Abstract
Background: Successful arthrodesis in challenging clinical scenarios is facilitated when the site is augmented with autograft bone. The iliac crest has long been the preferred source of autograft material, but graft harvest is associated with frequent complications and pain. Connective tissue progenitor cells aspirated from the iliac crest and concentrated with allograft matrix and demineralized bone matrix provide a promising alternative to traditional autograft harvest. The vertebral body, an even larger reservoir of myeloproliferative cells, should provide progenitor cell concentrations similar to those of the iliac crest.
Methods: Twenty-one adults (eleven men and ten women with a mean age of 59 ± 14 years) undergoing posterior lumbar arthrodesis and pedicle screw instrumentation underwent transpedicular aspiration of connective tissue progenitor cells. Aspirates were obtained from two depths within the vertebral body and were quantified relative to matched, bilateral aspirates from the iliac crest that were obtained from the same patient at the same time. Histochemical analysis was used to determine the prevalence of vertebral progenitor cells relative to the depth of aspiration, the vertebral level, age, and gender, as compared with the iliac crest standard. The cell count, progenitor cell concentration (cells/cc marrow), and progenitor cell prevalence (cells/million cells) were calculated.
Results: Aspirates of vertebral marrow demonstrated comparable or greater concentrations of progenitor cells compared with matched controls from the iliac crest. Progenitor cell concentrations were consistently higher than matched controls from the iliac crest (p = 0.05). The concentration of osteogenic progenitor cells was, on the average, 71% higher in the vertebral aspirates than in the paired iliac crest samples (p = 0.05). With the numbers available, there were no significant differences relative to vertebral body level, the side aspirated, the depth of aspiration, or gender. An age-related decline in cellularity was suggested for the iliac crest aspirates.
Conclusions: The vertebral body is a suitable site for aspiration of bone marrow for graft augmentation during spinal arthrodesis.
Spinal arthrodesis is the indicated treatment for severe or progressive spinal deformity, spinal instability following spinal decompression, spondylolisthesis, and some cases of degenerative disease. A successful outcome of surgical treatment often depends on a solid osseous fusion, and the failure of fusion (pseudarthrosis) may predispose the patient to fixation failure and pain.
Among the factors that the surgeon can control to maximize surgical success, bone graft placement at the surgical site plays an important role in generating a robust and consistent fusion. Autograft bone that is harvested from the iliac crest generally serves as the “gold standard” for spine fusion studies1. The harvesting of autograft bone is associated with a considerable complication rate and may prove to be a source of chronic pain in many patients2,3. Allograft bone, on its own, and the variety of graft extenders currently being introduced for spine fusion, are generally unreliable for stimulating fusion without the contribution of added autograft bone. Bone morphogenetic proteins (BMPs) continue to show promise in animal and clinical trials, but they remain expensive4.
Previous studies by Muschler et al. demonstrated that autologous connective-tissue progenitor cells—the osteogenic stem cell precursors—can be harvested in great numbers from the iliac crest marrow and concentrated on allograft cancellous matrix to form a suitable graft substitute5-7. The capacity of this composite material to stimulate arthrodesis has been demonstrated in both animal and clinical models8-11.
The purpose of the present study was to determine whether the vertebral body, a hematopoietic marrow reservoir, could provide progenitor cell volumes comparable with those in the iliac crest, thus providing an additional source of stem cells for graft augmentation during spinal arthrodesis. We tested the hypothesis that the concentration and prevalence of connective-tissue progenitor cells among vertebral body aspirates would not be significantly lower than those from the iliac crest of the same individual. The potential benefit of this finding, assuming that such a reservoir was verified, would be to eliminate the need for iliac crest harvest in many cases and to provide an additional source of progenitor cells both for patients requiring extensive grafting and for those with limited cancellous volume within the pelvis.
Materials and Methods
Twenty-one adult patients scheduled for posterior lumbar arthrodesis with pedicle screw instrumentation were enrolled in this prospective, institutional review board-approved study. Patients provided informed consent before being included in the study. The patients included eleven men and ten women who were scheduled to undergo lumbar surgery, with use of segmental pedicle screw instrumentation, for the treatment of degenerative disc disease or lumbar instability. Patients were excluded from the study if they had had previous iliac crest harvest, previous spinal surgery with instrumentation, or previous irradiation of either the spine or the pelvis. Patients also were excluded if they suffered from any myeloproliferative disorder or if they were being managed with chronic steroid medication, thyroxine, or chemotherapy. The mean age (and standard deviation) at the time of surgery was 59 ± 14 years (range, twenty-six to eighty-three years). A traditional bone-grafting technique provided bone as the source of fusion in these patients.
