbc.M107768200. [DOI] [PubMed] [Google Scholar]
- 35.Yun CC, Lamprecht G, Forster DV, Sidor A. NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J Biol Chem. 1998;273:25856–25863. doi: 10.1074/jbc.273.40.25856. [DOI] [PubMed] [Google Scholar]
- 36.Yun CC, Oh S, Zizak M, Steplock D, Tsao S, Tse CM, Weinman EJ, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA. 1997;94:3010–3015. doi: 10.1073/pnas.94.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na+/H+ exchanger NHE-3 by cAMP. Role of protein kinase a and NHE-3 phosphoserines 552 and 605. J Biol Chem. 1999;274:3978–3987. doi: 10.1074/jbc.274.7.3978. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H, Xiong H, Li H, Plevy SE, Walden PD, Sassaroli M, Prestwich GD, Unkeless JC. Microtubule-associated serine/threonine kinase-205 kDa and Fc gamma receptor control IL-12 p40 synthesis and NF-kappa B activation. J Immunol. 2004;172:2559–2568. doi: 10.4049/jimmunol.172.4.2559. [DOI] [PubMed] [Google Scholar]