The surgical procedure was carried out in a routine fashion, as indicated for the underlying disorder, with no alteration in the surgical approach or technique. At the point of pedicle screw instrumentation, the only alteration to the usual screw insertion technique12 was that a 2.8-mm-diameter aspiration needle was used to create the typical pilot hole for screw insertion. This needle was substituted for the blunt probe (or “gear shift”) that is commonly used, but it was inserted under fluoroscopic control in the usual fashion (Fig. 1). Previous studies have shown that the aspiration technique greatly affects the concentration of connective-tissue progenitor cells harvested from bone marrow, primarily through dilution with peripheral blood6. With use of an established technique that has been validated in previous studies5,6, bone marrow cells were aspirated directly into 10.0-mL syringes that had been preloaded with heparinized saline solution (1000 units of sodium heparin in 1.0 mL of saline solution). A smear was made at the time of each aspiration to confirm the presence of nucleated cells and the adequacy of the aspirate.
Fig. 1.

Fluoroscopic image of an aspiration needle in the transpedicular position for marrow aspiration. Posteroanterior and lateral views confirmed needle orientation and depth at each point of aspiration.
For the vertebral bodies, 2.0-cc aliquots were aspirated at two depths—first at the pedicle-vertebral body junction (2.0 to 2.5 cm), and then deeper within the vertebral body (3.5 to 4.0 cm)—from both the left and right sides. This yielded 8.0 cc of bone marrow aspirate from each of the two vertebrae from each patient. During the same operation, eight 2.0-cc aspirates (four aspirates from each side) were obtained percutaneously from the iliac crests. Therefore, for each patient, 8 cc of vertebral body bone marrow from each of the two vertebral levels (that is, a total of 16 cc from eight samples) were matched against 8 cc of marrow aspirated from each of the two iliac crests (that is, a total of 16 cc from eight samples).
Three parameters were measured directly or calculated from the results of cell culture: (1) the nucleated-cell count (the number of nucleated cells per 1.0 cc of marrow aspirate), (2) the prevalence of connective-tissue progenitor cells (the number of connective-tissue progenitor cells per 106 nucleated cells), and (3) the concentration of connective-tissue progenitor cells (the number of connective-tissue progenitor cells per 1.0 cc of aspirate).
The number of connective-tissue progenitor cells in a sample can be estimated by counting the number of colony-forming units (CFUs) expressing alkaline phosphatase activity in culture (CFU-APs)3. Alkaline phosphatase is an early marker for osteoblastic differentiation of these pluripotential cells13-15.
The heparinized marrow sample from each site was suspended in 20 mL of essential medium (Alpha-MEM; Gibco, Grand Island, New York) containing 2 U/mL Na-heparin and sealed in a test tube for immediate transport to the laboratory. Cell-count and connective-tissue progenitor cell (CFU-AP) assays were carried out without delay. Samples were centrifuged at 1500 rpm for ten minutes. The buffy coat was isolated and suspended in alpha-MEM containing 10% fetal bovine serum (BioWhittaker, Walkersville, Maryland), 50 mg/mL sodium ascorbate, antibiotic/antimycotic (Gibco, catalog #15420), and 10−8 M dexamethasone. The number of nucleated cells in each aspirate was then counted.
Each sample was placed into culture in four wells (two dual-chamber slides [Lab-Tek, Rochester, New York]) for connective-tissue progenitor cell assay on Day 6. Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 in air. Medium was exchanged after twenty-four hours and then again on Day 3. Alkaline phosphatase staining was carried out on Day 6 by rinsing cell layers twice with calcium-and-magnesium-free Hank's balanced salt solution (Gibco) and incubating them for thirty minutes with 120-mM Tris buffer (pH 8.4) containing 0.9-mM naphthol AS-MX phosphate and 1.8-mM fast red TR. After incubation, the cultures were washed in deionized water. The number of CFUs (eight or more cells in cluster) expressing alkaline phosphatase activity (CFU-APs) provided a quantitative measure of the prevalence of connective-tissue progenitor cells obtained from the vertebral body relative to depth of aspiration, vertebral level, age, and gender, as compared with the iliac crest standard. The total number of connective-tissue progenitor cells was defined as the maximal count of CFU-APs seen on Day 6 after plating.
Each outcome value (the total number of nucleated cells and the prevalence of connective tissue progenitor colonies in the sample per million nucleated cells) was summarized as a mean, median, and standard deviation. Because the data did not provide a normal distribution, a log transformation was applied to normalize the data and to eliminate the positive distribution skew noted in previous analyses5. The resulting variables have a normal distribution and can be analyzed with analysis of variance methods. Previous studies have shown that the number of nucleated cells per 2 cc aspirate from the pelvis is about 50 to 150 million6,7. The prevalence of connective-tissue progenitor cells (clusters of eight or more cells demonstrating alkaline phosphatase expression) is usually in the range of twenty to sixty connective-tissue progenitor cells/million cells. Data were analyzed relative to gender, age, the site of harvest, and the depth of harvest within the vertebral body. Pearson correlation coefficients were calculated for these specific comparisons. The level of significance was set at p < 0.05.
Results
Nucleated Cell Count
The total number of nucleated cells per 1.0 cc of aspirated marrow was consistently higher in the vertebral aspirates as compared with paired iliac crest specimens, but the differences were not significant. A mean of 19.76 × 106 nucleated cells (median, 18.0 × 106 nucleated cells; interquartile range, 13.3 to 24.2 × 106 nucleated cells) were contained in each 1.0 cc of vertebral marrow aspirated, compared with 16.95 × 106 (median, 14.7 × 106; interquartile range, 10.1 to 22.1 × 106) for iliac crest aspirates (Fig. 2). The cell count for vertebral body aspirates was, on the average, about 24% higher than the paired iliac crest values (p = 0.05). While there was variation from pedicle to pedicle, the largest variations in cell count were related to interindividual differences.
Fig. 2.

Plots showing the mean nucleated cell concentrations in the vertebral aspirates (Vert Asp) and iliac crest aspirates (IC Asp) for the twenty-one patients in the study.
While there was no significant age-related decline in cellularity for the aspirates from either the vertebral body or the iliac crest, such a trend was suggested (p = 0.10) for the iliac crest specimens. With the numbers available, there was no significant contribution to variation with respect to gender (p = 0.62), depth of harvest (p = 0.13), or side of the vertebral aspirate (p = 0.30) when analyzed separately.
Prevalence of Connective-Tissue Progenitor Cells
With the numbers available, there were no significant differences in the prevalence of connective-tissue progenitor cells (CFU-AP/106 nucleated cells) in relation to vertebral body level (Fig. 3). A mean of 19.62 connective-tissue progenitor cells (median, 16.0 connective-tissue progenitor cells; interquartile range, seven to seventeen connective-tissue progenitor cells) were identified per one million nucleated cells aspirated from the vertebral body. This value compared favorably with, but was not significantly better than, the mean of 17.76 connective-tissue progenitor cells (median, 12.0 connective-tissue progenitor cells; interquartile range, six to twenty-four connective-tissue progenitor cells) per million cells that were isolated from the iliac crest. The prevalence of connective-tissue progenitor cells in the vertebral body was a mean of 31% higher (95% confidence interval, 12% lower to 53% higher) than that in the paired iliac crest samples (p = 0.17) after adjusting for side, gender, and age. With the numbers available, there was no significant difference in marrow connective-tissue progenitor cell prevalence relative to side, depth of aspiration, or gender. On the average, the prevalence of connective-tissue progenitor cells in the deep pedicle aspirates was 22% higher (95% confidence interval, 10% lower to 54% higher) than that in the superficial pedicle aspirates, but the difference was not significant (p = 0.20) after adjusting for gender and side.
Fig. 3.
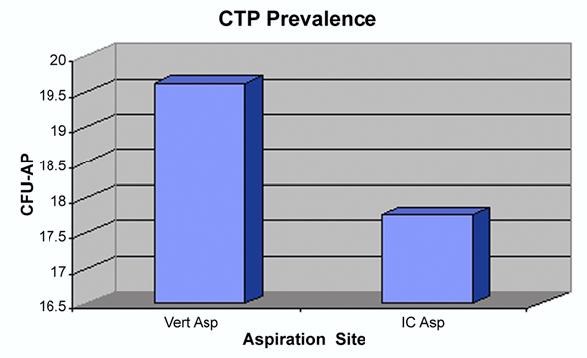
Plots showing the mean prevalences of connective-tissue progenitor (CTP) cells in the vertebral aspirates (Vert Asp) and iliac crest aspirates (IC Asp) for the twenty-one patients in the study. CFU-AP = colony-forming units expressing alkaline phosphatase activity in culture.
Concentration of Connective-Tissue Progenitor Cells
The concentration of osteogenic connective-tissue progenitor cells was calculated for each sample as the product of the nucleated cell count and the prevalence of osteogenic connective-tissue progenitor cells. Aspirates of vertebral marrow demonstrated similar or greater concentrations of connective-tissue progenitor cells compared with aspirates from the iliac crest (Fig. 4). The results of 168 aspirations from forty-two vertebral bodies revealed a mean of 465.67 ± 434 connective-tissue progenitor cells/cc of marrow (median, 248 connective-tissue progenitor cells/cc of marrow; interquar-tile range, 109 to 526 connective-tissue progenitor cells/cc of marrow), whereas the results of 168 aspirations from the iliac crest revealed a mean of 356 ± 469 connective-tissue progenitor cells/cc of marrow (median, 181 connective-tissue progenitor cells/cc of marrow; interquartile range, seventy to 401 connective-tissue progenitor cells/cc of marrow). On the average, the concentration of osteogenic connective-tissue progenitor cells in the vertebral aspirates was 71% higher (95% confidence interval, 3% lower to 138% higher) than that in the paired iliac crest samples (p = 0.05).
Fig. 4.

Plots showing the relative mean concentrations of connective-tissue progenitor (CTP) cells for the superficial vertebral, deep vertebral, and iliac crest aspiration sites for the twenty-one patients in the study.
A breakdown of superficial and deep vertebral aspirates revealed a median unadjusted connective-tissue progenitor cell concentration of 237 connective-tissue progenitor cells/cc bone marrow (interquartile range, ninety-two to 517 connective-tissue progenitor cells/cc bone marrow) in superficial aspirates, 260 connective-tissue progenitor cells/cc bone marrow (interquartile range, 114 to 567 connective-tissue progenitor cells/cc bone marrow) in deep vertebral aspirates, and 181 connective-tissue progenitor cells/cc bone marrow (interquar-tile range, seventy to 401 connective-tissue progenitor cells/cc bone marrow) in iliac crest aspirates. While these differences were not significant, vertebral aspirates were clearly not worse than iliac crest aspirates.
In only one patient was the concentration of connective-tissue progenitor cells in the iliac crest aspirates significantly higher than that in the vertebral aspirates, and in that patient both the iliac crest and vertebral values exceeded the iliac crest values for eighteen of the twenty-one patients (Fig. 5). One other patient demonstrated a severe deficit in connective-tissue progenitor cells within the iliac crest marrow while showing average nucleated cell volumes in the spine and pelvis and low-normal connective-tissue progenitor cell concentrations in vertebral marrow.
Fig. 5.

Illustration depicting the calculated concentration of connective-tissue progenitor (CTP) cells for the twenty-one patients in the study.
Discussion
The present study demonstrates that bone marrow that is aspirated from the vertebral body during pedicle screw preparation is comparable with that aspirated from the iliac crest with respect to providing osteogenic progenitor cells in sufficient concentration for use in graft augmentation. The present study also describes a harvesting technique that is useful during any spinal instrumentation procedure.
There are variations in the prevalence of connective-tissue progenitor cells from region to region within the pelvis, and variation should be expected from level to level within the spine. Other bones with hematopoietic marrow (such as the femur and the tibia) consistently have lower concentrations of connective-tissue progenitor cells relative to the pelvis. Vertebral marrow characteristics have not been well quantified, and vertebral autologous bone has not previously been considered to be a routine source of bone graft material. Even if this marrow reservoir were found to be a rich source of connective-tissue progenitor cells, it is only accessible to surgeons during rather specific surgical procedures, limiting its usefulness during most skeletal procedures. However, the operations that naturally provide access to the vertebral reservoir—spinal instrumentation using pedicle screws—depend on fusion for clinical success, so transpedicular or vertebral body aspiration likely will be of great clinical interest to spine surgeons. Removal of the marrow progenitor cells does not appear to compromise the mechanical integrity of the vertebral body and can be accomplished without incrementally increasing surgical risk. In fact, since entry into the vertebral pedicle can be accomplished without disruption of the facet joint or the articular tissues, it would be reasonable to use this point of access to harvest marrow cells for uninstrumented fusions as well.
One important technical point must be noted: As the aspiration tract serves as the pilot hole for pedicle screw fixation, the needle cannot be fanned out through the vertebral bone as is the practice during iliac crest aspiration. This limits access to a discrete cylinder of marrow bone coaxial with the pedicle and may limit utility to some degree.
We found a suitable, and often superior, source of nucleated cells in the vertebrae sampled in this patient group. Despite the wide range of ages and clinical histories, vertebral aspirates were consistently comparable with paired iliac crest samples, and most were higher in terms of both cell volume and the prevalence of connective-tissue progenitor cells. The interindividual variation in the number of marrow cells and prevalence of connective-tissue progenitor cells was consistent with the findings reported in previous studies5,6 and was reflected in aspirates from both anatomical regions. Previous studies have shown that there are large variations in the prevalence of iliac crest marrow progenitor cells from site to site in the same individual but that 70% of the observed variability in the prevalence of connective-tissue progenitor cells is accounted for by interindividual variation. The current study reflected those same observations, although vertebral aspirates did not demonstrate the paucity of cells seen at the lower end of the range seen in iliac crest aspirates.
One patient in the present study had iliac crest aspirates that were far below the norm, compared both with the other members of the cohort and with the established ranges from previous studies6,7. All eight iliac crest aspirates provided similar values, and it was concluded that the values were genuine. The vertebral aspirates from this patient demonstrated normal cell numbers and normal concentrations of connective-tissue progenitor cells, suggesting a regional disturbance to marrow health within the pelvis. In the case of this patient, the vertebral marrow would have provided a considerably better aspirate for graft augmentation than the iliac crest would have provided.
While autograft provides all of the elements needed to promote a successful fusion—osseous matrix, osteoinductive peptides, and osteoprogenitor cells—it is not always available in volume, and its harvest may result in considerable morbidity. There are also circumstances in which the harvest of adequate graft is not possible. When patients require long and extensive arthrodeses or revision surgery after previous graft harvest16-19, have paralytic deformities that require fixation into the pelvic wings20,21, or have had irradiation of the pelvis, there may not be an adequate volume of iliac crest bone to complete the fusion alone. Even when autograft material is available, extensive corticocancellous graft harvests are painful and debilitating and predispose the patient to serious complications.
If the surgeon wishes to limit graft site pain and complications altogether, yet gain the physiological advantages of autologous bone-grafting, then marrow aspiration becomes an attractive alternative to traditional graft harvest techniques. Compared with traditional iliac crest graft harvest techniques, both aspiration methods reduce operative time and morbidity. While aspiration of the iliac crest takes only a few minutes, aspiration of the pedicle adds no more time to the procedure because the pedicle will be sounded and a pilot hole will be created for the placement of each pedicle screw anyway.
In conclusion, a population of autologous osteoblast progenitors resides within the portion of the vertebral body that is routinely entered for pedicle screw placement. The biologic activity and prevalence of the connective-tissue progenitor cells within this site are comparable with those of cells from the iliac crest. This alternative marrow source may further reduce the time and morbidity associated with iliac crest harvest. Future clinical studies will attempt to confirm the ability to obtain fusion using only this source of connective-tissue progenitor cells.
Acknowledgments
The authors thank Edward Mascha, PhD, of the Department of Quantitative Health Sciences of The Cleveland Clinic Foundation, and Candy Rufo-Smith, RN, BSN, of the Cleveland Clinic Spine Institute, for their assistance in conducting this study.
Footnotes
Robert F. McLain, MD James E. Fleming, MD Cynthia A. Boehm, BS George F. Muschler, MD Department of Orthopaedic Surgery, Desk A 41, The Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195. E-mail address for R.F. McLain: mclainr@ccf.org
In support of their research or preparation of this manuscript, one or more of the authors received grants or outside funding from National Institutes of Health AR42997, OREF. In addition, one or more of the authors received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity (DePuy Spine, Inc.). No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, educational institution, or other charitable or nonprofit organization with which the authors are affiliated or associated.
References
- 1.Lane JM, Muschler GF, Kurz LT, Samburg LC, Herkowitz HN, Hanley EN, Phillips E, Harvell JC, Kahanowitz N, Balderstone RA. Principles of bone fusion. In: Herkowitz HN, Garfin SR, Eismont FR, Bell GR, Weisel SW, editors. Rothman-Simeone's The Spine. W.B. Saunders; Philadelphia: 1996. pp. 1739–55. [Google Scholar]
- 2.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–5. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Heary RF, Schlenk RP, Sacchieri TA, Barone D, Brotea C. Persistent iliac crest donor site pain: independent outcome assessment. Neurosurgery. 2002;50:510–7. doi: 10.1097/00006123-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine. 2002;27:2396–408. doi: 10.1097/00007632-200211010-00015. [DOI] [PubMed] [Google Scholar]
- 5.Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546–57. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- 6.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699–709. doi: 10.2106/00004623-199711000-00012. Erratum in: J Bone Joint Surg Am. 1998; 80:302. [DOI] [PubMed] [Google Scholar]
- 7.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–25. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 8.Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991;266:259–70. [PubMed] [Google Scholar]
- 9.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 10.Muschler GF, Nitto H, Matsukura Y, Boehm C, Valdevit A, Kambic H, Davros W, Powell K, Easley K. Spine fusion using cell matrix composites enriched in bone marrow-derived cells. Clin Orthop Relat Res. 2003;407:102–18. doi: 10.1097/00003086-200302000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieberman IH, Togawa D, McLain RF, Richmond BJ, Fleming JE, Reinhardt MK, Boehm C, Muschler GF. Lumbar interbody fusion using allograft enriched with bone marrow derived cells prepared by selective stem cell retention—results of clinical and radiographic studies and in-vitro assay. Read at the Annual Meeting of the North American Spine Society; Chicago, IL. Oct 26-30, 2004. [Google Scholar]
- 12.McLain RF. Transpedicular fixation. In: Bradford DS, Zdeblick TA, editors. Master techniques in orthopaedic surgery: the spine. 2nd Lippincott Williams and Wilkins; Philadelphia: 2004. pp. 293–312. [Google Scholar]
- 13.Bianco P, Riminucci M, Silvestrini G, Bonucci E, Termine JD, Fisher LW, Robey PG. Localization of bone sialoprotein (BSP) to Golgi and post-Golgi secretory structures in osteoblasts and to discrete sites in early bone matrix. J Histochem Cytochem. 1993;41:193–203. doi: 10.1177/41.2.8419459. [DOI] [PubMed] [Google Scholar]
- 14.Lee K, Deeds JD, Bond AT, Juppner H, Abou-Samra AB, Segre GV. In situ localization of PTH/PTHrP receptor mRNA in the bone of fetal and young rats. Bone. 1993;14:341–5. doi: 10.1016/8756-3282(93)90162-4. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji T, Hughes FJ, McCulloch CA, Melcher AH. Effects of donor age on osteogenic cells of rat bone marrow in vitro. Mech Ageing Dev. 1990;51:121–32. doi: 10.1016/0047-6374(90)90094-v. [DOI] [PubMed] [Google Scholar]
- 16.Kostuik JP, Israel J, Hall JE. Scoliosis surgery in adults. Clin Orthop Relat Res. 1973;93:225–34. doi: 10.1097/00003086-197306000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Ponder RC, Dickson JH, Harrington PR, Erwin WD. Results of Harrington instrumentation and fusion in the adult idiopathic scoliosis patient. J Bone Joint Surg Am. 1975;57:797–801. [PubMed] [Google Scholar]
- 18.Sponseller PD, Cohen MS, Nachemson AL, Hall JE, Wohl ME. Results of surgical treatment of adults with idiopathic scoliosis. J Bone Joint Surg Am. 1987;69:667–75. [PubMed] [Google Scholar]
- 19.van Dam BE, Bradford DS, Lonstein JE, Moe JH, Ogilvie JW, Winter RB. Adult idiopathic scoliosis treated by posterior spinal fusion and Harrington instrumentation. Spine. 1987;12:32–6. doi: 10.1097/00007632-198701000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Mayfield JK. Severe spine deformity in myelodysplasia and sacral agenesis: an aggressive surgical approach. Spine. 1981;6:498–509. doi: 10.1097/00007632-198109000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Osebold WR, Mayfield JK, Winter RB, Moe JH. Surgical treatment of paralytic scoliosis associated with myelomeningocele. J Bone Joint Surg Am. 1982;64:841–56. [PubMed] [Google Scholar]


